Modeling the Future Distribution of Trifolium repens L. in China: A MaxEnt Approach Under Climate Change Scenarios
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Species Occurrence Data
2.2. Collection and Preprocessing of Environmental Variables
2.2.1. Acquisition of Current Environmental Climate Variables
2.2.2. Accessing Future Climate Data
2.2.3. Spatial Standardization and Data Formatting
2.3. Correlation Screening of Environmental Variables
2.3.1. Pearson Correlation Coefficient Calculation
2.3.2. Variable Selection Based on Correlation and Contribution
2.4. Parameter Optimization of the Model
2.5. Model Evaluation and Identification of Dominant Environmental Variables
2.5.1. Model Accuracy Assessment According to AUC and Tss
2.5.2. Dominant Variables Identification Based on Jackknife Examination
2.6. Generation of Prediction Distribution Maps
3. Results
3.1. Analysis of the Pearson Correlation for Variables
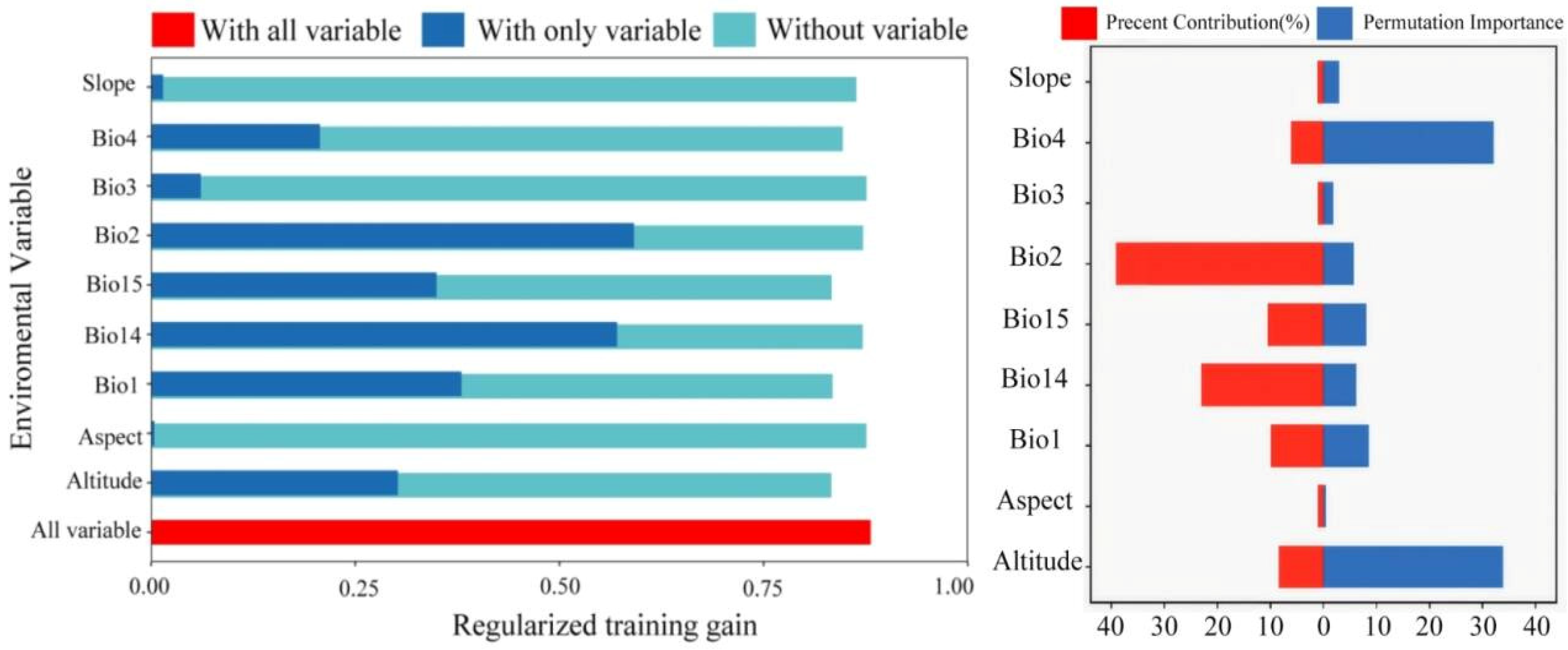
3.2. Importance Estimation of the Environmental Variables
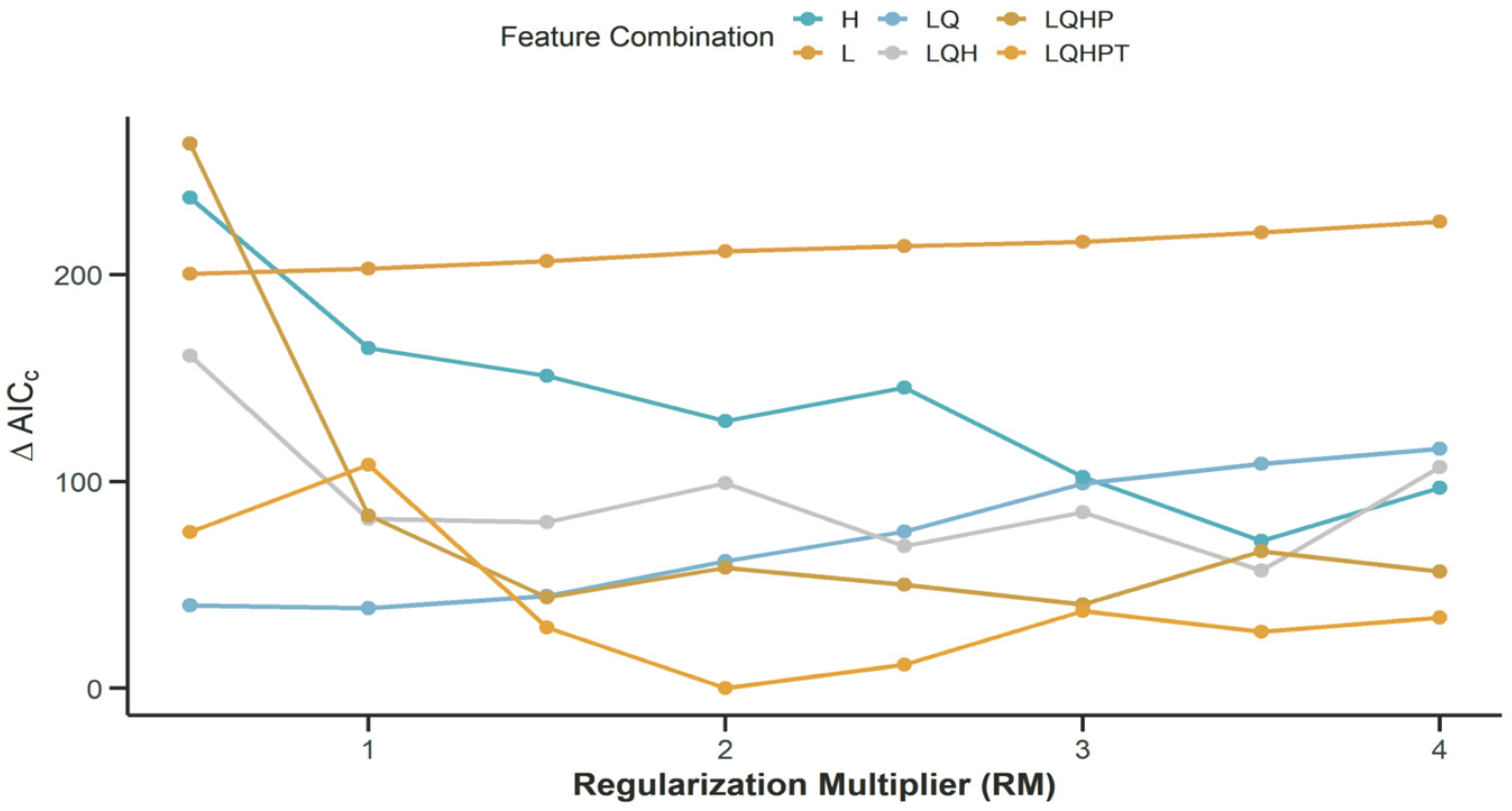
3.3. Determination of Optimal FC and RM Based on ΔAICC
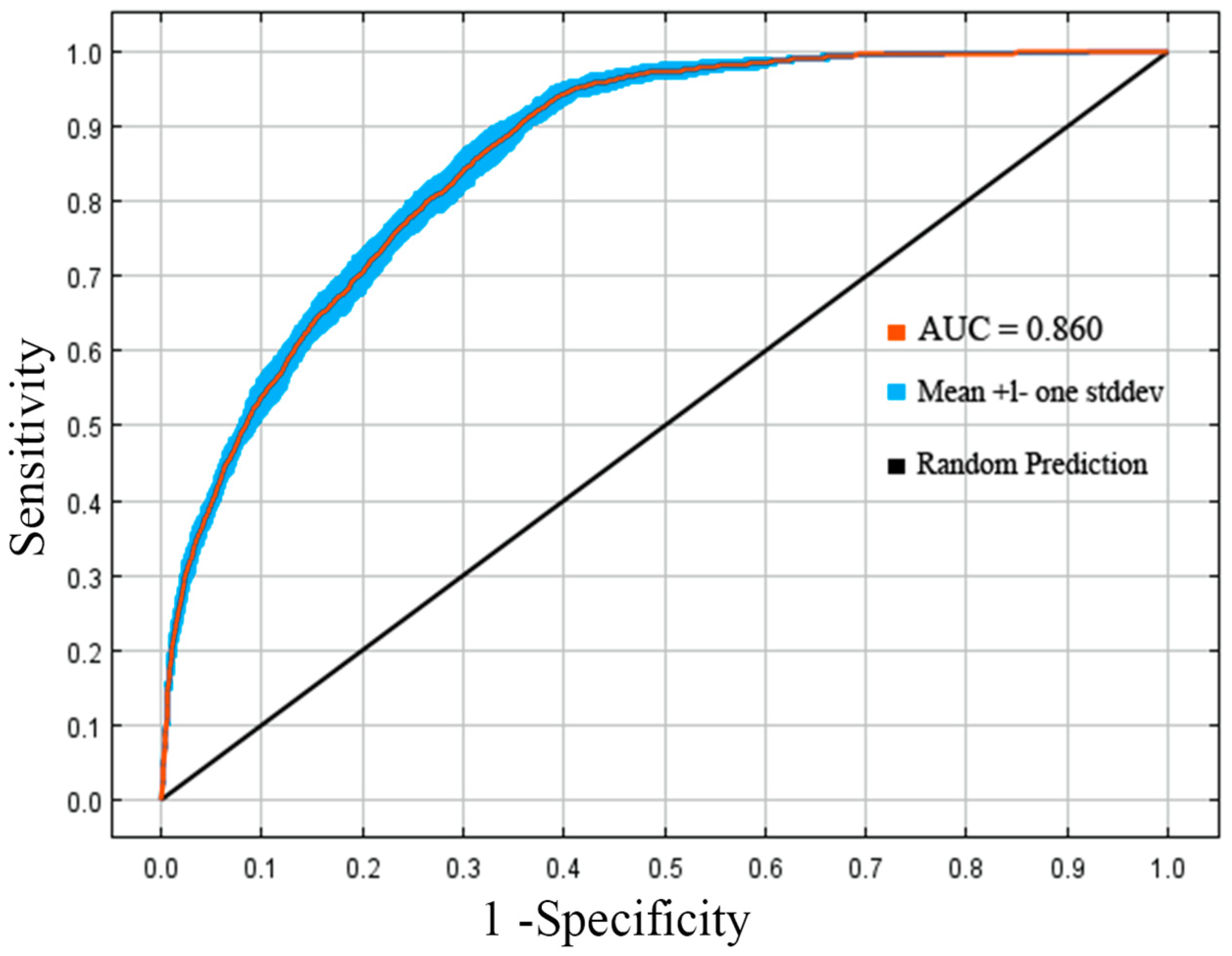
3.4. Evaluation of Predictive Accuracy Based on the AUC Curve and the TSS Value
3.5. Analysis of Dominant Variables and Evaluation of Model Prediction Accuracy
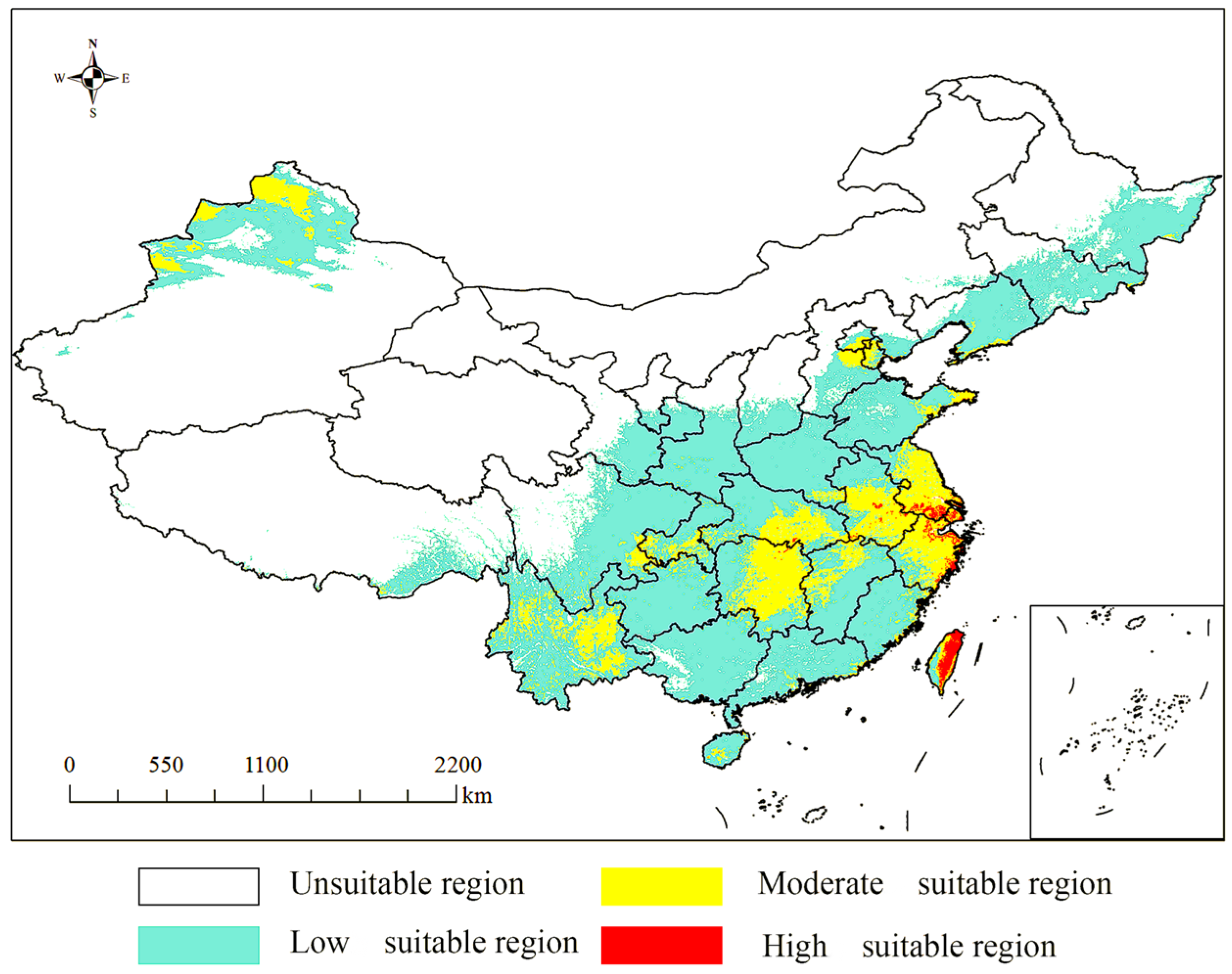
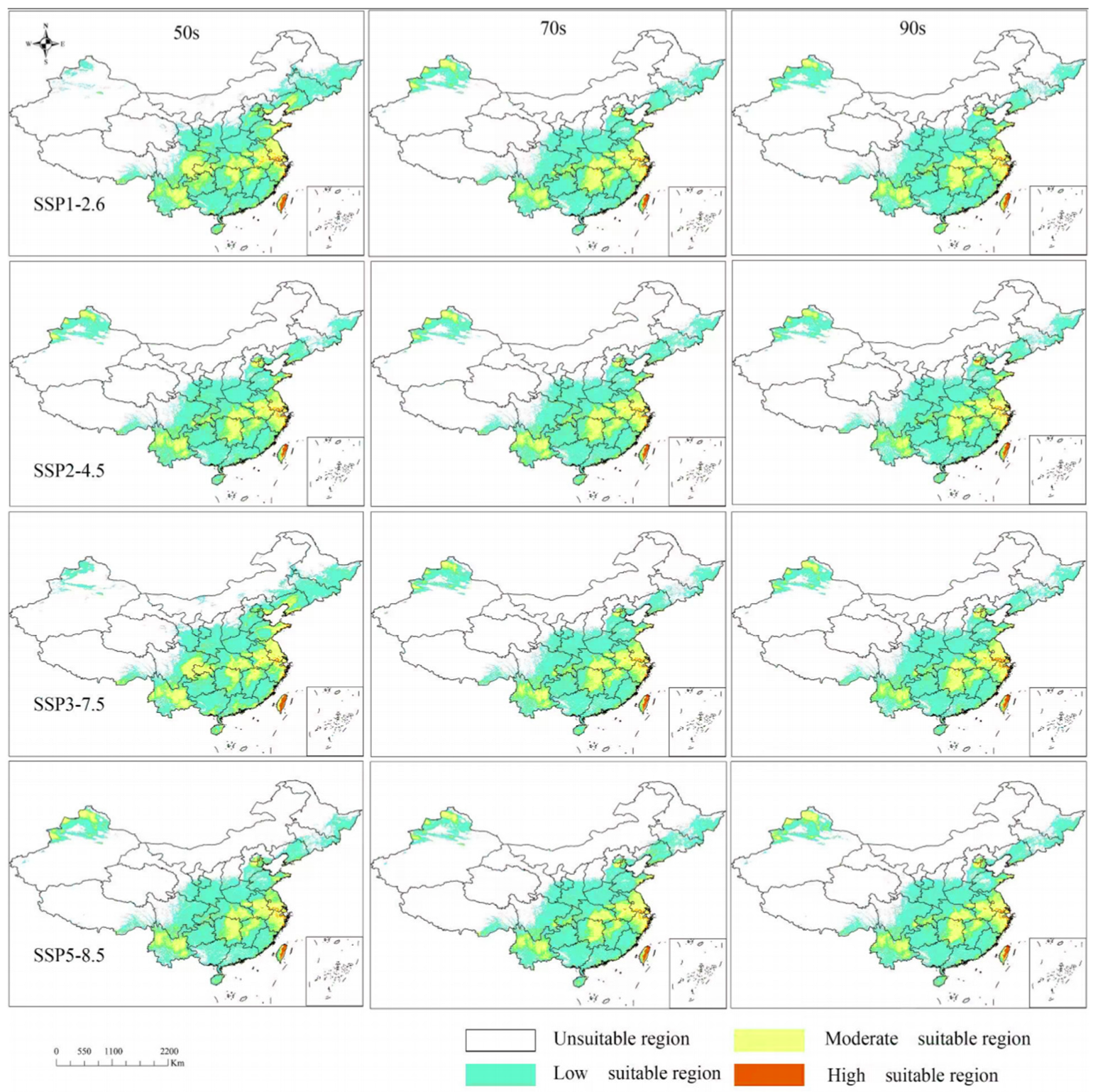
3.6. Distribution of Suitable Areas Under Four Climate Scenarios in China
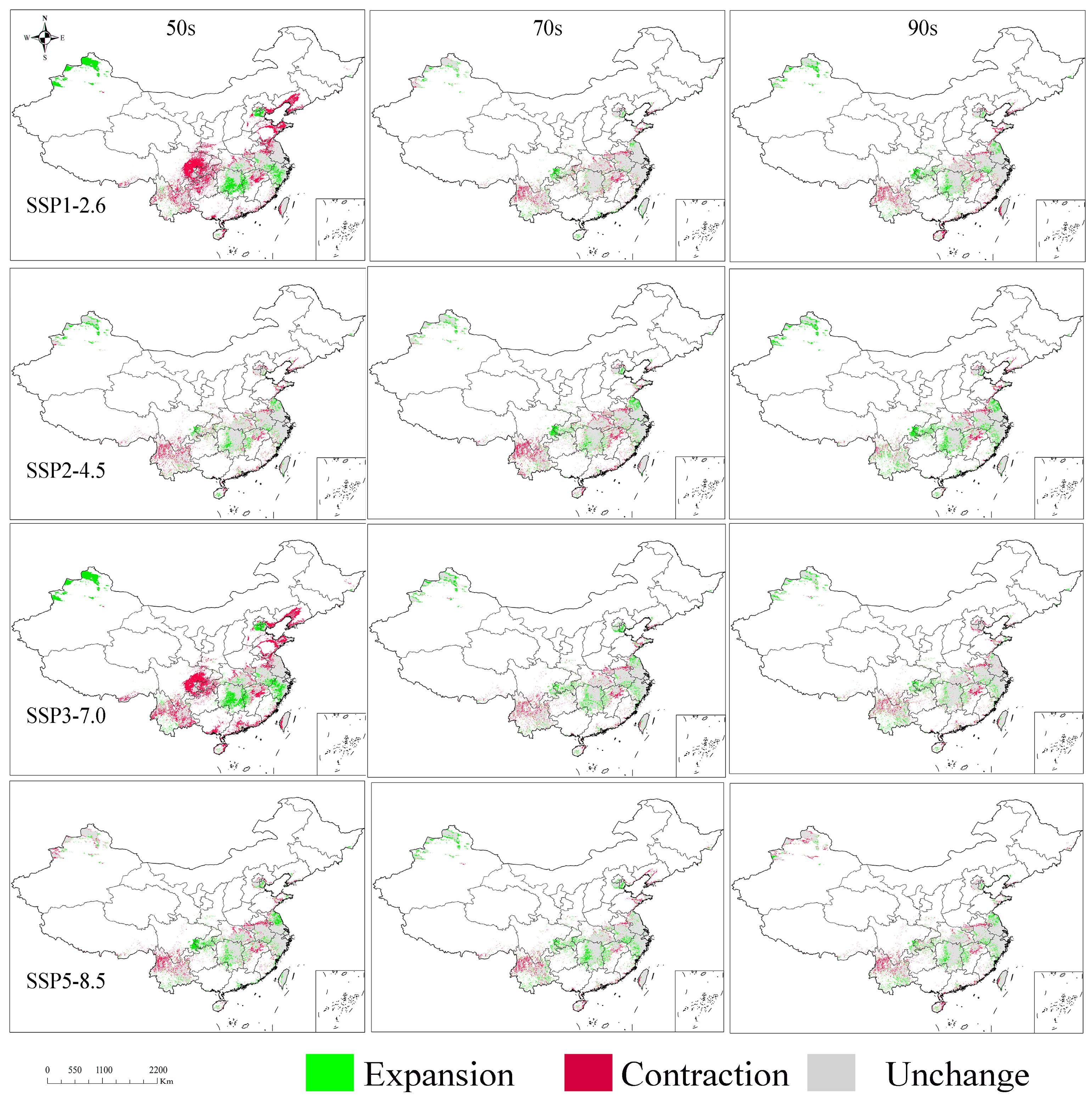
3.7. Dynamic Shifts in Distribution Under Various Climate Scenarios
4. Discussion
4.1. MaxEnt Model Selection, Optimization, and Performance Evaluation
4.2. Ecological Drivers and Current Distribution of T. repens
4.3. Future Niche Shifts and Heterogeneous Responses Under Climate Change
4.4. Implications for Invasion Risk Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vásquez, H.V.; Valqui, L.; Valqui-Valqui, L.; Bobadilla, L.G.; Reyna, M.; Maravi, C.; Pajares, N.; Altamirano-Tantalean, M.A. Influence of nitrogen fertilization and cutting dynamics on the yield and nutritional composition of white clover (Trifolium repens L.). Plants 2025, 14, 2765. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, X.; Mou, M.; Wang, Z.; Duo, L. Assessment of graphene oxide toxicity on the growth and nutrient levels of white clover (Trifolium repens L.). Ecotoxicol. Environ. Saf. 2022, 234, 113399. [Google Scholar] [CrossRef]
