Rulers of the Open Sky at Risk: Climate-Driven Habitat Shifts of Three Conservation-Priority Raptors in the Eastern Himalayas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
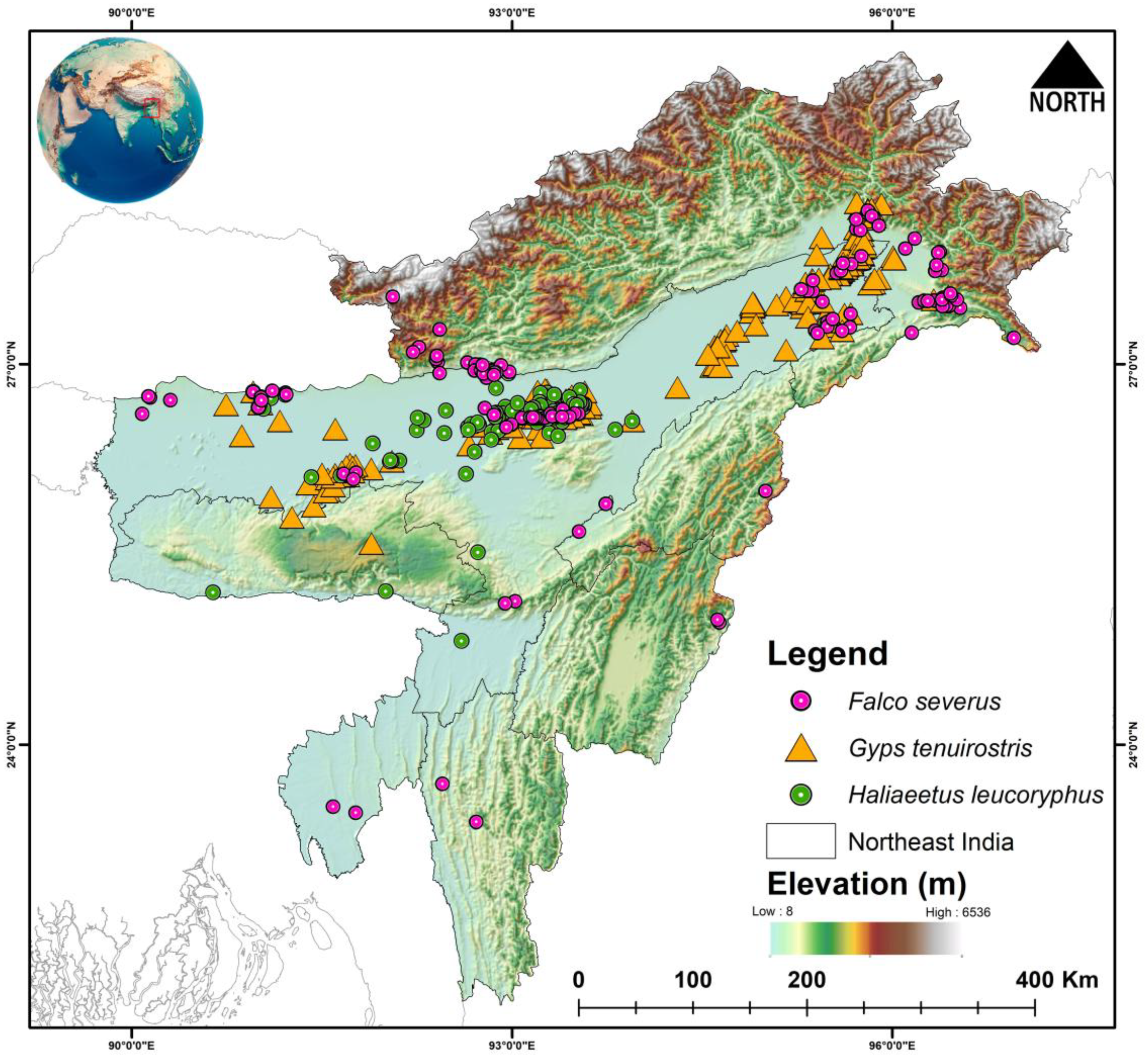
2.1. Study Area
2.2. Covariates for Suitable Habitat Evaluation
2.3. Species Distribution Modeling
2.4. Habitat Quality Assessment
3. Results
3.1. Model Performance
3.2. Variable Importance
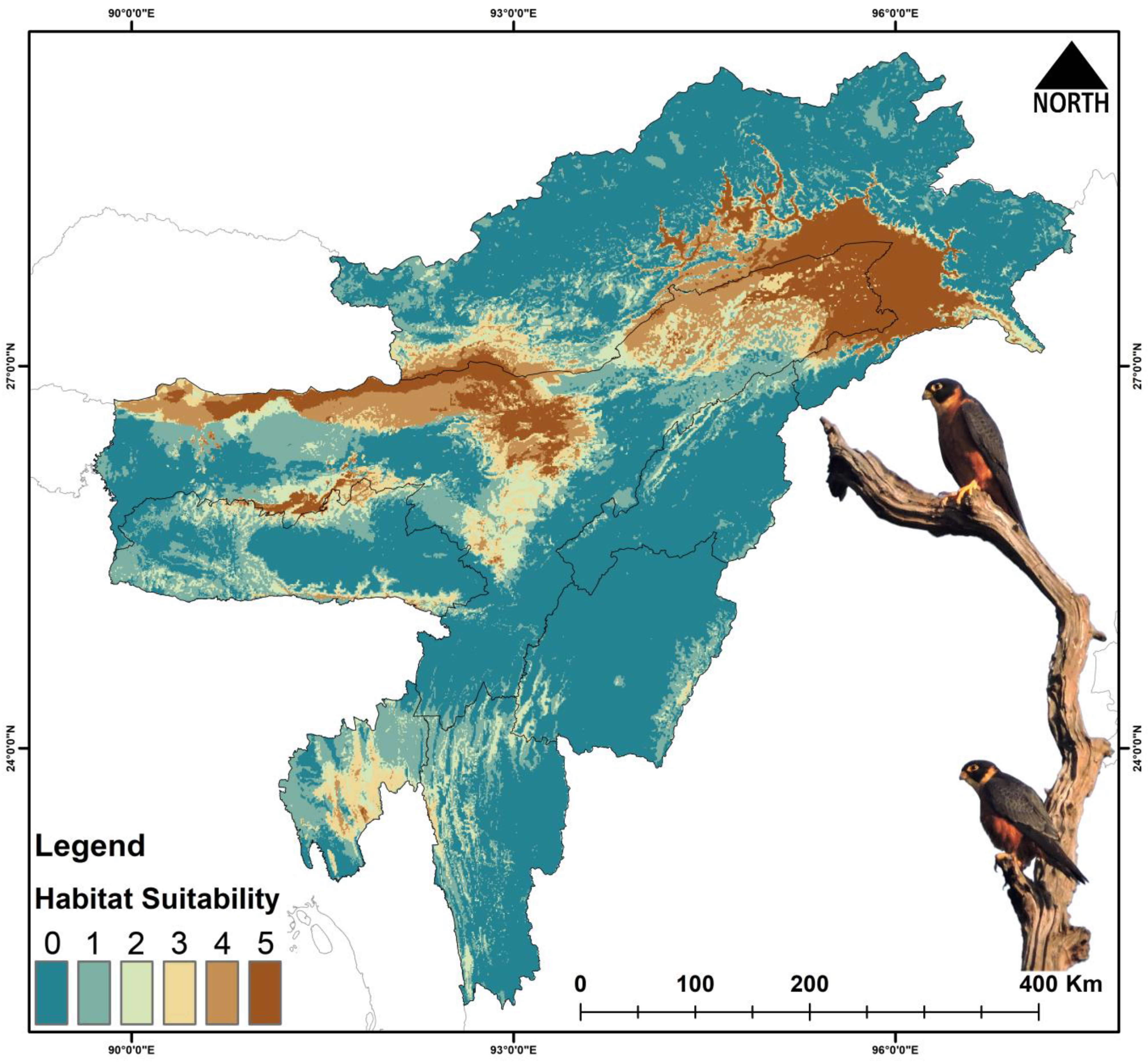
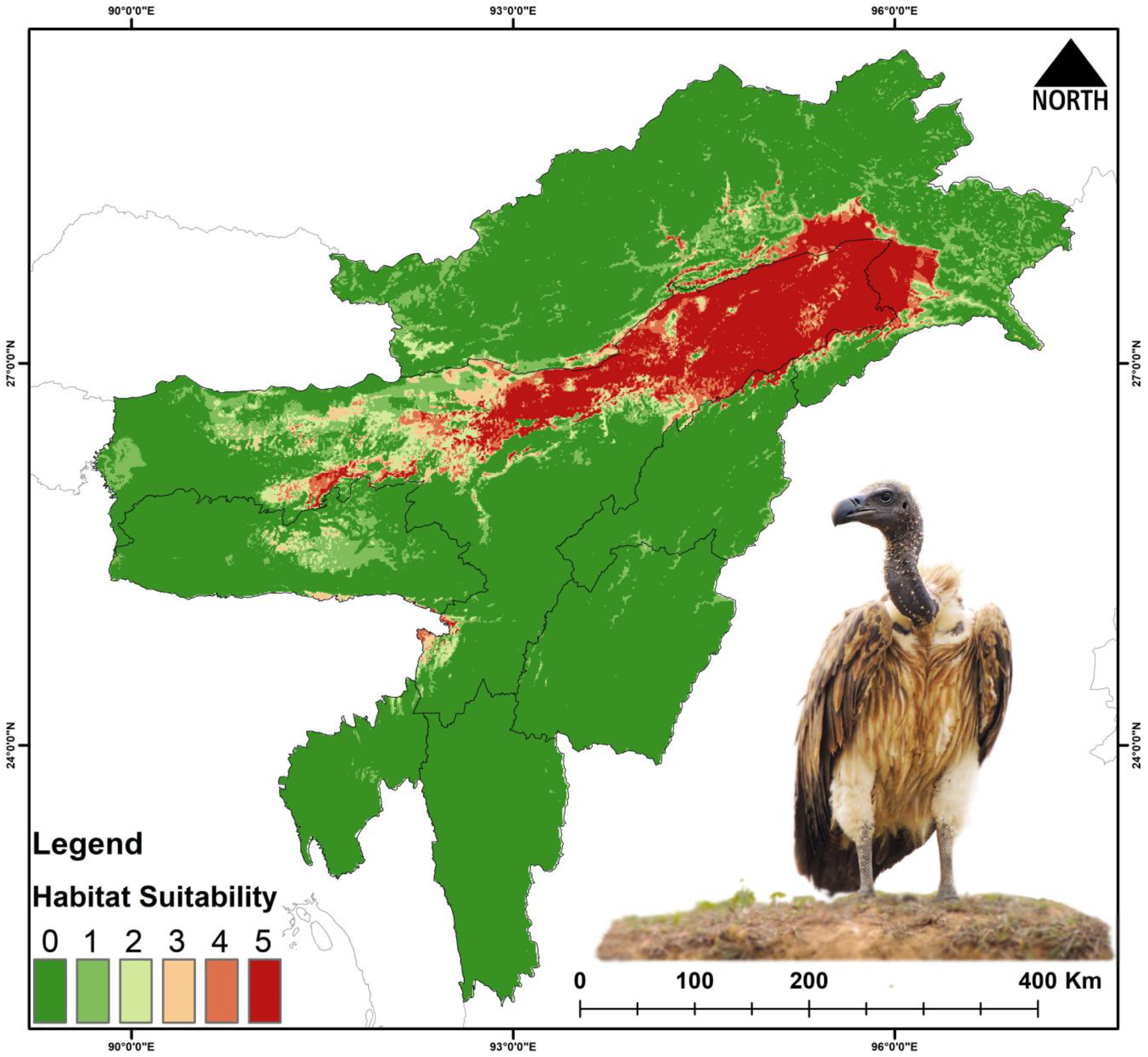
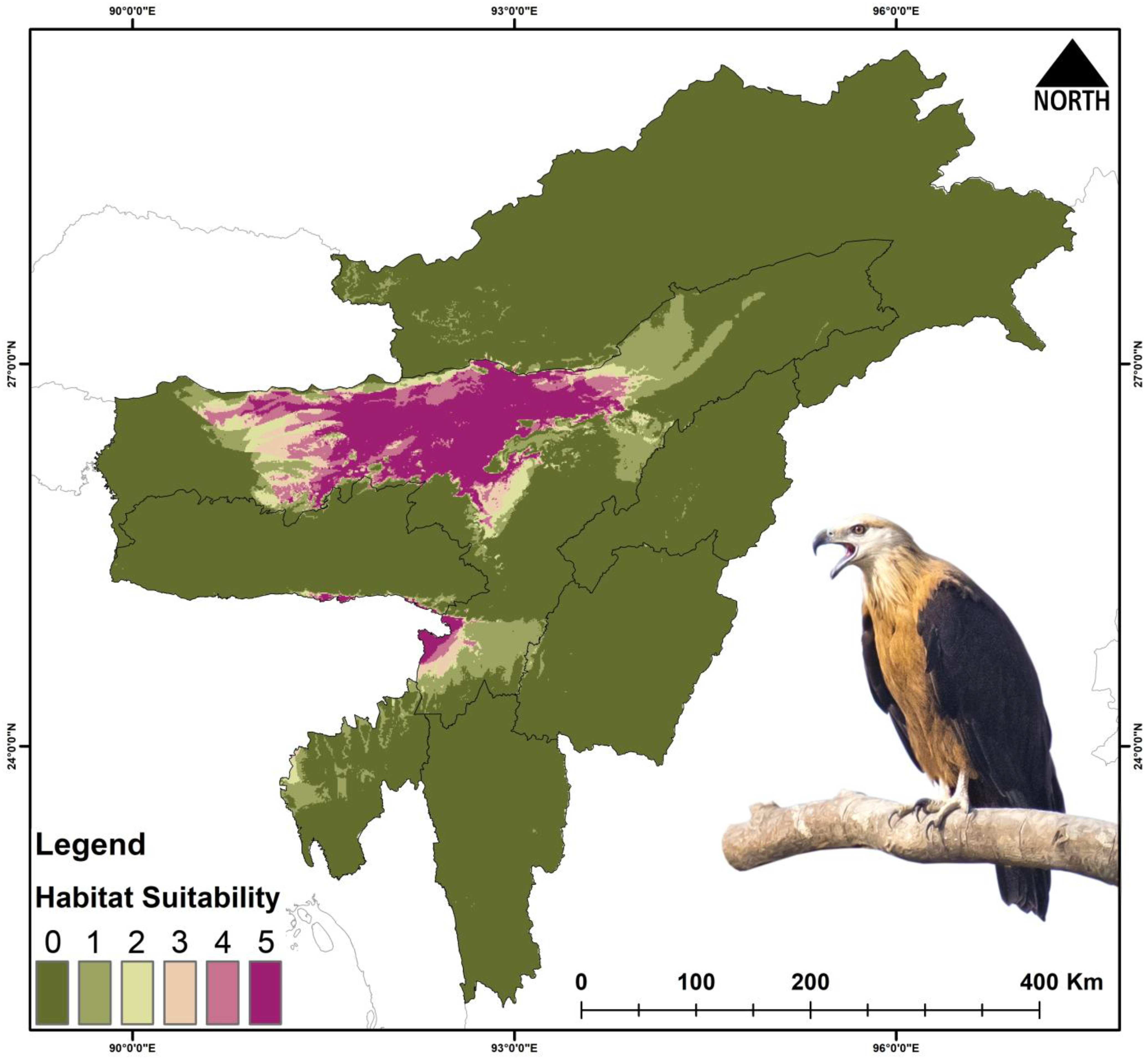
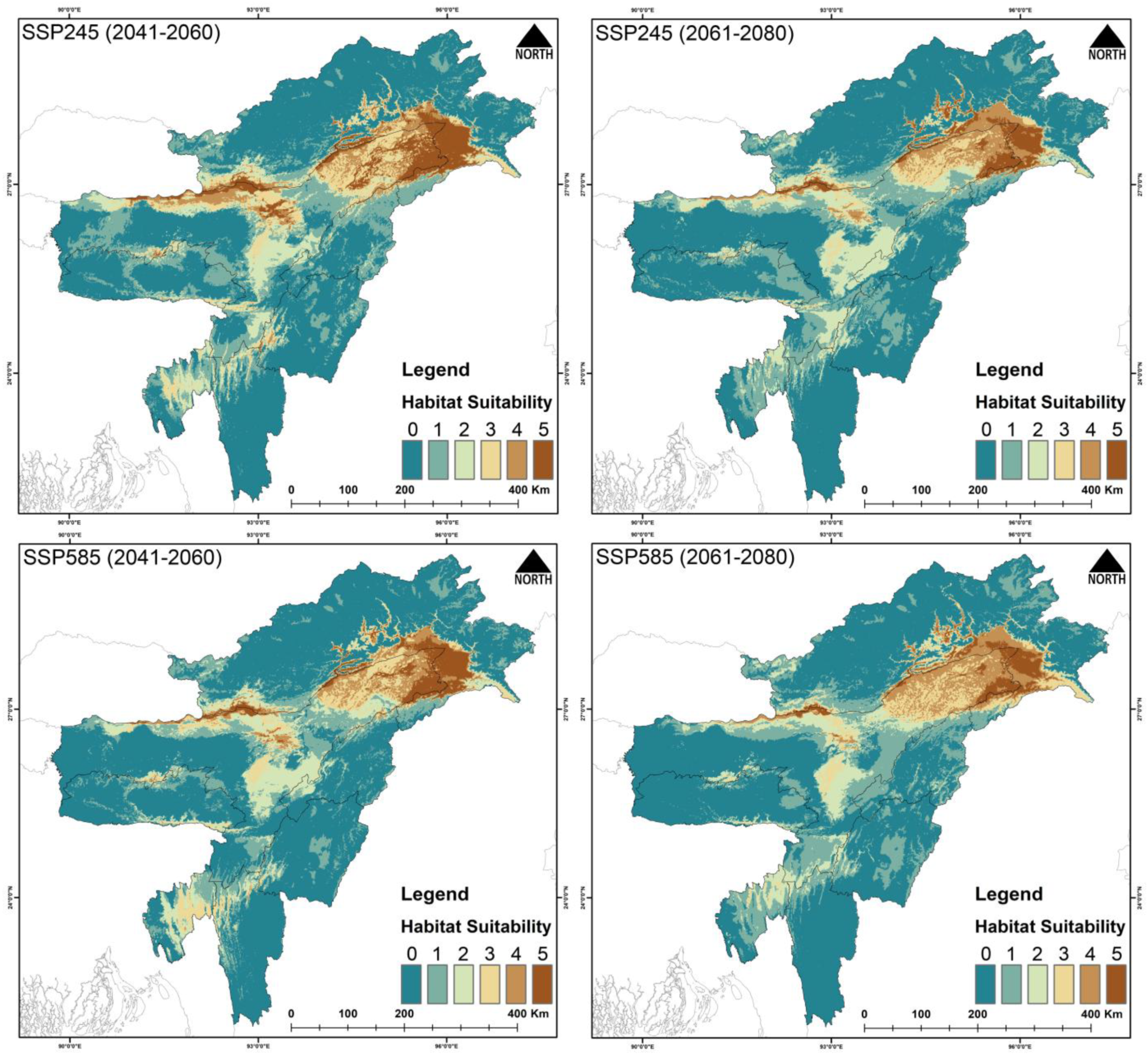
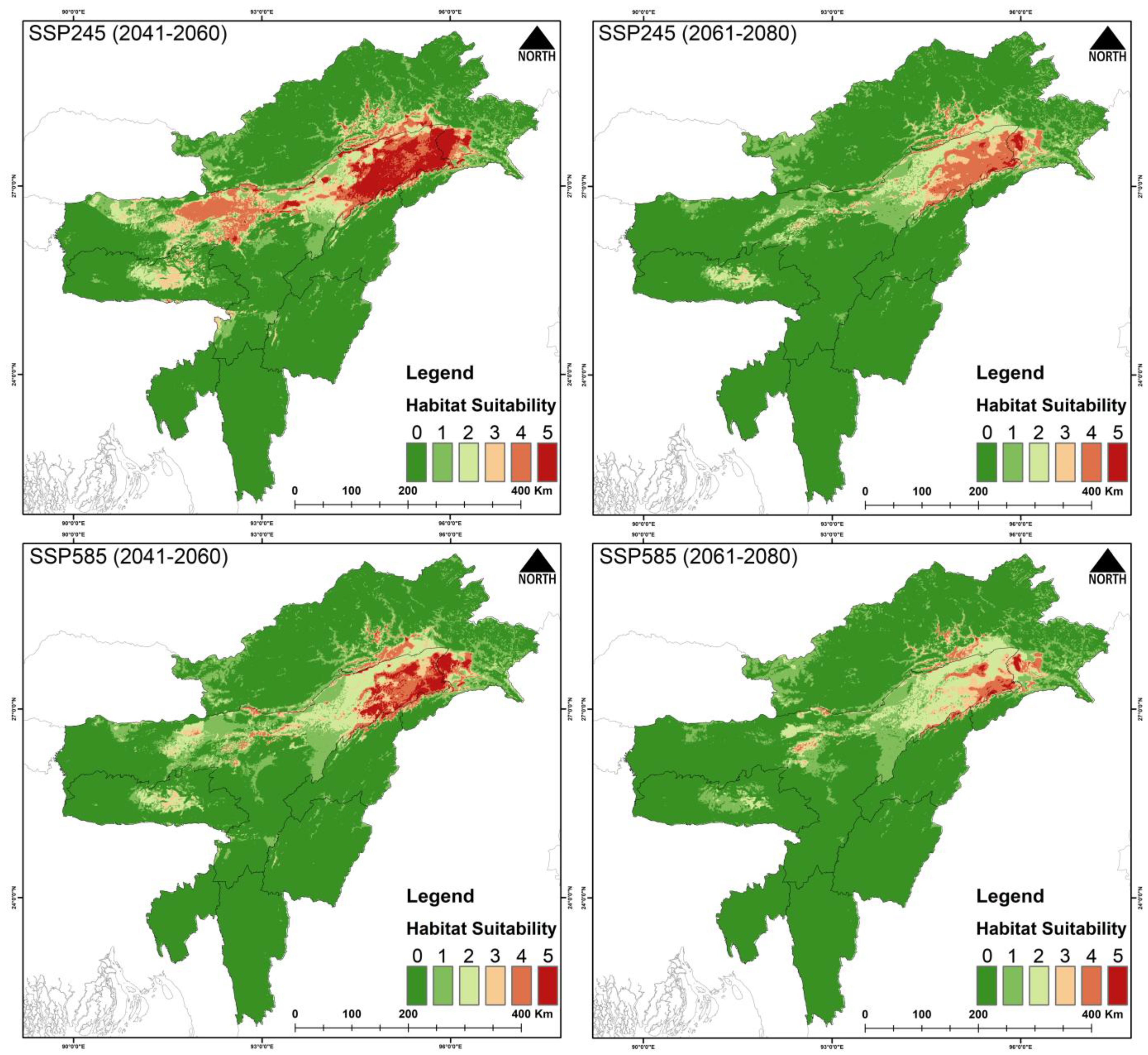
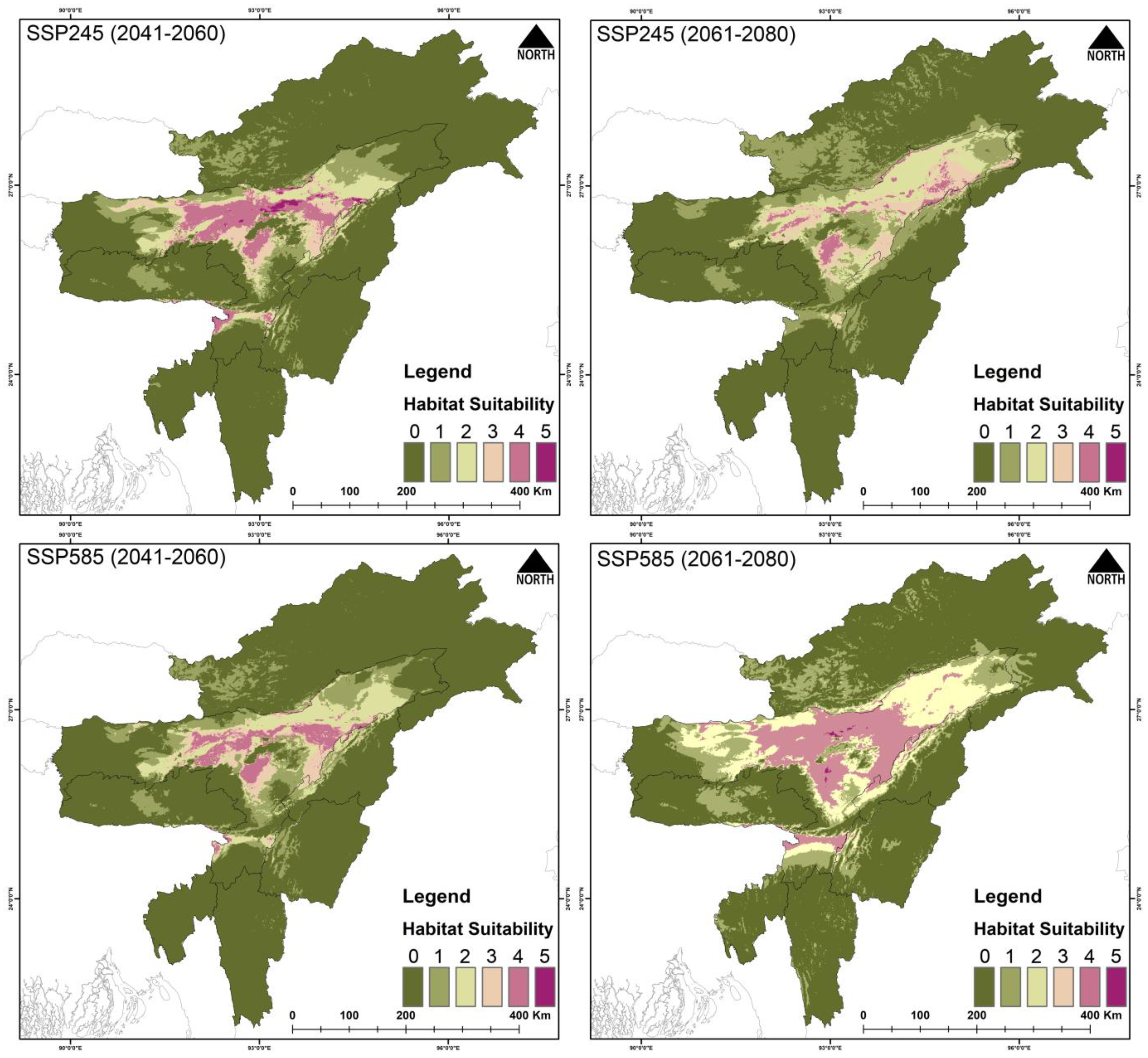
3.3. Habitat Suitability
3.4. Quality of the Suitable Habitat
4. Discussion
4.1. Present Suitable Habitats
4.2. Future Climatic Predictions
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halpern, B.S.; Frazier, M.; Afflerbach, J.C.; Lowndes, J.S.; Micheli, F.; O’Hara, C.C.; Scarborough, C.; Selkoe, K.A. Recent Pace of Change in Human Impact on the World’s Ocean. Sci. Rep. 2019, 9, 11609. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, M.; Dykstra, C.R.; Booms, T.L.; Henderson, M.T. Conservation Letter: Effects of Global Climate Change on Raptors. J. Raptor Res. 2023, 57, 92–105. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, N.; Kumar, M.; Singh, R. Modelling Habitat Suitability of Western Tragopan (Tragopan melanocephalus) a Range-Restricted Vulnerable Bird Species of the Himalayan Region, in Response to Climate Change. Clim. Risk Manag. 2020, 29, 100241. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, S.; Hua, J.; Liu, Z.; Xu, J. Effect of Climate and Land-Use Changes on a Threatened Forest Resident Bird. Forests 2024, 15, 348. [Google Scholar] [CrossRef]
- Sergio, F.; Marchesi, L.; Pedrini, P.; Penteriani, V. Coexistence of a Generalist Owl with Its Intraguild Predator: Distance-Sensitive or Habitat-Mediated Avoidance? Anim. Behav. 2007, 74, 1607–1616. [Google Scholar] [CrossRef]
- Sergio, F.; Newton, I.A.N.; Marchesi, L.; Pedrini, P. Ecologically Justified Charisma: Preservation of Top Predators Delivers Biodiversity Conservation. J. Appl. Ecol. 2006, 43, 1049–1055. [Google Scholar] [CrossRef]
