Analysis of Pigmentation Changes in Bracts of Bougainvillea × buttiana ‘Miss Manila’ During Different Developmental Periods
Simple Summary
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Measurement of Color Parameters
2.3. Chlorophyll Extraction and Content Determination
2.4. Flavonoid Extraction and Content Determination
2.5. Betalain Extraction and Content Determination
2.6. Gene Expression Quantification
2.7. Sequence Bioinformatics Analysis
2.8. Data Analysis
3. Results
3.1. Determination of Developmental Stages in B. × buttiana ‘Miss Manila’ Bracts
3.2. Color Parameters of B. × buttiana ‘Miss Manila’ Bracts at Different Developmental Stages
3.3. Pigment Content in B. × buttiana ‘Miss Manila’ Bracts at Different Developmental Stages
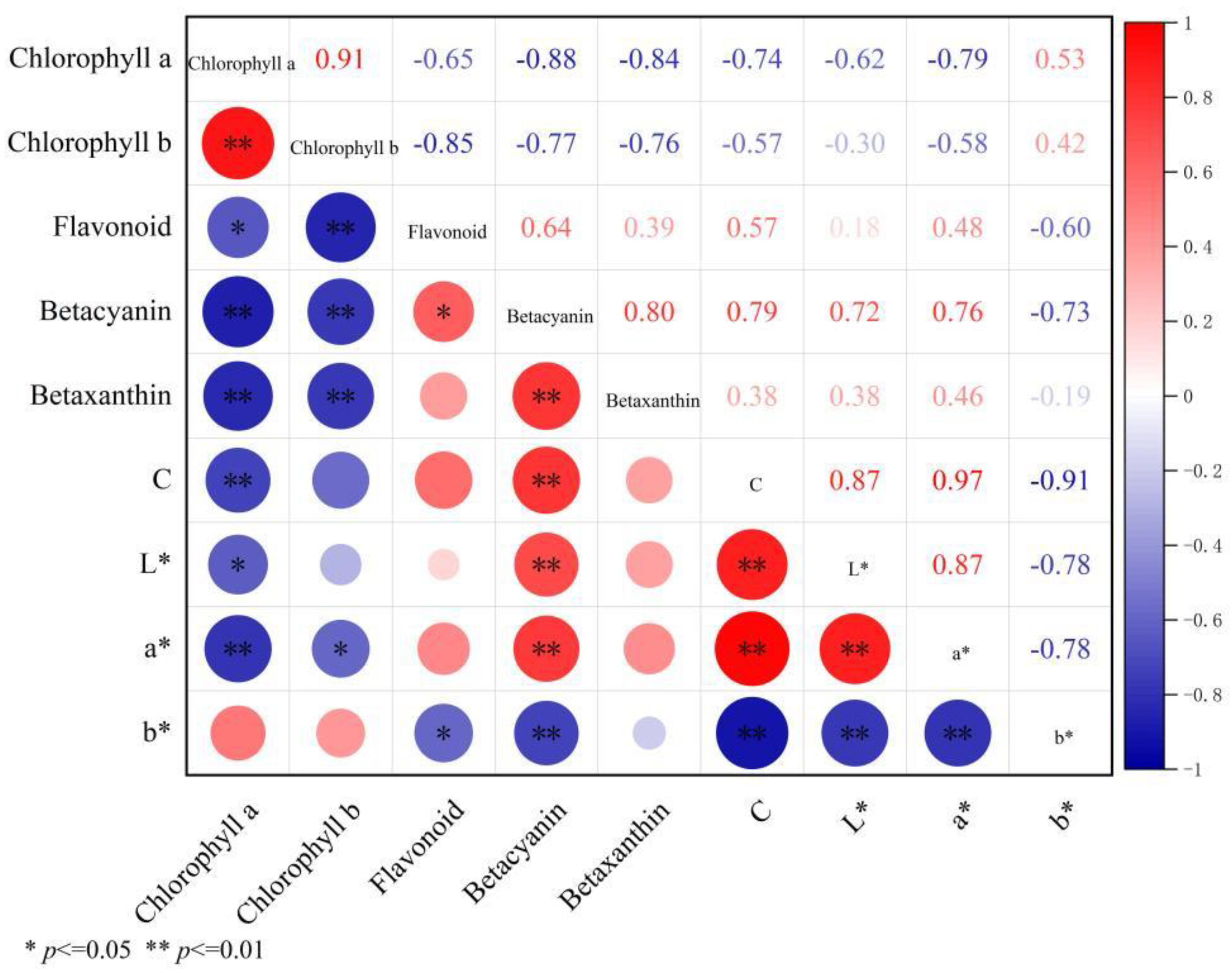
3.4. Correlation Between Color Parameters and Pigment Content in Bracts of a B. × buttiana ‘Miss Manila’ at Different Developmental Stages
3.5. Expression Patterns of Pigment Biosynthesis-Related Genes in Bracts of B. × buttiana ‘Miss Manila’ at Different Developmental Stages
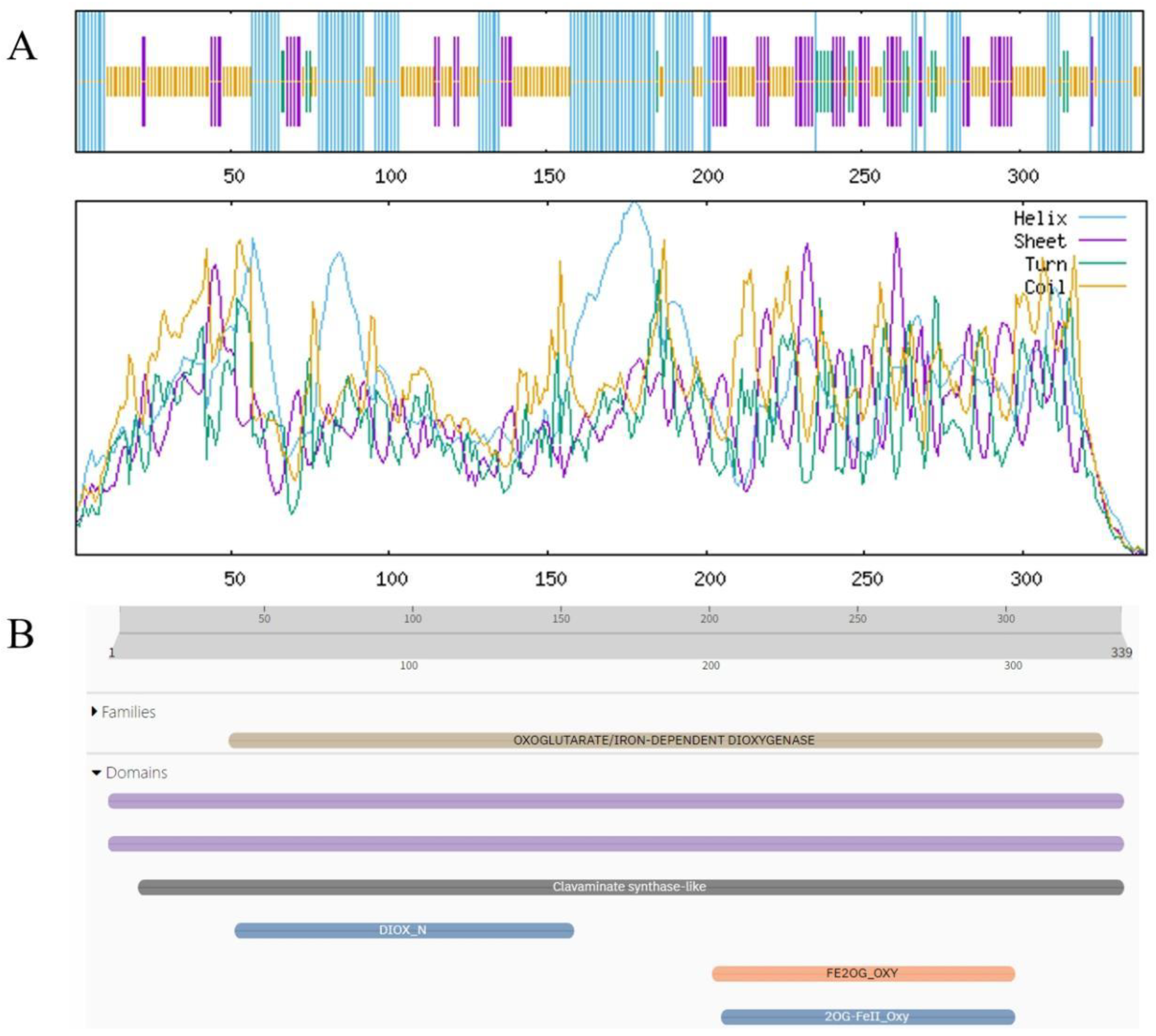
3.6. Physicochemical Properties and Structural Prediction of BgFLS and BgCHIL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| L* | Lightness |
| a* | Red–green component |
| b* | Blue–yellow component |
| C | Chroma |
| DODA | 4,5-DOPA dioxygenase gene |
| RT-qPCR | Real-time quantitative PCR |
| SGR | STAY-GREEN |
| POR | protochlorophyllide oxidoreductase |
| DFR | dihydroflavonol 4-reductase |
| FLS | flavonol synthase/flavanone 3-hydroxylase |
| CHIL | Chalcone-flavanone isomerase family protein |
References
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P.; et al. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Genet. 2016, 48, 657–666. [Google Scholar] [CrossRef]
