Simple Summary
The maize weevil, Sitophilus zeamais, is a globally distributed stored-grain pest that causes significant losses to cereals, posing a serious threat to food security. We investigated chemical composition and fumigation activity of Angelica archangelica root essential oil (AAEO) against S. zeamais. The main components of AAEO and its median lethal concentration (LC50) for fumigation against this pest were identified. The enzymatic activities of acetylcholinesterase, glutathione-S-transferase, and carboxylesterase were measured when fumigated by AAEO LC50. The study demonstrates that AAEO exhibits strong insecticidal activity against S. zeamais, affecting survival, detoxification enzymes, and gene transcription. The present study provide a scientific basis for the development of effective plant essential oil-based insecticide against S. zeamais.
Abstract
Sitophilus zeamais is one of the most destructive pests of stored grains. Both adults and larvae penetrate and consume the grains, thereby diminishing the grain quality and nutritional value. We determined the chemical composition of Angelica archangelica essential oil, its fumigation toxicity against S. zeamais, and its effects on the activities of detoxification enzymes in the insects. RNA-seq was performed to analyze the impact of the essential oil on the transcriptional level of S. zeamais, and qRT-PCR was conducted to validate the differentially expressed genes. Chemical analysis identified 35 components in essential oil, including δ-3-Carene (24.26%), Limonene (19.81%), and α-Pinene (14.96%). A significant positive correlation was observed between the fumigation activity of the essential oil and the applied dose. The median lethal concentrations (LC50) values were 164.38, 132.62, and 90.35 mg/L air at 24, 48, and 72 h, respectively. Fumigation significantly inhibited the activities of the three detoxification enzymes. RNA-seq revealed a total of 3718 significantly differentially expressed genes (DEGs). qRT-PCR confirmed that the expression patterns of the DEGs were consistent with the RNA-seq data. This study comprehensively evaluates the control efficacy of A. archangelica essential oil against S. zeamais and provides data support for developing novel, eco-friendly, plant-based pesticides.
1. Introduction
Sitophilus zeamais is a destructive pest of stored grain, including rice, maize, wheat and their products, causing extensive loss due to reduction in quality and nutritional value [1,2]. Adults of S. zeamais can directly lay eggs inside the grains to protect the developing larvae, resulting in grain losses and contamination. The larvae infest the grains, causing entrance of various fungi and bacteria, thereby increasing the risk of contamination [3]. To combat postharvest losses, chemical fumigants including methyl bromide and phosphine were frequently and widely used to control S. zeamais during storage [4,5]. Although they have brought remarkable economic benefits, long-term use of these chemicals has led to the development of pest resistance. Additionally, the chemical fumigants, such as methyl bromide, have been prohibited due to their environmental pollution [6,7]. Thus, it is critical to explore natural products as potential bioinsecticide sources to control stored-product pests.
Plant essential oil (EO), as one class of important volatile secondary metabolites, is extracted from flowers, seeds, leaves, roots and fruits of plants [8]. EOs exhibits not only high bioactivity but also outstanding advantages such as remarkable efficacy, low toxicity, and short persistence in ecosystems [9]. Up to now, many plant essential oils have been reported to have insecticidal activity [10,11,12]; the effects on S. zeamais could be classified as repellency, fumigation, contact action. Therefore, EOs can be regarded as a foundation of the development of biopesticides [13]. Plant essential oils, as an emerging “green insecticide,” demonstrate significant potential in the field of stored-grain pest control [9]. Research has progressed from simple toxicity assays to in-depth investigations of complex multi-target mechanisms, providing a compelling scientific foundation for their use as alternatives to traditional chemical fumigants [2]. In comparison, their key advantages include high efficacy and broad-spectrum activity, multi-targeted mechanisms of action, environmental friendliness and biodegradability, relative safety for non-target organisms, and a low tendency to induce pest resistance [7]. Comprehensive research positions them as a promising future direction for integrated pest management.