- Donázar, J.A.; Cortés-Avizanda, A.; Fargallo, J.A.; Margalida, A.; Moleón, M.; Morales-Reyes, Z.; Moreno-Opo, R.; Pérez-García, J.M.; Sánchez-Zapata, J.A.; Zuberogoitia, I.; et al. Roles of Raptors in a Changing World: From Flagships to Providers of Key Ecosystem Services. Ardeola 2016, 63, 181–234. [Google Scholar] [CrossRef]
- McClure, C.J.W.; Westrip, J.R.S.; Johnson, J.A.; Schulwitz, S.E.; Virani, M.Z.; Davies, R.; Symes, A.; Wheatley, H.; Thorstrom, R.; Amar, A.; et al. State of the World’s Raptors: Distributions, Threats, and Conservation Recommendations. Biol. Conserv. 2018, 227, 390–402. [Google Scholar] [CrossRef]
- Meserve, P.L.; Kelt, D.A.; Milstead, W.B.; Gutiérrez, J.R. Thirteen Years of Shifting Top-Down and Bottom-Up Control. BioScience 2003, 53, 633–646. [Google Scholar] [CrossRef]
- Schmidt, J.H.; McIntyre, C.L.; Roland, C.A.; MacCluskie, M.C.; Flamme, M.J. Bottom-Up Processes Drive Reproductive Success in an Apex Predator. Ecol. Evol. 2018, 8, 1833–1841. [Google Scholar] [CrossRef]
- Urban, M.C.; Zarnetske, P.L.; Skelly, D.K. Searching for biotic multipliers of climate change. Integr. Comp. Biol. 2017, 57, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Thiollay, J.-M. Current Status and Conservation of Falconiformes in Tropical Asia. J. Raptor Res. 1998, 32, 5–15. [Google Scholar]
- Goriup, P.; Tucker, G. Assessment of the Merits of a CMS Instrument Covering Migratory Raptors in Africa and Eurasia; DEFRA: Bristol, UK, 2007. [Google Scholar]
- Iknayan, K.J.; Beissinger, S.R. Collapse of a Desert Bird Community over the Past Century Driven by Climate Change. Proc. Natl. Acad. Sci. USA 2018, 115, 10583–10588. [Google Scholar] [CrossRef]
- Dykstra, C.R.; Hays, J.L.; Simon, M.M.; Wegman, A.R.; Dykstra, L.R.; Williams, K.A. Habitat and Weather Conditions Influence Reproductive Rates of Suburban and Rural Red-Shouldered Hawks Buteo lineatus. Ibis 2021, 163, 623–640. [Google Scholar] [CrossRef]
- Reside, A.E.; VanDerWal, J.J.; Kutt, A.S.; Perkins, G.C. Weather, Not Climate, Defines Distributions of Vagile Bird Species. PLoS ONE 2010, 5, e13569. [Google Scholar] [CrossRef]
- Curley, S.R.; Manne, L.L.; Veit, R.R. Differential Winter and Breeding Range Shifts: Implications for Avian Migration Distances. Divers. Distrib. 2020, 26, 415–425. [Google Scholar] [CrossRef]
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A framework for community interactions under climate change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef]