- Zhang, R.; Li, M.; Gao, X.; Duan, Y.; Cai, Y.; Li, H.; Zhao, X.; Wang, Y. Changes in the characteristics of soil dissolved organic matter over time since inter-planting with white clover (Trifolium repens L.) in apple orchards on the loess plateau in China. Plant Soil 2024, 499, 293–310, Correction in Plant Soil 2024, 499, 311. https://doi.org/10.1007/s11104-022-05721-w. [Google Scholar] [CrossRef]
- Qiao-lan, L.; Lie-xin, W.; Bing-liang, X.U.; Calderón-Urrea, A.; Dong, X. Study of viruses co-infecting white clover (Trifolium repens L.) in China. J. Integr. Agric. 2017, 16, 1990–1998. [Google Scholar]
- Battlay, P.; Hendrickson, B.T.; Mendez-Reneau, J.I.; Santangelo, J.S.; Albano, L.J.; Wilson, J.; Caizergues, A.E.; King, N.; Puentes, A.; Tudoran, A.; et al. Haploblocks contribute to parallel climate adaptation following global invasion of a cosmopolitan plant. Nat. Ecol. Evol. 2025, 9, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Cekstere, G.; Karlsons, A.; Grauda, D. Salinity-induced responses and resistance in Trifolium repens L. Urban For. Urban Green. 2015, 14, 225–236. [Google Scholar] [CrossRef]
- Tundis, R.; Marrelli, M.; Conforti, F.; Tenuta, M.C.; Bonesi, M.; Menichini, F.; Loizzo, M. Trifolium pratense and T. repens (leguminosae): Edible flower extracts as functional ingredients. Foods 2015, 4, 338–348. [Google Scholar] [CrossRef]
- Callahan, C.W. Present and future limits to climate change adaptation. Nat. Sustain. 2025, 8, 336–342. [Google Scholar] [CrossRef]
- Rahman, I.U.; EHart, R.; Ijaz, F.; Afzal, A.; Iqbal, Z.; Calixto, E.S.; Allah, E.F.A.; AAlqarawi, A.; Hashem, A.; Al-Arjani, A.-B.F.; et al. Environmental variables drive plant species composition and distribution in the moist temperate forests of northwestern himalaya, pakistan. PLoS ONE 2022, 17, e0260687. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, Z.; Wang, S.; Chen, L. Leaf physiological plasticity in Schima Superba and Schima Argentea is related to ecological niche width under varied altitude gradients. Ecol. Evol. 2025, 15, e71793. [Google Scholar] [CrossRef]
- Ahmad, M.; Sharma, P.; Rathee, S.; Singh, H.P.; Batish, D.R.; Lone, G.R.; Kaur, S.; Jaryan, V.; Kohli, R.K. Niche width analyses facilitate identification of high-risk endemic species at high altitudes in western himalayas. Ecol. Indic. 2021, 126, 107653. [Google Scholar] [CrossRef]
- Mathur, M.; Mathur, P.; Purohit, H. Ecological niche modelling of a critically endangered species Commiphora wightii (arn.) Bhandari using bioclimatic and non-bioclimatic variables. Ecol. Process. 2023, 12, 8. [Google Scholar] [CrossRef]
