Three Different Biopesticides Against Megalurothrips usitatus (Thysanoptera: Thripidae) and Their Toxicological and Biochemical Impacts
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects for Testing
2.2. Formulation of Different Concentrations
2.3. Determination of Laboratory Virulence of Biopesticides Against M. usitatus
2.4. Effects of Different Agents Treatments on Enzyme Activity of M. usitatus
2.5. Data Analysis
3. Results
3.1. Determination of Laboratory Toxicity of Biopesticides Against M. usitatus
3.1.1. Corrected Mortality and Lethal Time (LT50) of B. bassiana on M. usitatus
3.1.2. Corrected Mortality and Lethal Time (LT50) of B. safensis on M. usitatus
3.1.3. Corrected Mortality and Lethal Time (LT50) of A. ordosica essential oil on M. usitatus
3.1.4. Laboratory Toxicity of Different Agents Against M. usitatus
3.2. Protective Enzyme Peroxidase (POD) and Detoxifying Enzyme Glutathione S-Transferase (GST) Activity of M. usitatus
3.2.1. POD Enzyme Activity of M. usitatus
3.2.2. GST Enzyme Activity of M. usitatus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, R.; Seal, D.; Adhikari, R. Bean Flower Thrips Megalurothrips usitatus (Bagnall) (Insecta: Thysanoptera: Thripidae): EENY-777/IN1352, 10/2021. J. EDIS 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Miao, M.; Xu, Y.F.; Zhang, H.X.; Wang, Y.; Zhang, R.; Wei, S.; Ban, L.P. Dynamic and correlation of major pests with natural enemies and response to climatic factors in alfalfa fields in Ningxia. J. Plant Prot. 2024, 51, 1169–1178. [Google Scholar]
- Wei, S.H.; Liu, X.Q.; Wang, Y.; Liu, C.; Zhang, R. Effects of Intercropping Alfalfa and Functional Plants on Population of Thrips and Their Natural Enemies Orius similis. J. Chin. J. Biol. Control 2024, 40, 99–107. [Google Scholar]
- Chen, Y.; Yang, B.; Li, Z.; Yue, Y.; Tian, Q.; Chen, W.; Ali, S.; Wu, J. Immune Related Genes of Megalurothrips usitatus (Bagrall) Against Beauveria brongniartii and Akanthomyces attenuatus Identified Using RNA Sequencing. Front. Physiol. 2021, 12, 671599. [Google Scholar] [CrossRef]