- Bradley, M.J.; Kutz, S.J.; Jenkins, E.; O’Hara, T.M. The Potential Impact of Climate Change on Infectious Diseases of Arctic Fauna. Int. J. Circumpolar Health 2005, 64, 468–477. [Google Scholar] [CrossRef]
- Merino, S.; Møller, A.P. Host-Parasite Interactions and Climate Change. In Effects of Climate Change on Birds; Møller, A.P., Fiedler, W., Berthold, P., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 213–226. [Google Scholar]
- Dudek, B.M.; Henderson, M.T.; Hudon, S.F.; Hayden, E.J.; Heath, J.A. Haematophagous ectoparasites lower survival of and have detrimental physiological effects on golden eagle nestlings. Conserv. Physiol. 2021, 9, coab060. [Google Scholar] [CrossRef] [PubMed]
- Terraube, J.; Villers, A.; Ruffino, L.; Iso-Iivari, L.; Henttonen, H.; Oksanen, T.; Korpimäki, E. Coping with Fast Climate Change in Northern Ecosystems: Mechanisms Underlying the Population-Level Response of a Specialist Avian Predator. Ecography 2015, 38, 690–699. [Google Scholar] [CrossRef]
- Rosenfield, R.N.; Hardin, M.G.; Bielefeldt, J.; Keyel, E.R. Are Life History Events of a Northern Breeding Population of Cooper’s Hawks Influenced by Changing Climate? Ecol. Evol. 2017, 7, 399–408. [Google Scholar] [CrossRef]
- Dunn, P.O. Changes in timing of breeding and reproductive success in birds. In Effects of Climate Change on Birds, 2nd ed.; Dunn, P.O., Møller, A.P., Eds.; Oxford University Press: Oxford, UK, 2019; pp. 108–119. [Google Scholar]
- Callery, K.R.; Smallwood, J.A.; Hunt, A.R.; Snyder, E.R.; Heath, J.A. Seasonal Trends in Adult Apparent Survival and Reproductive Trade-Offs Reveal Potential Constraints to Earlier Nesting in a Migratory Bird. Oecologia 2022, 199, 91–102. [Google Scholar] [CrossRef]
- Curk, T.; Pokrovsky, I.; Lecomte, N.; Aarvak, T.; Brinker, D.F.; Burnham, K.; Dietz, A.; Dixon, A.; Franke, A.; Gauthier, G.; et al. Arctic Avian Predators Synchronise Their Spring Migration with the Northern Progression of Snowmelt. Sci. Rep. 2020, 10, 7220. [Google Scholar] [CrossRef]
- Condro, A.A.; Higuchi, H.; Mulyani, Y.A.; Raffiudin, R.; Rusniarsyah, L.; Setiawan, Y.; Prasetyo, L.B. Climate change leads to range contraction for Japanese population of the Oriental Honey-Buzzards: Implications for future conservation strategies. Glob. Ecol. Conserv. 2022, 34, e02044. [Google Scholar] [CrossRef]