Angelica archangelica L. (syn. A. officinalis Hoffm.), well known as garden angelica and folk medicine, is a bi-annual or perennial herb with a strong aroma, so it is often used as a food ingredient. The essential oil from roots of A. archangelica has multiple biological activities, including antioxidant and antibacterial effects, among other pharmacological effects [14,15,16]. However, there is a lack of evidence regarding the insecticidal activity of A. archangelica root essential oil against S. zeamais.
This study investigates the fumigant toxicity of A. archangelica root essential oil against S. zeamais adults and evaluates its impact on the insect’s detoxification and nervous system enzymes. Furthermore, we performed transcriptome sequencing on S. zeamais after fumigation with essential oil for 24 h to identify the genes altered by treatment. The results provide a theoretical basis for the management of stored-grain pests using A. archangelica root essential oil.
2. Materials and Methods
2.1. Essential Oil
The essential oil from A. archangelica was obtained from Ji’An HuaTianBao Herbs Biological Products Factory (purity ≥ 95.00%; Ji’an, China). It was extracted from root of A. archangelica by hydro-distillation.
2.2. Gas Chromatography and Mass Spectrometry (GC-MS)
Components of the essential oil of A. archangelica were identified by a GC-MS 7890B-5977B system (Agilent Technologies, Palo Alto, CA, USA) coated with a DB-5 MS fused silica capillary column (30 m × 0.25 mm × 0.25 μm). n-Hexane was used as the dilution solvent. The injection temperature was set at 250 °C, with 1 μL of a 10% solution injected each time. The temperature was maintained at 50 °C for 2 min, then increased at a rate of 10 °C per minute to 240 °C, and held for 5 min. The carrier gas was helium at flow rate of 1.0 μL/min. Spectra were scanned from 50 to 550 m/z. The retention indices were determined in relation to a homologous series of n-alkanes (C7–C40) under the same operating conditions. The components were identified by NIST 14.L and confirmed by comparing the Arithmetic Indices and comparison with authentic standards. Relative percentages of the individual components of the essential oil were quantified on the basis of the peak area.
2.3. Insect Culture
S. zeamais were maintained in the insectarium at Henan Institute of Science and Technology for over three years without exposure to insecticides. The insects were reared in a round glass jar with a 10 cm diameter and a height of 15 cm, containing 0.5 kg of sterilized whole wheat. The glass jars were placed in an incubator maintained at 28 ± 1 °C and 75 ± 5% RH, in total darkness.
2.4. Fumigant Toxicity
S. zeamais adults were used to evaluate the fumigant activity of A. archangelica essential oils (EOs). Solutions of A. archangelica EOs were prepared at concentrations of 34, 68, 102, 136, and 170 mg/L using hexane. A 100 μL aliquot of each concentration was dropped onto a Whatman filter paper strip (1.5 cm × 5.0 cm) and evaporated for 1 min at room temperature. It was then suspended in a triangular flask (50 mL). Thirty randomly selected S. zeamais adults were released into a fumigation bottle containing 3 g of wheat, and the cap was screwed tightly shut. The control consisted of hexane solvent. The numbers of dead S. zeamais adults were observed and recorded at 24, 48, and 72 h post-treatment. All treatments and controls were conducted independently five times.
2.5. Assessment of Enzyme Activity
We determined the activities of acetylcholinesterase (AChE), glutathione S-transferase (GST), and carboxylesterase (CarE). A series of treatments to S. zeamais were set with dose of 34, 68, 102, 136, and 170 mg/L of A. archangelica EOs, respectively. Living insects were collected at 24 h after EO treatment, and used for the extraction of total protein. In order to explore the enzyme activity in different stages, another set of test samples were treated with LC50 of EO (164.38 mg/L) at 4, 8, 12, 24, and 48 h, respectively. The live insects after treatment were weighed, rinsed, and homogenized, followed by the addition of 900 μL of pre-cooled normal saline. The mixture was centrifuged at 2500 rpm for 10 min at 4 °C. The supernatant was stored at −80 °C. The entire experimental procedure was performed on ice. The concentration of total protein was determined using the total protein quantitative assay (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The activity of each enzyme, including AChE, GST, and CarE was tested following the instruction of the AChE, GST, and CarE assay kits (Nanjing Jiancheng Bioengineering Institute), respectively. Three replicates were performed for each treatment and each replicate was performed three times.