- Kang, Y.; Li, Y.; Zhang, T.; Wang, P.; Liu, W.; Zhang, Z.; Yu, W.; Wang, J.; Wang, J.; Zhou, Y. Integrated metabolome, full-length sequencing, and transcriptome analyses unveil the molecular mechanisms of color formation of the canary yellow and red bracts of Bougainvillea × buttiana ‘Chitra’. Plant J. 2023, 116, 1441–1461. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fang, Z.; Liu, M.; Zhao, D.; Tao, J. Color characteristics, pigment accumulation and biosynthetic analyses of leaf color variation in herbaceous peony (Paeonia lactiflora Pall.). 3 Biotech 2020, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Wu, Q.; Fu, X.; Chen, Z.; Wang, H.; Wang, J.; Zhu, Z.; Zhu, G. Composition, Color Stability and Antioxidant Properties of Betalain-Based Extracts from Bracts of Bougainvillea. Molecules 2022, 27, 5120. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, Y.; Sheng, Q.; Zhu, Z. Variation characteristics of color parameters and pigment content in bracts at different flowering stages among various cultivars of Bougainvillea spectabilis. J. Cent. South Univ. For. Technol. 2024, 44, 157–165. [Google Scholar]
- Clement, J.S.; Mabry, T.J.; Wyler, H.; Dreiding, A.S. Chemical Review and Evolutionary Significance of the Betalains. In Caryophyllales; Springer: Berlin/Heidelberg, Germany, 1994; pp. 247–261. [Google Scholar]
- Lan, L.; Zhao, H.; Xu, S.; Kan, S.; Zhang, X.; Liu, W.; Liao, X.; Tembrock, L.R.; Ren, Y.; Reeve, W.; et al. A high-quality Bougainvillea genome provides new insights into evolutionary history and pigment biosynthetic pathways in the Caryophyllales. Hortic. Res. 2023, 10, uhad124. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F. Biosynthesis of betalains: Yellow and violet plant pigments. Trends Plant Sci. 2013, 18, 334–343. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, Q.; Lin, J.; Ma, X.; Dong, F.; Yan, H.; Zhong, W.; Lu, Y.; Yao, Y.; Shen, X.; et al. Transcriptome analyses shed light on floral organ morphogenesis and bract color formation in Bougainvillea. BMC Plant Biol. 2022, 22, 97. [Google Scholar] [CrossRef]
- Wang, F.; Yao, G.; Li, J.; Zhu, W.; Li, Z.; Sun, Z.; Xin, P. Mining and expression analysis of color related genes in Bougainvillea glabra bracts based on transcriptome sequencing. Sci. Rep. 2024, 14, 24491. [Google Scholar] [CrossRef]