- Weisenburger, D.D. Human health effects of agrichemical use. Hum. Pathol. 1993, 24, 571–576. [Google Scholar] [CrossRef]
- Yura, W.F.; Muhammad, F.R.; Mirza, F.F.; Maurend, Y.L.; Widyantoro, W.; Farida, S.S.; Aziz, Y.P.; Desti, A.; Edy, W.; Septy, M.; et al. Pesticide residues in food and potential risk of health problems: A systematic literature review. IOP Conf. Ser. Earth Environ. Sci. 2021, 894, 012025. [Google Scholar] [CrossRef]
- Yang, L.; Chen, E.H.; Liang, G.W. The role of biological pest control in ecological pest control. J. Cent. South Univ. For. Technol. 2003, 4, 111–115. [Google Scholar]
- Shourove, J.H.; Meem, F.C.; Chowdhury, R.S.; Eti, S.A.; Samaddar, M. Biocontrol agents and their potential use as nano biopesticides to control the tea red spider mite (Oligonychus coffeae): A comprehensive review. Heliyon 2024, 10, e34605. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Akutse, K.S.; Siddiqui, J.A.; Xu, Y. Model application of entomopathogenic fungi as alternatives to chemical pesticides: Prospects, challenges, and insights for next generation sustainable agriculture. Front. Plant Sci. 2021, 12, 741804. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Zhang, J.L.; Lu, Y.H. Toxic efficiency of biocontrol fungi Beauveria bassiana and Metarhizium anisopliae jointly with four insecticides on controlling cotton aphid Aphis gossypii. J. Plant Prot. 2024, 51, 1457–1465. [Google Scholar]
- Pan, X.L.; Yang, L.; Jin, H.F.; Lu, R.; Li, F.; Cao, F.; Wu, S. Research advances in occurrence and control of Megalurothrips usitatus in Hainan. J. Trop. Biol. 2021, 12, 508–513. [Google Scholar]
- Wang, H.H.; Liu, S.; Wang, S.Y.; Lei, Z.R. Research and development of wettable powder of Beauveria bassiana and its control and application to Frankliniella occidentalis. Chin. J. Biol. Control. 2020, 36, 858–861. [Google Scholar]