2.6. RNA Extraction and Sequencing
In order to explore the functional genes of S. zeamais against A. archangelica EOs, 164.83 mg/L (LC50 of 24 h) EOs was used to fumigate the adults for 24 h. The control was hexane solvent. Living insects were collected and frozen separately in liquid nitrogen and stored at −80 °C for RNA extraction.
Total RNA was sequentially extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA), followed by RNA integrity assessment with the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA), and subsequent library construction.
2.7. Transcript Assembly and Gene Function Annotation
Raw reads were firstly processed through removing low-quality reads, reads containing adapter, and reads containing N base to obtain clean reads. Transcriptome assembly was accomplished using Trinity with min_kmer_cov set to 2 by default and all other parameters set default [17]. To obtain the comprehensive information of gene functions, assembled unigenes were annotated according to Nr, Nt, Pfam, KOG/COG, Swiss-Prot, KO, and GO database.
2.8. Differentially Expressed Genes (DEGS) Analysis
To identify DEGs in A. archangelica EOs fumigated S. zeamais, clean reads were analyzed by DESeq2 R package (1.20.0) to calculate the Padj of differential expression [18]. In addition, in order to control error detection rate (FDR), a normalized absolute log2-fold change (fumigated/control) of 2 with adjusted p-value less than 0.05 were set as the threshold for significantly differentially expression.
2.9. GO and KEGG Pathway Enrichment Analysis
After classifying the annotation information, the DEGs were associated with the Gene Ontology (GO) for enrichment analysis. GO functional enrichment was considered as the differential expression of genes with a significant enrichment threshold of padj less than 0.05. Kyoto Encyclopedia of Genes and Genomes (KEGG) is a comprehensive database integrating genomic, chemical, and system functional information. The threshold for KEGG pathway enrichment was padj less than 0.05. The ClusterProfiler (3.8.1) software was used to conduct GO functional enrichment analysis and KEGG pathway enrichment analysis on the DEGs [19].
2.10. Real Time Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
From the DEGs, 14 genes were selected and a heat map was generated using the heatmap package in R software (Version 3.5.0) based on their expression levels in the treatment and control groups, including five P450 genes, three UGT genes, three GST genes, two CarE genes, and one JHEH gene. qRT-PCR was performed on a Real-time Detection System (QuantStuido 5; Waltham, USA) to verify the reliability of transcriptome. qRT-PCR was performed using a 20 μL reaction volume. The program conditions were 95 °C for 30 s, 40 cycles at 95 °C for 10 s, and 60 °C for 30 s. The primers used are listed in Table 1. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference gene [20]. Three technical repeats were performed for each sample. The relative expression level of genes was performed using 2−ΔΔCT.

Table 1.
qRT-PCR primers and internal reference gene sequences.
2.11. Statistical Analysis
The percentage of insect mortality was transformed using the arcsine square root transformation. All experimental data were analyzed using SPSS Statistics 22.0 (https://www.ibm.com/products/spss-statistics) with this method. Based on the assumptions of normality and homogeneity of variance, one-way analysis of variance (ANOVA) was subsequently performed, followed by Tukey’s honestly significant difference (HSD) test. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Chemical Composition of Essential Oil
The chemical composition of the essential oil is summarized in Table 2. A total of 35 compositions accounting for 96.94% of A. archangelica essential oil were identified. The major components were δ-3-Carene (24.26%), Limonene (19.81%) and α-Pinene (14.96%), followed by ο-Cymene (9.94%), β-Pinene (6.55%), Linalool (6.49%), Myrcene (5.60%).

Table 2.
Chemical composition of A. archangelica essential oil.
3.2. Fumigant Toxicity of the Essential Oil
Fumigation assays were conducted to evaluate the fumigant toxicity of essential oils (EOs) against adult S. zeamais. The results demonstrated a significant positive correlation between fumigation activity and dosage at 24, 48, and 72 h post-treatment with essential oil (Table 3). At the same dose, corrected mortality also gradually increased with the extension of time, from 24 h to 48 h to 72 h. The largest dose (170 mg/L) of essential oil caused the corrected mortality of 94.48% after 72 h of essential oil treatment (Table 4). The lethal concentration (LC50) values were 164.38, 132.62, and 90.35 mg/L air at 24, 48, and 72 h, respectively.