- Mao, Y.; Luo, J.; Cai, Z. Biosynthesis and Regulatory Mechanisms of Plant Flavonoids: A Review. Plants 2025, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Ito, H. Chlorophyll degradation and its physiological function. Plant Cell Physiol. 2025, 66, 139–152. [Google Scholar] [CrossRef]
- Berhe, M.; You, J.; Dossa, K.; Li, D.; Zhou, R.; Zhang, Y.; Wang, L. Examining Chlorophyll Extraction Methods in Sesame Genotypes: Uncovering Leaf Coloration Effects and Anatomy Variations. Plants 2024, 13, 1589. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Dong, B.; Huang, Y.; Bao, Z.; Zhao, H. Relationship between Pigment Composition and Peel Color for the Fruit of Chinese Flame Tree. J. Am. Soc. Hortic. Sci. 2018, 143, 184–193. [Google Scholar] [CrossRef]
- Conesa, A.; Manera, F.C.; Brotons, J.M.; Fernandez-Zapata, J.C.; Simón, I.; Simón-Grao, S.; Alfosea-Simón, M.; Martínez-Nicolás, J.J.; Valverde, J.M.; García-Sanchez, F. Changes in the content of chlorophylls and carotenoids in the rind of Fino 49 lemons during maturation and their relationship with parameters from the CIELAB color space. Sci. Hortic. 2019, 243, 252–260. [Google Scholar] [CrossRef]
- Mochizuki-Kawai, H.; Kishimoto, S.; Wada, Y.; Masuda, T.; Ichimura, K. Petal Saturation Affects Visible Flower Senescence in Cut Lilies. J. Jpn. Soc. Hortic. Sci. 2012, 81, 350–356. [Google Scholar] [CrossRef]
- Song, L.; Ma, Q.; Zou, Z.; Sun, K.; Yao, Y.; Tao, J.; Kaleri, N.A.; Li, X. Molecular Link between Leaf Coloration and Gene Expression of Flavonoid and Carotenoid Biosynthesis in Camellia sinensis Cultivar ‘Huangjinya’. Front. Plant Sci. 2017, 8, 803. [Google Scholar] [CrossRef]
- Tan, X.; Wang, W.; Gao, L.; Wei, J.; Zhang, W.; Li, L.; Wu, J.; Wang, J.; Zhang, X.; Liao, X.; et al. The difference in leaf color quality of Cotinus coggygria during the coloration peak period affected by soil and topographic heterogeneity. CATENA 2023, 228, 107140. [Google Scholar] [CrossRef]
- Yang, G.S.; Yao, H.X.; He, F.M.; Li, Z.L.; Wang, Y.Y. Unveiling CcR2R3-MYB: A Key Regulator of Leaf Pigmentation in Cymbidium Orchids. Horticulturae 2025, 11, 190. [Google Scholar] [CrossRef]