- Satomi, M.; La, D.M.T.; Venkateswaran, K. Bacillus Safensis sp. nov., isolated from spacecraft and assembly facility surfaces. Int. J. Syst. Evol. Microbiol. 2006, 56, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.X. Study on the Diversity and Function of Bacteria from Nasonia vitripennis Venom Reservoir. Master’s Thesis, Taian Shandong Agricultural University, Taian, China, 2020. [Google Scholar]
- Li, B.H.; Wang, J.F.; Wang, D.D. Effects of Fermentation Broths of Symbiotic, Bacteria in Coboldiafuscipes (Meigen) on Host Olfactory Behavioral Responses and Analysis of Volatiles. J. Life Sci. Res. 2024, 28, 232–240. [Google Scholar]
- Ünlü, E.; Çalış, Ö.; Say, A.; Karim, A.A.; Yetişir, H.; Yılmaz, S. Investigation of the effects of Bacillus subtilis and Bacillus thuringiensis as Bio-agents against powdery mildew (Podosphaera xanthii) disease in zucchini (Cucurbita pepo L.). Microb. Pathog. 2023, 185, 106430. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.Q.; Feng, J.T.; Wu, H.; Han, L. Review on research and development of botanical pesticides. Chin. J. Biol. Control 2015, 31, 685–698. [Google Scholar]
- Bedini, S.; Djebbi, T.; Ascrizzi, R.; Farina, P.; Pieracci, Y.; Echeverría, M.C.; Flamini, G.; Trusendi, F.; Ortega, S.; Chiliquinga, A.; et al. Repellence and attractiveness: The hormetic effect of aromatic plant essential oils on insect behavior. Ind. Crops Prod. 2024, 210, 118122. [Google Scholar] [CrossRef]
- He, Y.; Yu, M.; Ding, G.; Zhang, F. Precipitation pattern changed the content of nonstructural carbohydrates components in different organs of Artemisia ordosica. BMC Plant Biol. 2023, 23, 505. [Google Scholar] [CrossRef]
- Tang, G.; Yang, S.; Hu, W.; Jiang, J.; Yan, H.; Feng, J.; Zhang, C.; Wang, Y. Bioassay-guided isolation of broad spectrum fungicidal active compound from Artemisia ordosica. Metabolites 2021, 11, 629. [Google Scholar] [CrossRef]
- Bruno, S.; Qiang, L.; Mark, W.; Achraf, M.J.; Alexis, R.; Marce, L.Z.; Catherine, M.; Christophe, D. Redoxi Base: A database for ROS homeostasis regulated proteins. J. Redox Biol. 2019, 26, 101247. [Google Scholar]