- Kosicki, J.Z. Generalised additive models and random forest approach as effective methods for predictive species density and functional species richness. Environ. Ecol. Stat. 2020, 27, 273–292. [Google Scholar] [CrossRef]
- Froeschke, B.F.; Roux-Osovitz, M.; Baker, M.L.; Hampson, E.G.; Nau, S.L.; Thomas, A. The use of boosted regression trees to predict the occurrence and quantity of Staphylococcus Aureus in recreational marine waterways. Water 2024, 16, 1283. [Google Scholar] [CrossRef]
- Ahmadi, M.; Hemami, M.; Kaboli, M.; Shabani, F. Maxent brings comparable results when the input data are being completed; Model parameterization of four species distribution models. Ecol. Evol. 2023, 13, e9827. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, X.; Sun, Y.; Liu, Y. Species distribution modeling based on maxent to inform biodiversity conservation in the central urban area of chongqing municipality. Ecol. Indic. 2024, 158, 111491. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A comparison between ensemble and maxent species distribution modelling ap-proaches for conservation: A case study with egyptian medicinal plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Mack, B.; Waske, B. In-depth comparisons of MaxEnt, biased SVM and one-class SVM for one-class classification of remote sensing data. Remote Sens. Lett. 2017, 8, 290–299. [Google Scholar] [CrossRef]
- Mousazade, M.; Ghanbarian, G.; Pourghasemi, H.R.; Safaeian, R.; Cerdà, A. Maxent data mining technique and its comparison with a bivariate statistical model for predicting the potential distribution of Astragalus fasciculifolius Boiss. in Fars, Iran. Sustainability 2019, 11, 3452. [Google Scholar] [CrossRef]
- Ma, J.; Nie, G.; Yang, Z.; Ma, S.; Fan, J.; Hu, R.; Wu, F.; Zhang, X. Genome-wide identification, characterization, and expression profiling analysis of SPL gene family during the inflorescence development in Trifolium repens Genes. Genes 2022, 13, 900. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, W.; Chen, T.; Li, Y.; Xiong, Z.; Ji, M. Predicting Eriochloa villosa (thunb.) Kunth invasion in northeast China using an r-optimized maxent model. Eur. J. Agron. 2026, 172, 127829. [Google Scholar] [CrossRef]
- Nan, Q.; Li, C.; Li, X.; Zheng, D.; Li, Z.; Zhao, L. Modeling the potential distribution patterns of the invasive plant species Phytolacca americana in China in response to climate change. Plants 2024, 13, 1082. [Google Scholar] [CrossRef]