- Gao, Y.F.; Zhao, D.H.; Zhang, J.Q.; Chen, J.S.; Li, J.L.; Weng, Z.; Rong, L.P. De novo transcriptome sequencing and anthocyanin metabolite analysis reveals leaf color of Acer pseudosieboldianum in autumn. BMC Genom. 2021, 22, 383. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Peng, X.Q.; Shu, X.C.; Li, Y.H.; Wang, Z.; Zhuang, W.B. Genome-wide identification and characterization of PdbHLH transcription factors related to anthocyanin biosynthesis in colored-leaf poplar (Populus deltoids). BMC Genom. 2022, 23, 244. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Yamamoto, R.; Kojima, N.; Yahata, M.; Kato, M. Molecular characterization of a flavanone 3-hydroxylase gene from citrus fruit reveals its crucial roles in anthocyanin accumulation. BMC Plant Biol. 2023, 23, 233. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Chu, D.; Wang, X. Exploring the evolution of CHS gene family in plants. Front Genet. 2024, 15, 1368358. [Google Scholar] [CrossRef]
- Kriangphan, N.; Vuttipongchaikij, S.; Kittiwongwattana, C.; Suttangkakul, A.; Pinmanee, P.; Sakulsathaporn, A.; Suwimon, R.; Suputtitada, S.; Chanvivattana, Y.; Apisitwanich, S. Effects of Sequence and Expression of Eight Anthocyanin Biosynthesis Genes on Floral Coloration in Four Dendrobium Hybrids. Hortic. J. 2015, 84, 83–92. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Ye, C.; Liang, H. Characterization and expression analysis of a chalcone isomerase-like gene in relation to petal color of Actinidia chrysantha. Biologia 2017, 72, 753–763. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.J.; Wang, Y.; Liu, S.; Geng, Z.; Song, A.; Jiang, J.; Chen, S.; Chen, F. Functional identification of a flavone synthase and a flavonol synthase genes affecting flower color formation in Chrysanthemum morifolium. Plant Physiol. Biochem. 2021, 166, 1109–1120. [Google Scholar] [CrossRef]
- Lam, P.Y.; Wang, L.; Lui, A.C.W.; Liu, H.; Takeda-Kimura, Y.; Chen, M.-X.; Zhu, F.-Y.; Zhang, J.; Umezawa, T.; Tobimatsu, Y.; et al. Deficiency in flavonoid biosynthesis genes CHS, CHI, and CHIL alters rice flavonoid and lignin profiles. Plant Physiol. 2021, 188, 1993–2011. [Google Scholar] [CrossRef]