- McDermott, B.J.; Millar, B.C.; Piper, H.M. Effects of Neuropeptide Y: Receptor Interactions and Cellular Mechanisms. J. Cardiovasc. Res. 1993, 27, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Lozinskaya, Y.L.; Slepneva, I.A.; Khramtsov, V.V. Changes of the antioxidantstatus and system of generation of free radicals in hemolymph of Galleria mellonella larvae at microsporidiosis. J. Evol. Biochem. Physiol. 2004, 40, 119–125. [Google Scholar] [CrossRef]
- Yu, S.J. Biochemical characteristics of microsomal and cytosolicglutathione S-transferases in larvae of the fall armyworm, Spodop-tera frugiperda (J. E. Smith). Pestic. Biochem. Physiol. 2002, 72, 100–110. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Walker, A.C.; Farhan, U. Antioxidants in the mid-gut fluids of a tannin-tolerant and a tannin-sensitive caterpillar: Effects of seasonal changes in tree leaves. J. Chem. Ecol. 2003, 29, 1099–1116. [Google Scholar] [CrossRef]
- Després, L.; David, J.P.; Gallet, C. The evolutionary ecology of in-sect resistance to plant chemicals. Trends Ecol. Evol. 2007, 22, 298–307. [Google Scholar] [CrossRef]
- Chemendoza, A.; Penilla, R.P.; Américo Rodríguez, D. Insecticide resistance and glutathione S-transferases in mosquitoes: A review. Q. J. Med. 2009, 8, 503–513. [Google Scholar]
- Chen, X.H.; Wang, Z.Y.; Li, X.P.; Zhu, Y.M.; Liu, L.; Chen, W.; Chen, Q. Research progress on glutathione S-transferases. J. Northeast. Agric. Univ. 2013, 44, 149–153. [Google Scholar]
- Chamani, M.; Dadpour, M.; Dehghanian, Z.; Panahirad, S.; Chenari Bouket, A.; Oszako, T.; Kumar, S. From Digestion to Detoxification: Exploring Plant Metabolite Impacts on Insect Enzyme Systems for Enhanced Pest Control. Insects 2025, 16, 392. [Google Scholar] [CrossRef]
- Absher, M. Hemocytometer counting. In Tissue Culture; Academic Press: Cambridge, MA, USA, 1973; pp. 395–397. [Google Scholar]