- Martínez, I.F.B.; Rueda, M.; Ferrin, O.O.O.; Ochoa, J.A.D.; Vargasmachuca, S.C.; Selvaraj, J.J. A novel approach for improving the spa-tiotemporal distribution modeling of marine benthic species by coupling a new GIS procedure with machine learning. Deep.-Sea Res. Part I 2024, 203, 104222. [Google Scholar] [CrossRef]
- Salamanca-Palou, F.; Guzman-Echavarría, G.; Vanos, J.; Moseley, P.; Domino, M.E.; Georgescu, M. Effects of urbanization and climate change on heat stress under relatively dry and wet warm conditions in a semi-arid urban environment. Earth’s Future 2025, 13, e2024E–e4983E. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.242anh (accessed on 7 April 2025).
- Rusaati, B.I.W.; Kang, J.W. Maxent modeling for predicting the potential distribution of Lebrunia bushaie Staner (clusiaceae) under different climate change scenarios in democratic republic of congo. J. Asia-Pac. Biodivers. 2024, 17, 1–6. [Google Scholar] [CrossRef]
- Benjamin, M.; Mostafa, Q.E.; Benoît, J. Extension and significance testing of variable importance in projection (vip) indices in partial least squares regression and principal components analysis. Chemometr. Intell. Lab. Syst. 2023, 242, 104986. [Google Scholar]
- Ghafari, L.; Parvishi, A. Climate projection and drought assessment in the lake Urmia basin using LSTM-based downscaling of GCM models under SSP scenarios. Phys. Chem. Earth Parts A/B/C 2025, 141, 104134. [Google Scholar] [CrossRef]
- Hakimi, A.B.A.; Najmuddin, M.H.M.; Ammar, Z.; Ashraf, A.H.A. Pearson’s correlation coefficient analysis of non-invasive jaundice detection based on colour card technique. J. Phys. Conf. Ser. 2019, 1372, 12012. [Google Scholar]
- Pan, S.; Liu, Z.; Han, Y.; Zhang, D.; Zhao, X.; Li, J.; Wang, K. Using the pearson’s correlation coefficient as the sole metric to measure the accuracy of quantitative trait prediction: Is it sufficient? Front. Plant Sci. 2024, 15, 1480463. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Gao, Y.-Z.; Liu, X.; Wang, J.-S.; Guan, X.-Y.; Zhang, S.-B.; Lu, J.-Y. Solar photovoltaic SDM parameter estimation with improved sailfish optimizer based on random walking and multiple mutation strategies. Renew. Energy 2025, 254, 123771. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, D.; He, Q.; Li, L. A hybrid-strategy-improved dragonfly algorithm for the parameter identification of an SDM. Sustainability 2023, 15, 11791. [Google Scholar] [CrossRef]
- Kerem, Ç.Ş.; Gökhan, A. Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turk. J. Emerg. Med. 2023, 23, 195–198. [Google Scholar]
- Kim, H.; Shin, S.J. Variable selection in AUC-optimizing classification. Comput. Stat. Data Anal. 2026, 213, 108256. [Google Scholar] [CrossRef]