| Component Name | Volume |
|---|---|
| Oligo dT Primer (2.5 μM) | 1 μL |
| Template RNA | 2 μL |
| RNase free water | 7 μL |
| Component Name (200 U/μL) | Volume |
|---|---|
| the aforementioned reaction solution after denaturation and annealing | 10 μL |
| 5× RTase Reaction Buffer II | 4 μL |
| dNTP Mix (10 mM each) | 2 μL |
| Evo M-MLV II RTase | 0.5 μL |
| RNase free water | 4.5 μL |
| Steps | Temperature | Time | Number of Cycles |
|---|---|---|---|
| Step 1 | 95 °C | 30 s | 1 |
| Step 2 | 95 °C | 5 s | 40 |
| 60 °C | 30 s | ||
| Step 3 | Dissociation Stage | ||
| Gene Name | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|
| BgDODA | ACAAAGCGTGGACTTGACCAT | AAGGTTGTAGTGGTGGGTCC |
| BgDTX | TCAACCTTGCTTTGCGCTTG | TACATTTTCGCTGGACCGCT |
| BgF3H | GACACAAGGACTCAGGAGCGATAA | GGGTGGAAGAAGAAGGGAATGGA |
| BgCHIL | CAGTGGATACATTGTTGA | TTCTTCGTCTTCTTCATAA |
| BgCHS | TACTACTTCCGAGTCACT | GTATGTCTTCCGTTAGGT |
| BgFLS | AAGCCAACAATGGAAGTAGA | CTCGGACGAACTCTGATG |
| BgSGR | ATTGCCAAGAACTTACAC | AATGACCACCACTTATGT |
| BgDFRA | CTGCTGTCCTTACTAATAC | TCCAGTGATTCTTGAGAT |
| BgPORA | CTCACAATGCAGGAGTTC | CTCTGAACAAGCCAGTAG |
| ACTIN | TAGACCCTCCTATCCAAACA | TTTTCCAGCCTTCACTTATC |
| Stages | Lightness L* | Red–Green Component a* | Blue–Yellow Component b* | Chroma C |
|---|---|---|---|---|
| BR1 | 52.477 ± 1.829 c | 24.78 ± 0.945 b | −4.997 ± 4.096 ac | 25.473 ± 1.703 b |
| BR2 | 58.123 ± 3.291 b | 34.613 ± 3.78 b | −14.2 ± 2.722 b | 37.45 ± 4.187 b |
| BR3 | 64.773 ± 2.566 b | 39.467 ± 4.438 b | −21.247 ± 2.03 c | 44.831 ± 4.763 b |
| BR4 | 72.98 ± 72.98 a | 41.807 ± 1.728 a | −28.843 ± 7.06 d | 51.098 ± 2.426 a |
| Stages | Chlorophyll a mg/g | Chlorophyll b mg/g | Flavonoid mg/g | Betacyanin mg/g | Betaxanthin mg/g |
|---|---|---|---|---|---|
| BR1 | 1.23 ± 0.10 a | 0.97 ± 0.07 ac | 1.85 ± 0.01 d | 2.93 ± 0.26 a | 1.68 ± 0.009 a |
| BR2 | 0.75 ± 0.10 b | 0.63 ± 0.05 b | 2.54 ± 0.09 b | 4.45 ± 0.63 b | 2.56 ± 0.32 c |
| BR3 | 0.47 ± 0.03 c | 0.39 ± 0.05 c | 3.08 ± 0.04 | 4.94 ± 0.27 b | 2.16 ± 0.19 b |