- Morrison, J.L.; Baird, J.M. Using banding and encounter data to investigate movements of Red-tailed Hawks in the Northeast. J. Raptor Res. 2016, 50, 161–175. [Google Scholar] [CrossRef]
- Powers, B.F.; Winiarski, J.M.; Requena-Mullor, J.M.; Heath, J.A. Intra-Specific Variation in Migration Phenology of American Kestrels (Falco sparverius) in Response to Spring Temperatures. Ibis 2021, 163, 1448–1456. [Google Scholar] [CrossRef]
- Duerr, A.E.; Miller, T.A.; Lanzone, M.; Brandes, D.; Cooper, J.; O’Malley, K.; Maisonneuve, C.; Tremblay, J.A.; Katzner, T. Flight Response of Slope-Soaring Birds to Seasonal Variation in Thermal Generation. Funct. Ecol. 2015, 29, 779–790. [Google Scholar] [CrossRef]
- Mallon, J.M.; Bildstein, K.L.; Fagan, W.F. Inclement Weather Forces Stopovers and Prevents Migratory Progress for Obligate Soaring Migrants. Mov. Ecol. 2021, 9, 35. [Google Scholar] [CrossRef]
- Wichmann, M.C.; Jeltsch, F.; Dean, W.R.J.; Moloney, K.A.; Wissel, C. Implication of Climate Change for the Persistence of Raptors in Arid Savanna. Oikos 2003, 102, 186–202. [Google Scholar] [CrossRef]
- Monadjem, A.; Virani, M.Z.; Jackson, C.; Reside, A. Rapid Decline and Shift in the Future Distribution Predicted for the Endangered Sokoke Scops Owl Otus ireneae due to Climate Change. Bird Conserv. Int. 2013, 23, 247–258. [Google Scholar] [CrossRef]
- Lynch, J.F. Effects of Hurricane Gilbert on Birds in a Dry Tropical Forest in the Yucatan Peninsula. Biotropica 1991, 23, 488–496. [Google Scholar] [CrossRef]
- Mahananda, P.; Jelil, S.N.; Saikia, M.K. Raptor Research in India: Inadequate Data and Species’ Status Uncertainty for Many Species. J. Raptor Res. 2022, 56, 201–211. [Google Scholar] [CrossRef]
- Clark, W.S.; Boesman, P.F.D.; Marks, J.S. Oriental Hobby (Falco severus), Version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Owen, R.P. New Bird Records for Micronesia and Major Island Groups in Micronesia. Micronesica 1977, 13, 57–63. [Google Scholar]
- Rasmussen, P.C.; Anderton, J.C. Birds of South Asia: The Ripley Guide: Attributes and Status, 2nd ed.; Smithsonian Institution: Washington, DC, USA; Lynx Edicions: Barcelona, Spain, 2005. [Google Scholar]
- BirdLife International. Species Factsheet: Pallas’s Fish-Eagle Haliaeetus leucoryphus. Available online: https://datazone.birdlife.org/species/factsheet/pallass-fish-eagle-haliaeetus-leucoryphus (accessed on 8 August 2025).