- Nathan, F. Accuracy of vancomycin AUC values estimated with trough-only data in a veteran population. Am. J. Health-Syst. Pharm. 2022, 80, 390–394. [Google Scholar]
- Liu, L.; Cao, X.; Wang, H.; Xiang, J. Optimization of model parameters and hyperparameters in deep learning models for spatial interaction prediction. Expert Syst. Appl. 2025, 266, 126160. [Google Scholar] [CrossRef]
- Guang-Zhen, W.; Li, W.; Ling, J.; Juan, C. Evaluation of environmental factors affecting the quality of Codonopsis pilosula based on chromatographic fingerprint and maxent model. Ind. Crops Prod. 2021, 170, 113783. [Google Scholar]
- Halvorsen, R.; Mazzoni, S.; Dirksen, J.W.; Næsset, E.; Gobakken, T.; Ohlson, M. How important are choice of model selection method and spatial autocorrelation of presence data for distribution modelling by maxent? Ecol. Model. 2016, 328, 108–118. [Google Scholar] [CrossRef]
- Lissovsky, A.A.; Dudov, S.V. Species-distribution modeling: Advantages and limitations of its application. 2. Maxent. Biol. Bull. Rev. 2021, 11, 265–275. [Google Scholar] [CrossRef]
- Saribaeva, S.U.; Allamuratov, A.; Mavlanov, B.; Adilov, B. Assessment of the state of Trifolium repens L. Cenopopulations in arid conditions in the surkhandrarya region (Uzbekistan). Arid. Ecosyst. 2025, 15, 74–80. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, C.; Zhang, Y.; Wang, H.; Song, J.; Zhou, J.; Wang, L.; Sun, J.; Shen, M.; Chen, C.; et al. Prediction of potential geographic distribution of Oncomelania Hupensis in yunnan province using random forest and maximum entropy models. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi= Chin. J. Schistosomiasis Control. 2024, 36, 562–571. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Yi, K.; Yuan, D.; Zhang, Z. Spatiotemporal prediction of ideal butterfly habitats in Kun-Ming’s urban green areas: Enabled by maxent and ArcGIS. Ecol. Evol. 2025, 15, e72300. [Google Scholar] [CrossRef]
- Campos, J.C.; Garcia, N.; Alírio, J.; Arenas-Castro, S.; Teodoro, A.C.; Sillero, N. Ecological niche models using maxent in google earth engine: Evaluation, guidelines and recommendations. Ecol. Inform. 2023, 76, 102147. [Google Scholar] [CrossRef]
- Lowther, W.; Pryor, H.; Littlejohn, R.; Hussain, S.; Willliams, W. Rhizobium specificity of Trifolium ambiguum M. Bieb × T. repens L. hybrid clovers. Euphytica 2002, 127, 309–315. [Google Scholar] [CrossRef]
- Tsybulskiy, O.; Trylis, V.; Hupalo, O.; Savytskiy, O. Biological diversity of Sula river (Ukraine) under different hydrological conditions. Transylv. Rev. Syst. Ecol. Res. 2023, 25, 65–76. [Google Scholar] [CrossRef]