Table 3.
Corrected mortality rate of essential oil of A. archangelica against S. zeamais adults.

Table 4.
Fumigant toxicity of essential oil of A. archangelica against S. zeamais adults.
3.3. Enzyme Activity
The activity of three detoxification enzymes, AchE, GST and CarE of S. zeamais was determined. A. archangelica essential oil fumigation significantly reduced the activity of AchE, and enzyme activity was negatively correlated with the treatment dosage (Figure 1A). At 24 h after fumigation, the activity of AchE was the lowest, after which it showed an increasing trend but remained lower than that of the control group (Figure 1D). A. archangelica essential oil inhibited the AchE activity in adult S. zeamais. The effects of A. archangelica essential oil on GST and AChE in adult S. zeamais were generally consistent, both showing an inhibitory effect on enzyme activity. Enzyme activity was negatively correlated with the treatment dosage, and the lowest GST activity was observed at 24 h after fumigation (Figure 1B,E). A. archangelica essential oil fumigation significantly reduced CarE activity, with treatment dosage and time having only a minor influence (Figure 1C,F).
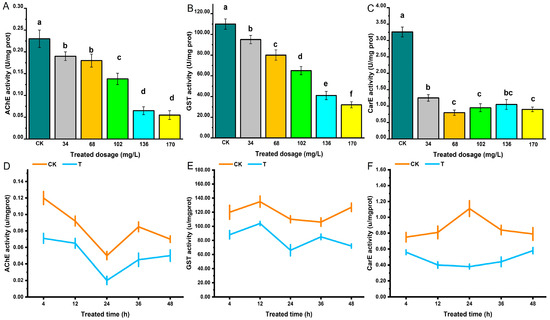
Figure 1.
Effects of A. archangelica essential oil on the Activities of AChE (A,D), GST (B,E) and CarE (C,F) in S. zeamais. (A–C) show the effects of essential oil on enzyme activities in 24 h under different dosages; (D–E) demonstrate the effects of essential oils on enzyme activities at different time points under the LD50 dosage. Different lowercase letters indicate statistically significant differences among treatments (p < 0.05).
3.4. Transcriptome Analysis
RNA-Seq was carried out to explore the differential genes of S. zeamais in response to A. archangelica essential oil. As a result, 6.17, 5.99, 6.65 G clean bases for three fumigated groups, and 5.77, 6.75, 5.77 G clean bases for three control groups were produced in this library (Table 5). The percentage of clean reads exceeded 95% of the total reads in each sample. The Q20 and Q30 values of the samples were all above 98% and 95%, respectively, indicating high-quality sequencing data with low error rates and good overall quality. After quality assessment and data filtering, 37,924 unigenes were obtained using Trinity software (Version 2.15.1). Based on the Nr annotation results, we analyzed the species distribution of the annotated sequences. The results revealed that 76.4% of Sitophilus oryzae genes matched those in S. zeamais, indicating a high degree of genomic similarity between the two species. This was followed by matches to Rhynchophorus ferrugineus (2.7%), Anthonomus grandis grandis (1.65%), and Acanthoscelides obtectus (2%) (Figure 2A). Transcriptomic analysis of S. zeamais treated with A. archangelica essential oil identified 3718 significantly differentially expressed genes. Expression pattern analysis revealed that 2265 genes were up-regulated, while the remaining 1453 genes were down-regulated (Figure 2B).

Table 5.
Summary of statistical data for the transcriptome of S. zeamais.
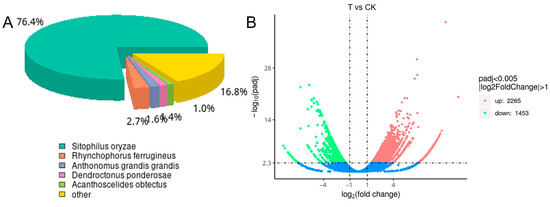
Figure 2.