- Green, R.E.; Newton, I.A.N.; Shultz, S.; Cunningham, A.A.; Gilbert, M.; Pain, D.J.; Prakash, V. Diclofenac Poisoning as a Cause of Vulture Population Declines across the Indian Subcontinent. J. Appl. Ecol. 2004, 41, 793–800. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species, Version 2024-2. Available online: https://www.iucnredlist.org (accessed on 10 June 2025).
- Chauhan, H.K.; Gallacher, D.; Bhatt, A.; Bisht, A.K. The Himalayas: A Climate Change Laboratory. Environ. Dev. 2023, 45, 100814. [Google Scholar] [CrossRef]
- Naoroji, R. Birds of Prey of the Indian Subcontinent; Christopher Helm, A&C Black Publishers Ltd.: London, UK, 2006. [Google Scholar]
- SOIB. State of India’s Birds: Range, Trends, and Conservation Status; The SoIB Partnership: Bengaluru, India, 2023; p. 119. [Google Scholar] [CrossRef]
- Chettri, N.; Rastogi, A.; Singh, O. Assessment of raptor migration and status along the Tsangpo-Brahmaputra corridor (India) by a local communities participatory survey. Avocetta-Parma 2006, 30, 61. [Google Scholar]
- Juhant, M.A.; Bildstein, K.L. Raptor migration across and around the Himalayas. In Bird Migration Across the Himalayas: Wetland Functioning amidst Mountains and Glaciers; Cambridge University Press: Cambridge, UK, 2017; Volume 6, pp. 98–116. [Google Scholar]
- Banerjee, S.; Niyogi, R.; Sarkar, M.S.; John, R. Assessing the vulnerability of protected areas in the eastern Himalayas based on their biological, anthropogenic, and environmental aspects. Trees For. People 2022, 8, 100228. [Google Scholar] [CrossRef]
- Casajus, N.; Périé, C.; Logan, T.; Lambert, M.-C.; de Blois, S.; Berteaux, D. An Objective Approach to Select Climate Scenarios When Projecting Species Distribution Under Climate Change. PLoS ONE 2016, 11, e0152495. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, J.G.; Langham, G.M.; Soykan, C.U.; Wilsey, C.B.; Auer, T.; Sanchez, C.C. Making Spatial Prioritizations Robust to Climate Change Uncertainties: A Case Study with North American Birds. Ecol. Appl. 2015, 25, 1819–1831. [Google Scholar] [CrossRef]
- Morley, J.W.; Selden, R.L.; Latour, R.J.; Frölicher, T.L.; Seagraves, R.J.; Pinsky, M.L. Projecting Shifts in Thermal Habitat for 686 Species on the North American Continental Shelf. PLoS ONE 2018, 13, e0196127. [Google Scholar] [CrossRef] [PubMed]
- Butet, A.; Rantier, Y.; Bergerot, B. Land Use Changes and Raptor Population Trends: A Twelve-Year Monitoring of Two Common Species in Agricultural Landscapes of Western France. Glob. Ecol. Conserv. 2022, 34, e02027. [Google Scholar] [CrossRef]
- Shaw, P.; Ogada, D.; Dunn, L.; Buij, R.; Amar, A.; Garbett, R.; Herremans, M.; Virani, M.Z.; Kendall, C.J.; Croes, B.M.; et al. African Savanna Raptors Show Evidence of Widespread Population Collapse and a Growing Dependence on Protected Areas. Nat. Ecol. Evol. 2024, 8, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, R.; Guichard, F.; Lutscher, F. Persistence and Extinction Dynamics Driven by the Rate of Environmental Change in a Predator–Prey Metacommunity. Theor. Ecol. 2020, 13, 629–643. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Gil, P.R.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; CEMEX: Mexico City, Mexico, 2005. [Google Scholar]
- Olson, D.M.; Dinerstein, E. The Global 200: Priority Ecoregions for Global Conservation. Ann. Mo. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Pawar, S.S.; Birand, A.C.; Ahmed, M.F.; Sengupta, S.; Raman, T.R.S. Conservation Biogeography in North-East India: Hierarchical Analysis of Cross-Taxon Distributional Congruence. Divers. Distrib. 2007, 13, 53–65. [Google Scholar] [CrossRef]
- Mani, M.S. Ecology and Biogeography in India; Springer Science & Business Media: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List Threat Assessments with GeoCAT: Geospatial Conservation Assessment Tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef] [PubMed]
- GBIF.org. GBIF Occurrence Download: Falco severus. 2025. Available online: https://doi.org/10.15468/dl.pfc2ej (accessed on 23 September 2025).
- GBIF.org. GBIF Occurrence Download. 2025. Available online: https://doi.org/10.15468/dl.duqyk2 (accessed on 23 September 2025).
- GBIF.org. GBIF Occurrence Download: Haliaeetus leucoryphus. 2025. Available online: https://doi.org/10.15468/dl.pttttq (accessed on 23 September 2025).
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The Next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Abedin, I.; Mukherjee, T.; Kim, A.R.; Kim, H.-W.; Kang, H.-E.; Kundu, S. Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios. Biology 2024, 13, 198. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cruz, M.; Rojas, E.; Guarino, L. DIVA-GIS, Version 1.4. A Geographic Information System for the Management and Analysis of Genetic Resources Data. Plant Genet. Resour. Newsl. 2001, 127, 15–19. [Google Scholar]
- Morisette, J.T.; Jarnevich, C.S.; Holcombe, T.R.; Talbert, C.B.; Ignizio, D.; Talbert, M.K.; Silva, C.; Koop, D.; Swanson, A.; Young, N.E. VisTrails SAHM: Visualization and Workflow Management for Species Habitat Modeling. Ecography 2013, 36, 129–135. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Riahi, K.; Ebi, K.L.; Hallegatte, S.; Carter, T.R.; Mathur, R.; van Vuuren, D.P. A New Scenario Framework for Climate Change Research: The Concept of Shared Socioeconomic Pathways. Clim. Change 2014, 122, 387–400. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and Their Energy, Land Use, and Greenhouse Gas Emissions Implications: An Overview. Glob. Environ. Change 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Andrews, M.B.; Ridley, J.K.; Wood, R.A.; Andrews, T.; Blockley, E.W.; Booth, B.; Burke, E.; Dittus, A.J.; Florek, P.; Gray, L.J.; et al. Historical Simulations with HadGEM3-GC3.1 for CMIP6. J. Adv. Model. Earth Syst. 2020, 12, e2019MS001995. [Google Scholar] [CrossRef]
- Desmet, Q.; Ngo-Duc, T. A Novel Method for Ranking CMIP6 Global Climate Models over the Southeast Asian Region. Int. J. Climatol. 2022, 42, 97–117. [Google Scholar] [CrossRef]
- Norgate, M.; Tiwari, P.R.; Das, S.; Kumar, D. On the Heat Waves over India and Their Future Projections under Different SSP Scenarios from CMIP6 Models. Int. J. Climatol. 2024, 44, 973–995. [Google Scholar] [CrossRef]
- Bhuyan, A.; Hazarika, S.; Baidya, S.; Sarma, K.; Thakur, B.; Prakash, A.; Devi, A. Assessing Current and Future Potential Habitat of Vatica lanceaefolia (Roxb.) Blume, a Critically Endangered Tree Species of Northeastern India. Theor. Appl. Climatol. 2025, 156, 1–13. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing Whether Ensemble Modelling Is Advantageous for Maximising Predictive Performance of Species Distribution Models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Talbert, C.B.; Talbert, M.K. User Manual for SAHM Package for VisTrails; U.S. Geological Survey: Reston, VA, USA, 2012.
- Salas, E.A.L.; Valdez, R.; Michel, S.; Boykin, K.G. Habitat Assessment of Marco Polo Sheep (Ovis ammon polii) in Eastern Tajikistan: Modeling the Effects of Climate Change. Ecol. Evol. 2018, 8, 5124–5138. [Google Scholar] [CrossRef] [PubMed]
- Lavazza, L.; Morasca, S.; Rotoloni, G. On the Reliability of the Area under the ROC Curve in Empirical Software Engineering. In Proceedings of the 27th International Conference on Evaluation and Assessment in Software Engineering, Oulu, Finland, 19–21 June 2023; Association for Computing Machinery: New York, NY, USA, 2023; pp. 93–100. [Google Scholar] [CrossRef]
- Phillips, S.J.; Elith, J. POC Plots: Calibrating Species Distribution Models with Presence-Only Data. Ecology 2010, 91, 2476–2484. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Acevedo, P.; Barbosa, A.M.; Lobo, J.M.; Real, R. Discrimination Capacity in Species Distribution Models Depends on the Representativeness of the Environmental Domain. Glob. Ecol. Biogeogr. 2013, 22, 508–516. [Google Scholar] [CrossRef]
- McGarigal, K. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; USDA Forest Service, General Technical Report PNW-GTR-351; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; p. 122.