- Xue, X.; Chen, W. Early hydrothermal conditions have a vital role in the responses of vegetation to extreme drought in southwest China. Agric. For. Meteorol. 2025, 367, 110523. [Google Scholar] [CrossRef]
- Adamopoulos, I.; Frantzana, A.; Syrou, N. Climate crises associated with epidemiological, environmental, and ecosystem effects of a storm: Flooding, landslides, and damage to urban and rural areas (extreme weather events of Storm Daniel in Thessaly, Greece). Med. Sci. Forum 2024, 25, 7. [Google Scholar]
- Dunn, J.A.M.; Grekin, P.; Darnton, J.B.; Soth, S.B.; Austin, E.J.; Woolworth, S.; Bhatraju, E.P.; Gojic, A.; Williams, E.C.; Hallgren, K.A.; et al. Disruption of opioid treatment program services due to an extreme weather event: An example of climate change effects on the health of persons who use drugs. J. Addict. Med. 2024, 19, 245–247. [Google Scholar] [CrossRef]
- Sabo, A.M.; Roy, S.S.; Pestle, W.J. Effects of climate change and extreme weather events on natural and archaeological landscapes in southwestern Puerto Rico. Remote Sens. Appl. Soc. Environ. 2024, 36, 101370. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, B.; Wu, C.; Chen, G.; Wang, Z.; Cui, J.; Hu, T.; Wiatrak, P. Models analyses for allelopathic effects of chicory at equivalent coupling of nitrogen supply and PH level on F. arundinacea, T. repens and M. sativa. PLoS ONE 2017, 7, e31670. [Google Scholar]
- Marshall, A.H.; Lowe, M.; Collins, R.P. Variation in response to moisture stress of young plants of interspecific hybrids between white clover (T. repens L.) and caucasian clover (T. ambiguum m. Bieb.). Agriculture 2015, 5, 353–366. [Google Scholar]
- Struebig, M.J.; Wenzler, M.; Runting, R.K.; Law, E.; Budiharta, S.; Seaman, D.; Kramer-Schadt, S. Connectivity conservation to mitigate climate and land-cover change impacts on Borneo. Biol. Conserv. 2024, 299, 110838. [Google Scholar] [CrossRef]
- Ahmad, S.; Zeb, A. Phytochemical profile and pharmacological properties of Trifolium repens. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 20200015. [Google Scholar] [CrossRef] [PubMed]
- Pingnan, Z.; Jie, Y.; Xiaoyuan, Z.; Zhixing, R.; Ming, L.; Song, H. Trifolium repens and biochar addition affecting soil nutrients and bacteria community. Environ. Sci. Pollut. Res. Int. 2022, 30, 33927–33941. [Google Scholar]