- Stock, S.P. Field manual of techniques in invertebrate pathology. J. Econ. Entomol. 2009, 102, 1726. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Su, X.; Lu, L.Y.; Zhen, C.A.; Zhu, B.; Li, Y.S.; Dong, W.Y.; Wang, G.; Xu, Y.B.; Kong, F.B.; et al. Effects of three insecticides at the sublethal dose on the expression of cytochrome P450 genes in Spodoptera frugiperda (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2020, 63, 565–573. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. J. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Yang, B.; Du, C.; Ali, S.; Wu, J. Molecular characterization and virulence of fungal isolates against the bean flower thrips, Megalurothrips usitatus Bagnall (Thysanoptera: Thripidae). Egypt. J. Biol. Pest Control 2020, 30, 50. [Google Scholar] [CrossRef]
- Ramírez-Larrota, J.S.; Eckhard, U. An introduction to bacterial biofilms and their proteases, and their roles in host infection and immune evasion. Biomolecules 2022, 12, 306. [Google Scholar] [CrossRef]
- India-Aldana, S.; Yao, M.; Midya, V.; Colicino, E.; Chatzi, L.; Chu, J.; Gennings, C.; Jones, D.P.; Loos, R.J.F.; Setiawan, V.W.; et al. PFAS exposures and the human metabolome: A systematic review of epidemiological studies. Curr. Pollut. Rep. 2023, 9, 510–568. [Google Scholar] [CrossRef]
- Wend, K.; Zorrilla, L.; Freimoser, F.M.; Gallet, A. Microbial pesticides challenges and future perspectives for testing and safety assessment with respect to human health. Environ. Health 2024, 23, 49. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ren, Y.; Zhao, J.; Liu, Y.; Xu, B.; Yang, M.; Zhao, W.; Zheng, X.; Wang, J.; Deng, L. Research on the Bioactivity of Plant Essential Oils on Armyworm [Mythimna separata (Walker)] Larvae. Front. Chem. 2022, 10, 936873. [Google Scholar] [CrossRef] [PubMed]
- Nishida, R. Chemical ecology of insect plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Cheng, J.H.; Han, H.B.; Lu, Y.B. Behavioral manipulation of the plant secondary metabolites to thrips and their application in thrips management. Acta Entomol. Sin. 2022, 65, 1222–1246. [Google Scholar]