| BR4 | 0.27 ± 0.03 d | 0.34 ± 0.04 d | 2.04 ± 0.003 c | 4.69 ± 0.06 b | 2.27 ± 0.05 bc |
| Color Parameters | Multiple Linear Regression | R2 |
|---|---|---|
| Chroma C | y = −27.80681 − 35.32951 × chlorophyll a content + 78.26294 × chlorophyll b content + 22.6553 × flavonoid content + 0.47252 × betacyanin content − 6.04786 × betaxanthin content | 0.925 |
| L* | y = 32.19816 − 27.053 × chlorophyll a content + 55.10084 × chlorophyll b content + 5.82794 × flavonoid content + 4.08936 × betacyanin content − 8.20242 × betaxanthin content | 0.997 |
| a* | y = 15.43201 − 27.15873 × chlorophyll a content + 46.64347 × chlorophyll b content + 10.36714 × flavonoid content − 0.83358 × betacyanin content − 5.91821 × betaxanthin content | 0.851 |
| b* | y = 103.13033 + 26.2152 × chlorophyll a content − 88.89036 × chlorophyll b content − 31.11977 × flavonoid content − 2.15693 × betacyanin content − 0.89268 × betaxanthin content | 0.946 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ma, Y.; Yan, J.; Liu, Y.; Huang, Y.; Deng, S.; Dong, J.; Hu, Y. Analysis of Pigmentation Changes in Bracts of Bougainvillea × buttiana ‘Miss Manila’ During Different Developmental Periods. Biology 2025, 14, 1607. https://doi.org/10.3390/biology14111607
Liu X, Ma Y, Yan J, Liu Y, Huang Y, Deng S, Dong J, Hu Y. Analysis of Pigmentation Changes in Bracts of Bougainvillea × buttiana ‘Miss Manila’ During Different Developmental Periods. Biology. 2025; 14(11):1607. https://doi.org/10.3390/biology14111607
Chicago/Turabian StyleLiu, Xiangdong, Yuwan Ma, Jiawen Yan, Yan Liu, Yaqi Huang, Siyin Deng, Jiawen Dong, and Yulin Hu. 2025. "Analysis of Pigmentation Changes in Bracts of Bougainvillea × buttiana ‘Miss Manila’ During Different Developmental Periods" Biology 14, no. 11: 1607. https://doi.org/10.3390/biology14111607
APA StyleLiu, X., Ma, Y., Yan, J., Liu, Y., Huang, Y., Deng, S., Dong, J., & Hu, Y. (2025). Analysis of Pigmentation Changes in Bracts of Bougainvillea × buttiana ‘Miss Manila’ During Different Developmental Periods. Biology, 14(11), 1607. https://doi.org/10.3390/biology14111607