- Lan, Y.; Wu, X.; Xu, M.; Li, K.; Huan, Y.; Zhou, G.; Lun, F.; Shang, W.; Zhang, R.; Xie, Y. High-resolution global distribution projections of 10 rodent genera under diverse SSP-RCP scenarios, 2021–2100. Sci. Data 2025, 12, 1467. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fang, X.; Yin, Z.; Hu, R. Warming-induced hydrothermal anomaly over the earth’s three poles amplifies concurrent extremes in 2022. NPJ Clim. Atmos. Sci. 2024, 7, 8. [Google Scholar] [CrossRef]
- Ignacio-Reardon, S.J.I.; Luo, J.-J.; Omondi, O.A.; Ayugi, B.O. Intensified changes in extreme climate events over the Philippines region under future climate scenarios. Model. Earth Syst. Environ. 2025, 11, 409. [Google Scholar] [CrossRef]
- Zou, L.; Zhou, T. Mean and extreme precipitation changes over China under SSP scenarios: Results from high-resolution dynamical downscaling for CORDEX East Asia. Clim. Dyn. 2021, 58, 1015–1031. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, T.; Ning, Y.; Chen, Y.; Yang, T.; Yue, H.; Yang, Y.; Zhao, H.; Wu, H.; Jin, Z.; et al. Global climate change and macadamia habitat suitability: Maxent-based prediction under future scenarios. Front. Plant Sci. 2025, 16, 1658566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Zhang, X.; Qin, Z.; Wang, P.; Liu, H. Prediction of potential suitable habitats of Malania Oleifera under future climate scenarios based on the maxent model. Sci. Rep. 2025, 15, 26422. [Google Scholar] [CrossRef] [PubMed]
| Environmental Variables | Description |
|---|---|
| Bio1 | Mean Annual Temperature (°C) |
| Bio2 | Mean Diurnal Range (Mean of monthly (max temp—min temp)) (°C) |
| Bio3 | Thermal Uniformity (Bio2/Bio7(Temperature annual range)) (× 100) (%) |
| Bio4 | Temperature Seasonality (Standard Deviation × 100) |
| Bio14 | Precipitation of Driest Month (mm) |
| Bio15 | Precipitation Seasonality (Coefficient of Variation) (%) |
| Altitude | Altitude (m) |
| Slope | Terrain Gradient (°) |
| Aspect | Orientation of the Slope |
| Environmental Variable | Percent Contribution (%) | Permutation Importance (%) |
|---|---|---|
| Bio2 | 39.1 | 5.7 |
| Bio14 | 23 | 6.2 |
| Bio15 | 10.5 | 8.1 |
| Bio1 | 9.9 | 8.6 |
| Altitude | 8.4 | 33.9 |
| Bio4 | 6.1 | 32.1 |
| Slope | 1.1 | 3 |
| Bio3 | 1 | 1.9 |
| Aspect | 1 | 0.5 |
| Decades | Low Suitable Region (104 km2) | LOW/TOTAL (%) | Moderate Suitable Region (104 km2) | MOD/TOTAL (%) | High Suitable Region (104 km2) | HIGH/TOTAL (%) | Total (104 km2) | |
|---|---|---|---|---|---|---|---|---|
| - | current | 284.68 | 78.74 | 73.09 | 20.22 | 3.76 | 1.04 | 361.53 |
| SSP126 | 50s | 278.19 | 74.11 | 92.21 | 24.57 | 4.97 | 1.32 | 375.37 |
| 70s | 274.96 | 77.58 | 76.09 | 21.47 | 3.36 | 0.95 | 354.41 | |