- Brown, J.L.; Collopy, M.W.; Smallwood, J.A. Habitat Fragmentation Reduces Occupancy of Nest Boxes by an Open-Country Raptor. Bird Conserv. Int. 2014, 24, 364–378. [Google Scholar] [CrossRef]
- Wang, X.; Blanchet, F.G.; Koper, N. Measuring Habitat Fragmentation: An Evaluation of Landscape Pattern Metrics. Methods Ecol. Evol. 2014, 5, 634–646. [Google Scholar] [CrossRef]
- Midha, N.; Mathur, P.K. Assessment of Forest Fragmentation in the Conservation Priority Dudhwa Landscape, India Using FRAGSTATS Computed Class Level Metrics. J. Indian Soc. Remote Sens. 2010, 38, 487–500. [Google Scholar] [CrossRef]
- Gülçin, D.; Yılmaz, T. Evaluation of Forest Fragmentation with Particular Reference to Landscape-Based Ecological Assessment and Wildlife Conservation. Turk. J. For. 2020, 21, 84–93. [Google Scholar] [CrossRef][Green Version]
- Barwicka, S.; Milecka, M. The Use of Selected Landscape Metrics to Evaluate the Transformation of the Rural Landscape as a Result of the Development of the Mining Function—A Case Study of the Puchaczów Commune. Sustainability 2021, 13, 12279. [Google Scholar] [CrossRef]
- Biju, T.; Kimsing, A.T.; Ngukir, J.; Saurav, G.K.; Mize, D. Habitat Suitability and Distribution Range Modelling for Slender-Billed Vulture G. tenuirostris in Arunachal Pradesh, North East India Using MaxEnt. Curr. Sci. 2024, 127, 1093–1099. [Google Scholar] [CrossRef]
- Bellocq, M.I.; Gómez-Insausti, R. Raptorial Birds and Environmental Gradients in the Southern Neotropics: A Test of Species-Richness Hypotheses. Austral Ecol. 2005, 30, 892–898. [Google Scholar] [CrossRef]
- Jankowiak, L.; Polakowski, M.; Kułakowski, T.; Świętochowski, P.; Tumiel, T.; Broniszewska, M.; Takács, V. Habitat and Weather Requirements of Diurnal Raptors Wintering in River Valleys. Biologia 2015, 70, 1136–1142. [Google Scholar] [CrossRef]
- Amato, M.; Ossino, A.; Angelini, J.; Brunelli, M.; De Lisio, L.; De Rosa, D.; Andreotti, A.; Leonardi, G. Effects of Rainfall and Temperature on Timing and Breeding Performances of a Threatened Large Falcon: Case Study of the Lanner Falcon (Falco biarmicus feldeggii) in Italy. Biologia 2021, 76, 3751–3760. [Google Scholar] [CrossRef]
- Jha, R.; Jha, K.K. Environmental Factors Shaping Habitat Suitability of Gyps Vultures: Climate Change Impact Modelling for Conservation in India. Ornithol. Res. 2023, 31, 119–140. [Google Scholar] [CrossRef]
- Mcdonald, P.G.; Olsen, P.D.; Cockburn, A. Weather dictates reproductive success and survival in the Australian brown falcon Falco berigora. J. Anim. Ecol. 2004, 73, 683–692. [Google Scholar]
- Sharikov, A.V.; Volkov, S.V.; Sviridova, T.V.; Buslakov, V.V. Cumulative effect of trophic and weather–climatic factors on the population dynamics of the vole-eating birds of prey in their breeding habitats. Biol. Bull. 2019, 46, 1097–1107. [Google Scholar] [CrossRef]
- Anctil, A.; Franke, A.; Bety, J. Heavy rainfall increases nestling mortality of an arctic top predator: Experimental evidence and long-term trend in peregrine falcons. Oecologia 2014, 174, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Ngila, P.M.; Chiawo, D.O.; Owuor, M.A.; Wasonga, V.O.; Mugo, J.W. Mapping Suitable Habitats for Globally Endangered Raptors in Kenya: Integrating Climate Factors and Conservation Planning. Ecol. Evol. 2023, 13, e10443. [Google Scholar] [CrossRef]
- Kumar, S.; Sohil, A.; Kichloo, M.A.; Sharma, N. Landscape heterogeneity affects diurnal raptor communities in a sub-tropical region of northwestern Himalayas, India. PLoS ONE 2022, 17, e0246555. [Google Scholar] [CrossRef]
- Nayeri, D.; Cushman, S.; Ganey, J.; Hysen, L.; Gunther, M.S.; Willey, D.; Wan, H.Y. Multiscale habitat suitability modeling for a threatened raptor offers insight into ecological model transferability. Ecol. Model. 2024, 496, 110845. [Google Scholar] [CrossRef]
- Monteagudo, N.; Rebollo, S.; Pérez-Camacho, L.; Martínez-Hesterkamp, S.; Fernández-Pereira, J.M.; Pataro, L.; Rey-Benayas, J.M. Relative importance of vegetation features and intra- and inter-specific interactions on habitat preferences of a raptor guild in eucalypt plantations. For. Ecol. Manag. 2024, 554, 121656. [Google Scholar] [CrossRef]
- Koufaki, T.; Barboutis, C.; Theodorou, K. Differential impact of climate and land use change on habitat suitability of migrant passerines according to habitat preferences. Anthropocene 2024, 47, 100447. [Google Scholar] [CrossRef]
- Sutton, L.J.; Anderson, D.L.; Franco, M.; McClure, C.J.; Miranda, E.B.; Vargas, F.H.; Vargas González, J.D.J.; Puschendorf, R. Reduced Range Size and Important Bird and Biodiversity Area Coverage for the Harpy Eagle (Harpia harpyja) Predicted from Multiple Climate Change Scenarios. Ibis 2022, 164, 649–666. [Google Scholar] [CrossRef]
- Pearce-Higgins, J.W.; Green, R.E. Birds and Climate Change: Impacts and Conservation Responses; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Jaffré, M.; Beaugrand, G.; Goberville, É.; Jiguet, F.; Kjellén, N.; Troost, G.; Dubois, P.J.; Leprêtre, A.; Luczak, C. Long-Term Phenological Shifts in Raptor Migration and Climate. PLoS ONE 2013, 8, e79112. [Google Scholar] [CrossRef]
- Sourav, S.H.; Ahmed, B.; Thompson, P. Pallas’s fish eagle Haliaeetus leucoryphus in Bangladesh. Birding ASIA 2011, 16, 101–105. [Google Scholar]
- Choudhury, B.A.; Saha, S.K.; Konwar, M.; Sujith, K.; Deshamukhya, A. Rapid drying of Northeast India in the last three decades: Climate change or natural variability? J. Geophys. Res. Atmos. 2019, 124, 227–237. [Google Scholar] [CrossRef]
- Ahirwal, J.; Thangjam, U.; Sahoo, U.K. Land use change and its impacts on soil carbon dynamics in Mizoram, Northeast India. In Soil Carbon Dynamics in Indian Himalayan Region; Springer: Singapore, 2023; pp. 217–234. [Google Scholar] [CrossRef]
- Lalthakimi, C.; Singh, N.S.; Vanlalfakawma, D.C.; Upadhyay, K.K.; Tripathi, S.K. Land use change effects on soil physical and biochemical properties during wet and dry season in forest and shifting cultivation (jhum) sites in Northeast India. Environ. Ecol. 2023, 41, 2584–2594. [Google Scholar] [CrossRef]