- Birnbaum, S.S.L.; Rinker, D.C.; Gerardo, N.M.; Abbot, P. Transcriptional profile and differential fitness in a specialist milkweed insect across host plants varying in toxicity. Mol. Ecol. 2017, 26, 6742–6761. [Google Scholar] [CrossRef]
- Tang, J.G.; Zhao, D.Y.; Chen, L.H.; Cao, F.L. Effects of Ginkgo biloba episperm on the growth and enzymatic activity of Plutella xylostella. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2013, 42, 233–236. [Google Scholar]
- Yin, F.; Chen, H.Y.; Feng, X.; Hu, Z.D.; Lin, Q.S.; Li, Z.Y.; Bao, H.L. The role of detoxifying enzymes in the resistance of Plutella xylostella to spinetoram. Chin. J. Appl. Entomol. 2016, 53, 314–319. [Google Scholar]

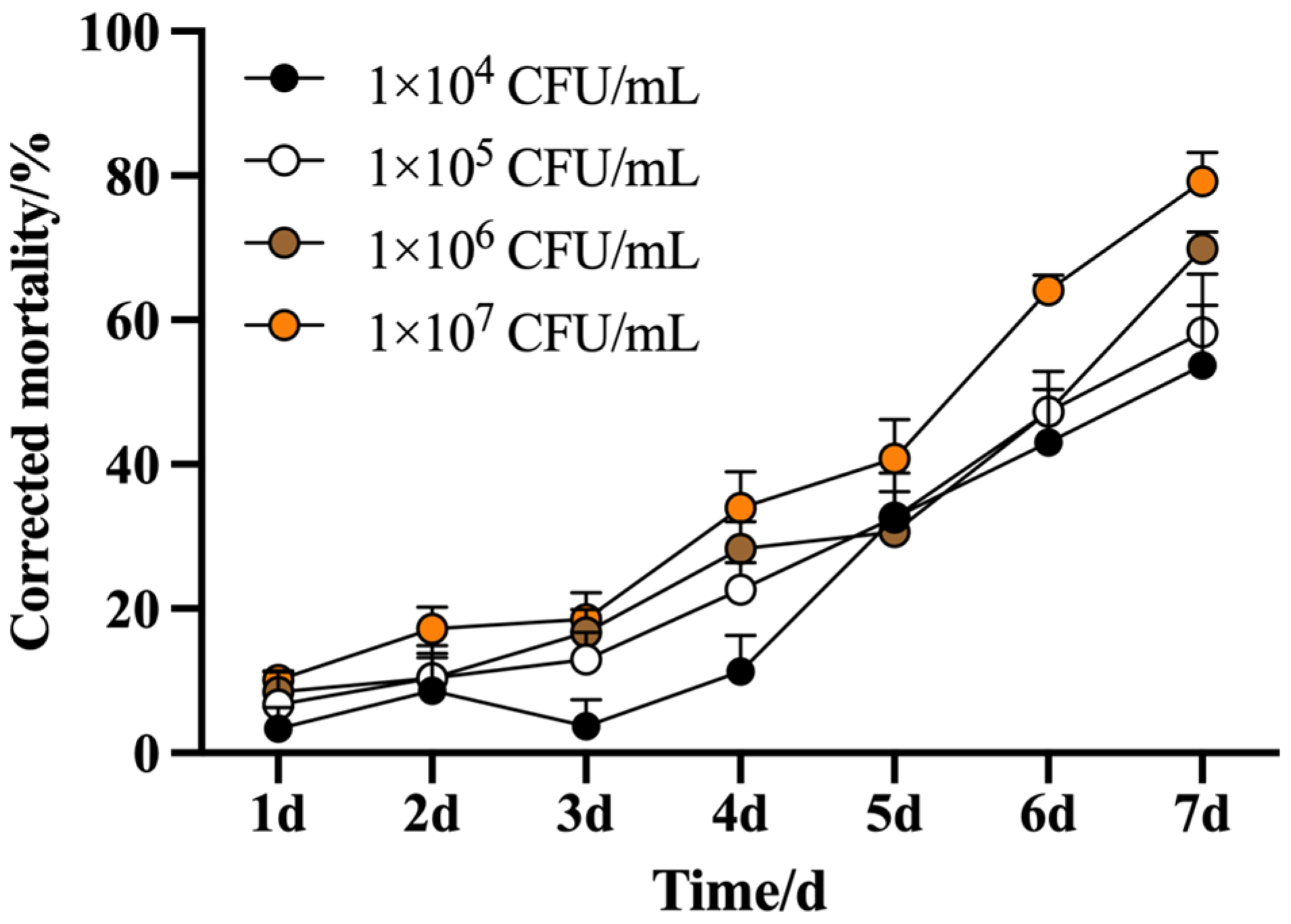
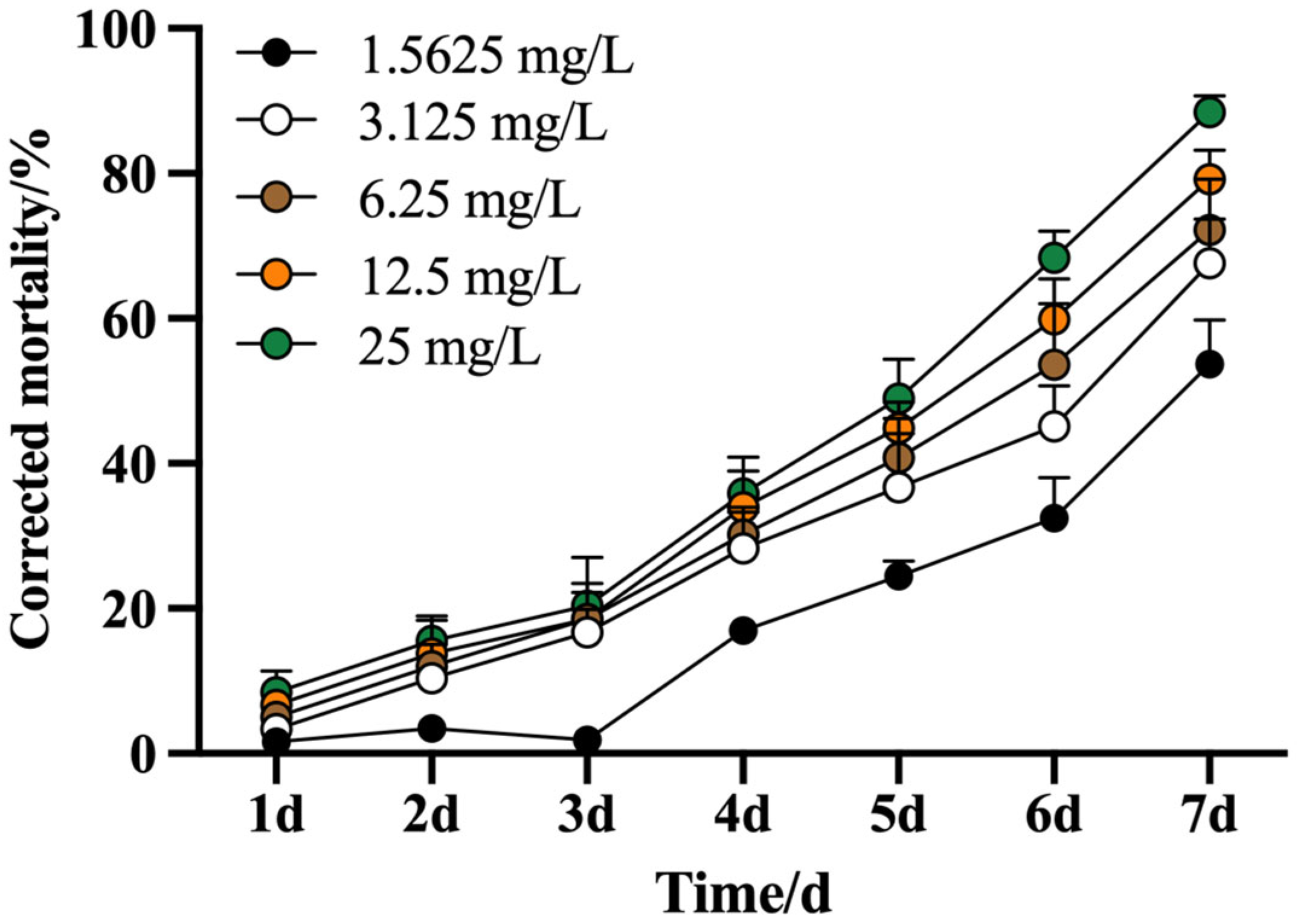

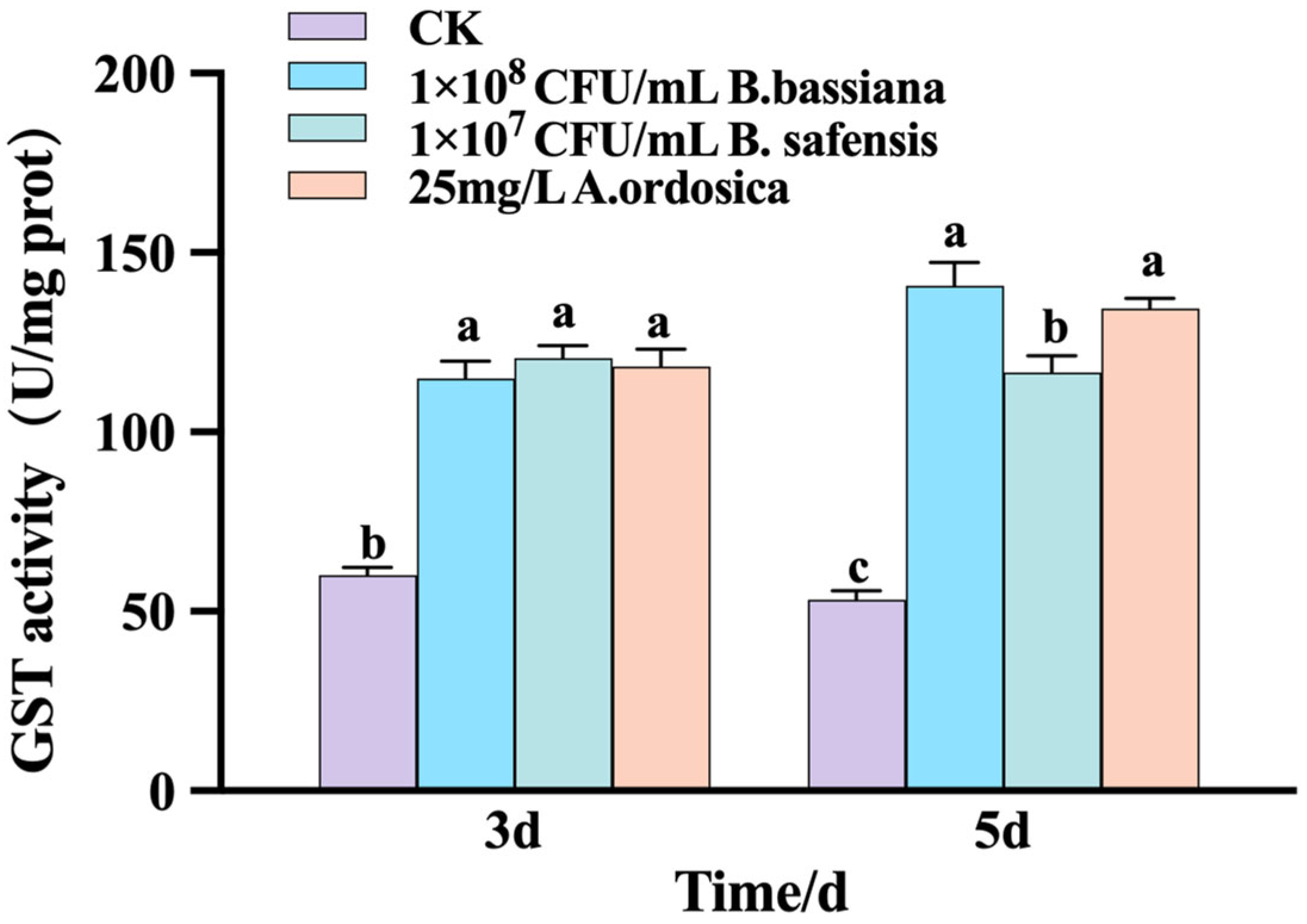
| Treatment | Concentration (CFU/mL) | Toxicity Regression Equation | LT50/d | 95% Confidence Interval | X2 | Correlation Coefficient | p |
|---|---|---|---|---|---|---|---|
| B. bassiana | 1 × 104 | y = 3.07x − 5.54 | 6.09 | 5.63~6.72 | 17.30 | 0.89 | <0.01 |
| 1 × 105 | y = 2.42x − 4.2 | 5.66 | 5.15~6.26 | 13.98 | 0.91 | <0.01 | |
| 1 × 106 | y = 2.44x − 4 | 5.12 | 4.71~5.65 | 12.60 | 0.93 | <0.01 | |
| 1 × 107 | y = 2.07x − 3.27 | 4.87 | 4.42~5.30 | 10.46 | 0.91 | <0.01 | |
| 1 × 108 | y = 2.59x − 3.85 | 4.51 | 4.11~4.84 | 5.54 | 0.91 | <0.01 |
| Treatment | Concentration (CFU/mL) | Toxicity Regression Equation | LT50/d | 95% Confidence Interval | X2 | Correlation Coefficient | p |
|---|---|---|---|---|---|---|---|
| B. safensis | 1 × 104 | y = 1.91x − 3.66 | 6.72 | 5.88~9.41 | 11.33 | 0.88 | =0.01 |
| 1 × 105 | y = 2.29x − 4.17 | 6.13 | 5.62~6.89 | 12.13 | 0.87 | <0.01 | |
| 1 × 106 | y = 2.23x − 3.9 | 5.76 | 5.21~6.40 | 9.20 | 0.90 | <0.01 | |
| 1 × 107 | y = 2.05x − 3.29 | 5.08 | 4.52~5.56 | 5.99 | 0.91 | <0.01 |
| Treatment | Concentration (mg/L) | Toxicity Regression Equation | LT50/d | 95% Confidence Interval | X2 | Correlation Coefficient | p |
|---|---|---|---|---|---|---|---|
| A. ordosica | 1.5625 | y = 2.76x − 5.24 | 6.62 | 6.12~7.51 | 23.63 | 0.89 | <0.01 |