| 90s | 275.80 | 78.54 | 71.91 | 20.48 | 3.45 | 0.98 | 351.16 | |
| SSP245 | 50s | 274.13 | 77.63 | 75.27 | 21.32 | 3.72 | 1.05 | 353.11 |
| 70s | 273.94 | 77.73 | 75.32 | 21.37 | 3.18 | 0.90 | 352.44 | |
| 90s | 279.34 | 80.94 | 62.51 | 18.11 | 3.27 | 0.95 | 345.12 | |
| SSP370 | 50s | 293.03 | 75.96 | 88.67 | 22.98 | 4.09 | 1.06 | 385.79 |
| 70s | 272.26 | 78.40 | 71.77 | 20.67 | 3.25 | 0.94 | 347.28 | |
| 90s | 270.45 | 77.96 | 72.19 | 20.81 | 4.26 | 1.23 | 346.90 | |
| SSP585 | 50s | 273.71 | 78.36 | 72.80 | 20.84 | 2.77 | 0.79 | 349.28 |
| 70s | 287.77 | 79.93 | 68.90 | 19.14 | 3.38 | 0.94 | 360.04 | |
| 90s | 279.43 | 78.29 | 74.19 | 20.79 | 3.28 | 0.92 | 356.91 |
| Future Climatic Conditions | Decades | Expansion | Contraction | Unchanged | Total Area Change |
|---|---|---|---|---|---|
| SSP126 | 50s | 20.77 | 40.36 | 54.64 | 61.14 |
| 70s | 7.98 | 10.74 | 67.42 | 18.72 | |
| 90s | 12.43 | 11.20 | 62.99 | 23.63 | |
| SSP245 | 50s | 11.06 | 12.80 | 64.36 | 23.86 |
| 70s | 11.82 | 13.67 | 63.56 | 25.49 | |
| 90s | 18.40 | 7.62 | 56.98 | 26.02 | |
| SSP370 | 50s | 22.82 | 38.95 | 52.58 | 61.78 |
| 70s | 12.73 | 10.36 | 62.69 | 23.10 | |
| 90s | 10.98 | 10.05 | 64.46 | 21.03 | |
| SSP585 | 50s | 13.60 | 12.57 | 61.76 | 26.17 |
| 70s | 14.61 | 10.02 | 60.78 | 24.64 | |
| 90s | 10.73 | 11.09 | 64.72 | 21.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, Q.; Shen, J.; Ding, J.; Zeng, Y.; Zhou, Z.; Yan, X.; Zhang, J.; Ma, X.; Yu, Q.; et al. Modeling the Future Distribution of Trifolium repens L. in China: A MaxEnt Approach Under Climate Change Scenarios. Biology 2025, 14, 1608. https://doi.org/10.3390/biology14111608
Wang H, Liu Q, Shen J, Ding J, Zeng Y, Zhou Z, Yan X, Zhang J, Ma X, Yu Q, et al. Modeling the Future Distribution of Trifolium repens L. in China: A MaxEnt Approach Under Climate Change Scenarios. Biology. 2025; 14(11):1608. https://doi.org/10.3390/biology14111608
Chicago/Turabian StyleWang, Haojun, Qilin Liu, Jinyu Shen, Jiayu Ding, Yu Zeng, Zixin Zhou, Xiangrong Yan, Jianbo Zhang, Xiao Ma, Qingqing Yu, and et al. 2025. "Modeling the Future Distribution of Trifolium repens L. in China: A MaxEnt Approach Under Climate Change Scenarios" Biology 14, no. 11: 1608. https://doi.org/10.3390/biology14111608
APA StyleWang, H., Liu, Q., Shen, J., Ding, J., Zeng, Y., Zhou, Z., Yan, X., Zhang, J., Ma, X., Yu, Q., Xiong, Y., & Xiong, Y. (2025). Modeling the Future Distribution of Trifolium repens L. in China: A MaxEnt Approach Under Climate Change Scenarios. Biology, 14(11), 1608. https://doi.org/10.3390/biology14111608