- Pain, D.J.; Cunningham, A.A.; Donald, P.F.; Duckworth, J.W.; Houston, D.C.; Katzner, T.; Parry-Jones, J.; Poole, C.; Prakash, V.; Round, P.; et al. Causes and effects of temporal and spatial declines of Gyps vultures in Asia. Conserv. Biol. 2003, 17, 661–671. [Google Scholar] [CrossRef]
- Markandya, A.; Taylor, T.; Longo, A.; Murty, M.N.; Murty, S.; Dhavala, K. Counting the cost of vulture decline—An appraisal of the human health and other benefits of vultures in India. Ecol. Econ. 2008, 67, 194–204. [Google Scholar] [CrossRef]
- Melis, C.; Selva, N.; Teurlings, I.; Skarpe, C.; Linnell, J.D.C.; Andersen, R. Soil and vegetation nutrient response to bison carcasses in Białowieza Primeval Forest, Poland. Ecol. Res. 2007, 22, 807–813. [Google Scholar] [CrossRef]
- Fargallo, J.A.; Martínez-Padilla, J.; Viñuela, J.; Blanco, G.; Torre, I.; Vergara, P.; De Neve, L. Kestrel–prey dynamic in a Mediterranean region: The effect of generalist predation and climatic factors. PLoS ONE 2009, 4, e4311. [Google Scholar] [CrossRef] [PubMed]
- Millon, A.S.; Petty, J.; Little, B.; Giménez, O.; Cornulier, T.; Lambin, X. Dampening prey cycle overrides the impact of climate change on predator population dynamics: A long-term demographic study on tawny owls. Glob. Change Biol. 2014, 20, 1770–1781. [Google Scholar] [CrossRef]
- Sergio, F.; Newton, I.; Marchesi, L. Top predators and biodiversity: Much debate, few data. J. Appl. Ecol. 2008, 45, 992–999. [Google Scholar] [CrossRef]
- Parejo, D. Informational mismatches: A neglected threat of climate change to interspecific interactions. Front. Ecol. Evol. 2016, 4, 31. [Google Scholar] [CrossRef]
- Hof, A.R.; Jansson, R.; Nilsson, C. Future climate change will favour non-specialist mammals in the (sub)Arctics. PLoS ONE 2012, 7, e52574. [Google Scholar] [CrossRef] [PubMed]
- Lurgi, M.; López, B.C.; Montoya, J.M. Novel communities from climate change. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2913–2922. [Google Scholar] [CrossRef]
- Boonman, C.C.F.; Huijbregts, M.A.J.; Benítez-López, A.; Schipper, A.M.; Thuiller, W.; Santini, L. Trait-based projections of climate change effects on global biome distributions. Divers. Distrib. 2022, 28, 25–37. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Singha, H.; Go, Y.; Kang, H.E.; Kim, H.W.; Kundu, S. Predicting Climate-Driven Habitat Dynamics of Adjutants for Implementing Strategic Conservation Measures in South and Southeast Asian Landscape. Sci. Rep. 2025, 15, 5986. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.; Watson, R.T.; Ahmed, S.; Asim, M.; Johnson, J.A. Vulture restaurants and their role in reducing diclofenac exposure in Asian vultures. Bird Conserv. Int. 2007, 17, 63–77. [Google Scholar] [CrossRef]
- Davaasuren, B.; Boesman, P.F.D.; Hansasuta, C.; Pyle, P. Pallas’s Fish-Eagle (Haliaeetus leucoryphus), version 2.0. In Birds of the World; Billerman, S.M., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2025. [Google Scholar] [CrossRef]
| Species | Model | Dataset | AUC | ΔAUC | PCC | TSS | Kappa | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|---|---|
| Falco severus | BRT | Train | 0.921 | 0.053 | 85.500 | 0.852 | 0.860 | 0.711 | 0.709 |
| CV | 0.868 | 80.000 | 0.845 | 0.747 | 0.592 | 0.593 | |||
| GLM | Train | 0.951 | 0.122 | 88.100 | 0.883 | 0.879 | 0.761 | 0.760 | |
| CV | 0.829 | 76.500 | 0.776 | 0.750 | 0.526 | 0.525 | |||
| MARS | Train | 0.910 | 0.066 | 83.000 | 0.828 | 0.832 | 0.660 | 0.658 | |
| CV | 0.844 | 79.600 | 0.819 | 0.769 | 0.588 | 0.588 | |||
| MaxEnt | Train | 0.934 | 0.062 | 87.600 | 0.874 | 0.879 | 0.753 | 0.751 | |
| CV | 0.872 | 79.500 | 0.825 | 0.762 | 0.587 | 0.584 | |||
| RF | Train | 0.908 | 0.017 | 85.100 | 0.852 | 0.850 | 0.702 | 0.700 | |
| CV | 0.891 | 82.500 | 0.860 | 0.786 | 0.647 | 0.648 | |||
| Gyps tenuirostris | BRT | Train | 0.985 | 0.068 | 92.700 | 0.924 | 0.935 | 0.859 | 0.838 |
| CV | 0.917 | 86.100 | 0.871 | 0.845 | 0.715 | 0.693 | |||
| GLM | Train | 0.926 | 0.058 | 85.200 | 0.848 | 0.860 | 0.708 | 0.677 | |
| CV | 0.868 | 82.500 | 0.836 | 0.808 | 0.644 | 0.619 | |||
| MARS | Train | 0.955 | 0.053 | 87.900 | 0.879 | 0.879 | 0.758 | 0.733 | |
| CV | 0.902 | 84.600 | 0.849 | 0.845 | 0.694 | 0.664 | |||
| MaxEnt | Train | 0.948 | 0.041 | 89.700 | 0.897 | 0.897 | 0.794 | 0.772 | |
| CV | 0.907 | 84.500 | 0.853 | 0.835 | 0.688 | 0.662 | |||
| RF | Train | 0.934 | 0.003 | 85.200 | 0.853 | 0.850 | 0.703 | 0.675 | |
| CV | 0.937 | 88.200 | 0.907 | 0.835 | 0.741 | 0.734 | |||
| Haliaeetus leucoryphus | BRT | Train | 0.988 | 0.030 | 93.300 | 0.933 | 0.933 | 0.866 | 0.866 |
| CV | 0.958 | 88.600 | 0.874 | 0.900 | 0.774 | 0.772 | |||
| GLM | Train | 0.955 | 0.022 | 90.900 | 0.911 | 0.908 | 0.819 | 0.818 | |
| CV | 0.933 | 87.900 | 0.890 | 0.867 | 0.756 | 0.759 | |||
| MARS | Train | 0.970 | 0.038 | 92.500 | 0.926 | 0.924 | 0.850 | 0.850 | |
| CV | 0.932 | 90.600 | 0.911 | 0.900 | 0.811 | 0.811 | |||
| MaxEnt | Train | 0.978 | 0.023 | 92.500 | 0.926 | 0.924 | 0.850 | 0.849 | |
| CV | 0.955 | 90.500 | 0.904 | 0.908 | 0.811 | 0.810 | |||
| RF | Train | 0.957 | 0.001 | 89.400 | 0.787 | 0.787 | 0.891 | 0.896 | |
| CV | 0.958 | 89.400 | 0.789 | 0.787 | 0.900 | 0.889 |
| Species | Predictors | BRT | GLM | MARS | MaxEnt | RF | μ (Mean) | μ (Mean) % |
|---|---|---|---|---|---|---|---|---|
| Falco severus | aspect | 0.000 | 0.007 | 0.005 | 0.017 | 0.000 | 0.006 | 1.030 |
| bio15 | 0.000 | 0.285 | 0.060 | 0.098 | 0.003 | 0.089 | 15.390 | |
| bio18 | 0.000 | 0.222 | 0.000 | 0.052 | 0.001 | 0.055 | 9.490 | |
| bio19 | 0.289 | 0.315 | 0.139 | 0.077 | 0.023 | 0.169 | 29.020 | |
| bio2 | 0.000 | 0.128 | 0.074 | 0.096 | 0.010 | 0.062 | 10.630 | |
| bio4 | 0.000 | 0.087 | 0.059 | 0.103 | 0.001 | 0.050 | 8.580 | |
| elevation | 0.139 | 0.195 | 0.170 | 0.165 | 0.019 | 0.137 | 23.660 | |
| slope | 0.000 | 0.000 | 0.000 | 0.061 | 0.002 | 0.013 | 2.210 | |
| Gyps tenuirostris | aspect | 0.000 | 0.004 | 0.008 | 0.006 | 0.001 | 0.004 | 1.110 |
| bio15 | 0.019 | 0.000 | 0.058 | 0.017 | 0.007 | 0.020 | 5.960 | |