| 3.125 | y = 2.16x − 3.82 | 5.85 | 5.35~6.49 | 17.19 | 0.83 | <0.01 | |
| 6.25 | y = 2.15x − 3.67 | 5.57 | 5.07~6.13 | 13.14 | 0.86 | <0.01 | |
| 12.5 | y = 2.09x − 3.41 | 5.18 | 4.71~5.63 | 7.84 | 0.88 | <0.01 | |
| 25 | y = 2.37x − 3.67 | 4.91 | 4.50~5.26 | 12.00 | 0.87 | <0.01 |
| Agents | Time/d | Toxicity Regression Equation | LC50 | LC90 | X2 | Correlation Coefficient | F | p |
|---|---|---|---|---|---|---|---|---|
| B. bassiana (CFU/mL) | 7 | y = 0.27x − 0.08 | 4.48 × 105 | 4.08 × 108 | 2.17 | 0.84 | 10 | 0.01 |
| B. safensis (CFU/mL) | 7 | y = 0.31x − 0.02 | 1.67 × 105 | 1.84 × 108 | 2.27 | 0.79 | 8 | 0.03 |
| A. ordosica (mg/L) | 7 | y = 0.06x − 0.18 | 2.91 | 22.13 | 4.17 | 0.75 | 12 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Z.; Cao, Z.; Xiong, C.; Cui, Y.; Cheng, Y.; Wang, Y.; Zhang, R.; Liu, C.; Sun, W.; Ban, L.; et al. Three Different Biopesticides Against Megalurothrips usitatus (Thysanoptera: Thripidae) and Their Toxicological and Biochemical Impacts. Biology 2025, 14, 1619. https://doi.org/10.3390/biology14111619
Fu Z, Cao Z, Xiong C, Cui Y, Cheng Y, Wang Y, Zhang R, Liu C, Sun W, Ban L, et al. Three Different Biopesticides Against Megalurothrips usitatus (Thysanoptera: Thripidae) and Their Toxicological and Biochemical Impacts. Biology. 2025; 14(11):1619. https://doi.org/10.3390/biology14111619
Chicago/Turabian StyleFu, Zuying, Ziyu Cao, Changyu Xiong, Yifan Cui, Yuanrun Cheng, Ying Wang, Rong Zhang, Chang Liu, Wei Sun, Liping Ban, and et al. 2025. "Three Different Biopesticides Against Megalurothrips usitatus (Thysanoptera: Thripidae) and Their Toxicological and Biochemical Impacts" Biology 14, no. 11: 1619. https://doi.org/10.3390/biology14111619
APA StyleFu, Z., Cao, Z., Xiong, C., Cui, Y., Cheng, Y., Wang, Y., Zhang, R., Liu, C., Sun, W., Ban, L., Tan, Y., & Wei, S. (2025). Three Different Biopesticides Against Megalurothrips usitatus (Thysanoptera: Thripidae) and Their Toxicological and Biochemical Impacts. Biology, 14(11), 1619. https://doi.org/10.3390/biology14111619