| bio18 | 0.017 | 0.052 | 0.000 | 0.017 | 0.004 | 0.018 | 5.250 | |
| bio19 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.110 | |
| bio2 | 0.020 | 0.036 | 0.075 | 0.028 | 0.006 | 0.033 | 9.620 | |
| bio4 | 0.081 | 0.184 | 0.048 | 0.077 | 0.007 | 0.079 | 23.180 | |
| elevation | 0.082 | 0.254 | 0.052 | 0.187 | 0.061 | 0.127 | 37.140 | |
| slope | 0.017 | 0.057 | 0.076 | 0.016 | 0.005 | 0.034 | 9.940 | |
| bio14 | 0.000 | 0.017 | 0.055 | 0.059 | 0.000 | 0.026 | 7.700 | |
| Haliaeetus leucoryphus | aspect | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.130 |
| bio15 | 0.000 | 0.081 | 0.022 | 0.004 | 0.001 | 0.022 | 7.430 | |
| bio18 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.120 | |
| bio19 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.120 | |
| bio2 | 0.000 | 0.000 | 0.007 | 0.020 | 0.001 | 0.006 | 1.960 | |
| bio4 | 0.028 | 0.093 | 0.034 | 0.019 | 0.000 | 0.035 | 11.940 | |
| elevation | 0.286 | 0.241 | 0.056 | 0.149 | 0.056 | 0.158 | 54.020 | |
| slope | 0.000 | 0.000 | 0.060 | 0.035 | 0.002 | 0.019 | 6.670 | |
| bio14 | 0.046 | 0.064 | 0.044 | 0.085 | 0.000 | 0.048 | 16.410 | |
| bio16 | 0.000 | 0.000 | 0.016 | 0.000 | 0.001 | 0.003 | 1.190 |
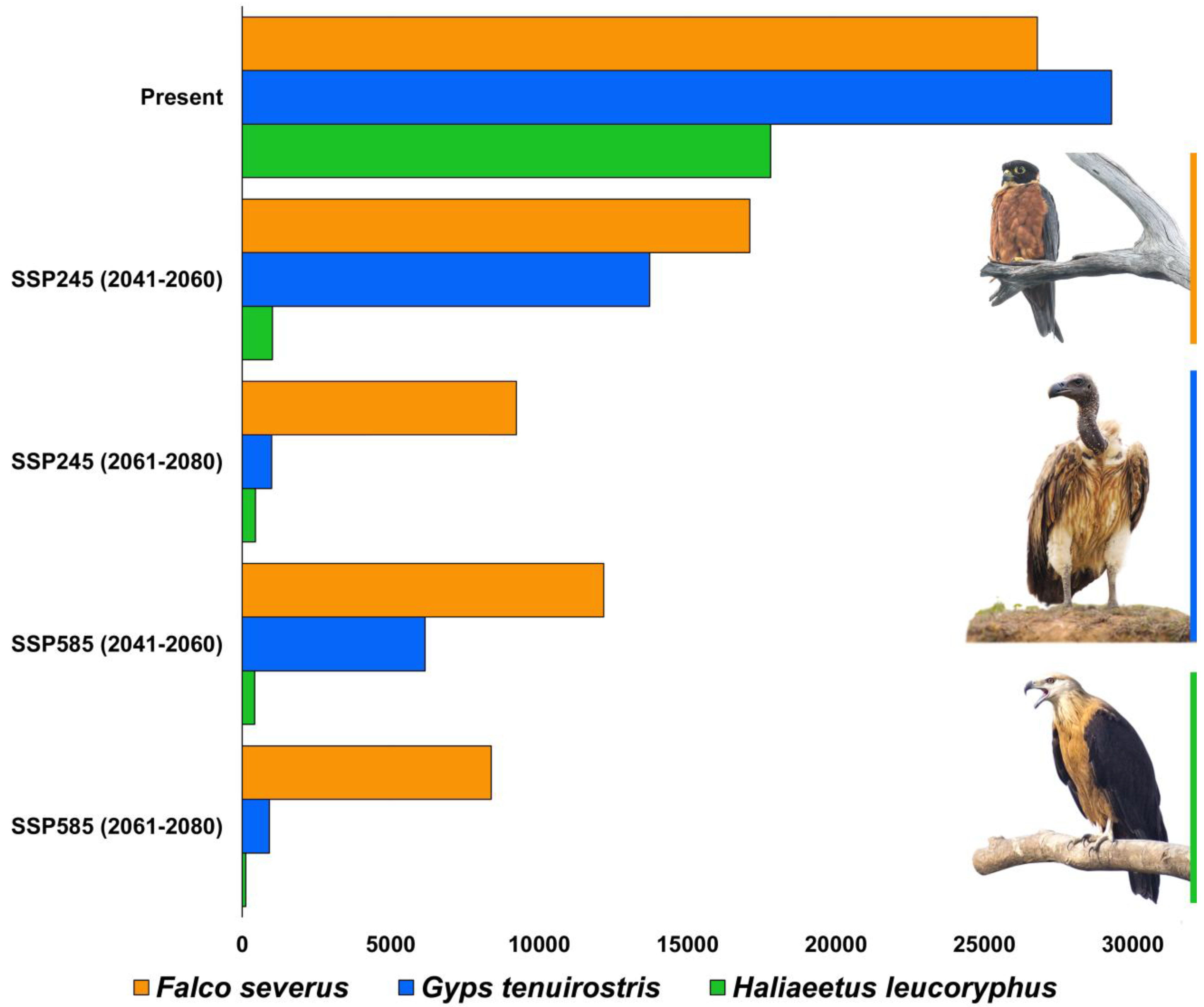
| Species | Present | SSP245 (2041–2060) | Habitat Loss from Present in SSP245 (2041–2060) (%) | SSP245 (2061–2080) | Habitat Loss from Present in SSP245 (2061–2080) (%) | SSP585 (2041–2060) | Habitat Loss from Present in SSP585 (2041–2060) (%) | SSP585 (2061–2080) | Habitat Loss from Present in SSP585 (2061–2080) (%) |
|---|---|---|---|---|---|---|---|---|---|
| Falco severus | 26,801 | 17,109 | −36.16 | 9231 | −65.55 | 12,184 | −54.53 | 8389 | −68.69 |
| Gyps tenuirostris | 29,297 | 13,731 | −53.13 | 989 | −96.62 | 6160 | −78.97 | 919 | −96.86 |
| Haliaeetus leucoryphus | 17,808 | 1021 | −94.26 | 444 | −97.50 | 419 | −97.64 | 119 | −99.33 |
| Species | Scenario | NP | LPI | TE | LSI | AI |
|---|---|---|---|---|---|---|
| Falco severus | Present | 200 | 5.091 | 80.368 | 15.314 | 91.187 |
| SSP245 (2041–2060) | 303 | 3.198 | 75.392 | 17.985 | 86.895 | |
| SSP245 (2061–2080) | 134 | 1.816 | 43.696 | 14.150 | 86.108 | |
| SSP585 (2041–2060) | 212 | 2.517 | 50.096 | 14.167 | 87.949 | |
| SSP585 (2061–2080) | 118 | 1.051 | 39.184 | 13.310 | 86.351 | |
| Gyps tenuirostris | Present | 255 | 8.371 | 84.640 | 15.423 | 91.507 |
| SSP245 (2041–2060) | 203 | 3.637 | 53.040 | 14.106 | 88.688 | |
| SSP245 (2061–2080) | 76 | 0.132 | 8.080 | 8.016 | 76.919 | |
| SSP585 (2041–2060) | 185 | 0.668 | 38.736 | 15.420 | 81.386 | |
| SSP585 (2061–2080) | 58 | 0.089 | 7.712 | 7.902 | 76.308 | |
| Haliaeetus leucoryphus | Present | 61 | 4.998 | 41.024 | 9.603 | 93.502 |
| SSP245 (2041–2060) | 46 | 0.171 | 8.512 | 8.313 | 76.340 | |
| SSP245 (2061–2080) | 14 | 0.003 | 0.944 | 4.214 | 39.189 | |
| SSP585 (2041–2060) | 8 | 0.002 | 0.432 | 3 | 37.931 | |
| SSP585 (2061–2080) | 6 | 0.002 | 0.401 | 2.330 | 32.681 |
| Species | Threats (IUCN) | Necessary Responses | Scale of Action |
|---|---|---|---|
| Falco severus | Climate change, habitat loss and fragmentation. The percentage of suitable area loss from present area is 36.16% during 2041–2060 (SSP245), and loss during 2061–2080 (SSP245) is 65.55%. Similarly, the percent suitable area loss during 2041–2060 (SSP585) is 54.53%, and there is 68.69% loss during 2061–2080 (SSP585). | Long-term population trend monitoring by citizen science program. Impact assessment. Strict law enforcement. Awareness and education. Climate change adaptive management. | Regional |
| Gyps tenuirostris | Climate change, habitat loss and fragmentation. The percentage of suitable area loss from present area is 53.13% during 2041–2060 (SSP245), and loss during 2061–2080 (SSP245) is 96.62%. Similarly, the percent suitable area loss during 2041–2060 (SSP585) is 78.97%, and there is 96.86% loss during 2061–2080 (SSP585). | Updated vulture action plan. Updated areas of vulture safe zones. Awareness and advocacy. Long-term population trend monitoring by citizen science program. Impact assessment. Climate change adaptive management. | Regional |
| Haliaeetus leucoryphus | Climate change, Habitat loss and fragmentation. The percentage of suitable area loss from present area is 94.26% during 2041–2060 (SSP245), and loss during 2061–2080 (SSP245) is 97.5%. Similarly, the percent suitable area loss during 2041–2060 (SSP585) is 97.64% and there is 99.33% loss during 2061–2080 (SSP585). | Identification of suitable sites for protection. Recommend sustainable wetland management practices. Regulate pollution of wetlands. Nesting trees plantation around waterbodies. Conduct education and awareness programs in rural areas having wetlands. Long-term population trend monitoring by citizen science program. Impact assessment. | Regional and International |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahananda, P.; Abedin, I.; Bhuyan, A.; Saikia, M.K.; Saikia, P.K.; Singha, H.; Kundu, S. Rulers of the Open Sky at Risk: Climate-Driven Habitat Shifts of Three Conservation-Priority Raptors in the Eastern Himalayas. Biology 2025, 14, 1376. https://doi.org/10.3390/biology14101376
Mahananda P, Abedin I, Bhuyan A, Saikia MK, Saikia PK, Singha H, Kundu S. Rulers of the Open Sky at Risk: Climate-Driven Habitat Shifts of Three Conservation-Priority Raptors in the Eastern Himalayas. Biology. 2025; 14(10):1376. https://doi.org/10.3390/biology14101376
Chicago/Turabian StyleMahananda, Pranjal, Imon Abedin, Anubhav Bhuyan, Malabika Kakati Saikia, Prasanta Kumar Saikia, Hilloljyoti Singha, and Shantanu Kundu. 2025. "Rulers of the Open Sky at Risk: Climate-Driven Habitat Shifts of Three Conservation-Priority Raptors in the Eastern Himalayas" Biology 14, no. 10: 1376. https://doi.org/10.3390/biology14101376
APA StyleMahananda, P., Abedin, I., Bhuyan, A., Saikia, M. K., Saikia, P. K., Singha, H., & Kundu, S. (2025). Rulers of the Open Sky at Risk: Climate-Driven Habitat Shifts of Three Conservation-Priority Raptors in the Eastern Himalayas. Biology, 14(10), 1376. https://doi.org/10.3390/biology14101376