Annotation of species distribution map (A) and differential gene volcano map (B).
3.5. GO and KEGG Pathway Enrichment Analysis of DEGs
Functional enrichment results indicated that DEGs in GO terms were mainly related to carbohydrate metabolic process, hydrolase activity, oxidoreductase activity, and catalytic activity. They mainly belong to biological processes and molecular functions, respectively (Figure 3A). The DEGs were significantly involved in KEGG pathways including those related to drug metabolism-cytochrome P450, insect hormone biosynthesis, retinol metabolism, terpenoid backbone biosynthesis, glutathione metabolism, fluid shear stress and atherosclerosis, and platinum drug resistance (Figure 3B).
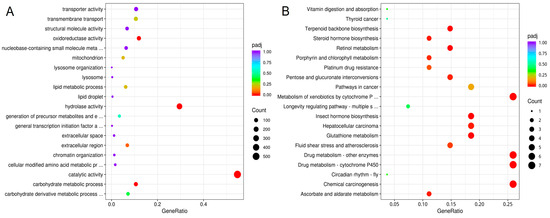
Figure 3.
Scatter plot of GO (A) and KEGG (B) enrichment of DEGs. The horizontal axis represents the ratio of the number of differentially expressed genes annotated to the GO Term/KEGG pathway to the total number of differentially expressed genes, while the vertical axis represents the GO Term and KEGG pathway.
3.6. qRT-PCR Analyses
The expression levels of SzeaCYP6A14, SzeaCYP6M5, SzeaUGT2C1, SzeaUGT2C10, SzeaGST3, and SzeaJHEH1 were significantly higher in the treatment group compared to the control group (p < 0.05), whereas SzeaCYP12V2 showed the opposite trend with significantly lower expression in the treatment group (p < 0.05) (Figure 4). As expected, the qRT-PCR results for the 14 genes showed similar differential expression patterns between the treatment and control groups as the RNA-seq data, confirming the reliability of our data and analysis.
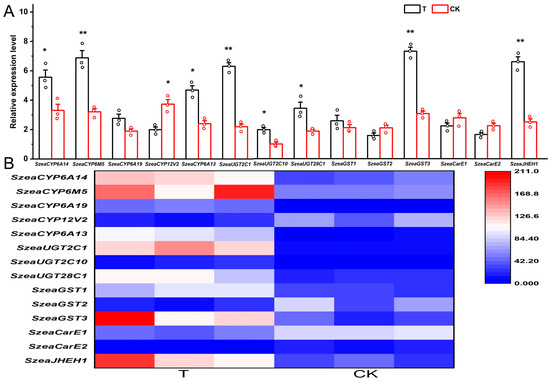
Figure 4.
Gene qRT-PCR expression level (A) and expression heatmap (B). Error bars indicate ± SE. Statistical differences between the treatment and control at the same instar age: *, p < 0.05; **, p < 0.01.
4. Discussion
Several researchers have examined the chemical constituents of the A. archangelica roots essential oil. Fraternale et al. reported the major components of the essential oil were α-pinene (21.3%), δ-3-carene (16.5%), limonene (16.4%) [16]. Wedge et al. also reported α-pinene (17.5%), δ-3-carene (16.3%), limonene (8.5%) [21]. Factors such as variety, geographic and seasonal variations, as well as extraction techniques, may account for the quantitative discrepancies between the values presented in this study and those reported in earlier research.
Angelica essential oils exhibit insecticidal properties against insect pests. The present study is the first to reveal that A. archangelica essential oil possesses significant fumigant toxicity against S. zeamais adults. Accumulating studies have also reported that the essential oils from other (Angelica) species, including A. pubescens [22], A. sinensis [23], A. dahurica [24,25], and A. archangelica [26], showed deterrent, repellent, and acute toxicity. Main compounds such as δ-3-Carene, α-pinene, and limonene may be possible mortality of factors for A. archangelica essential oil, which are capable of interfering with AChE, CAT and GST activity [27,28,29]. These enzymes are directly related to the insecticidal mechanism.
Li et al. used Illicium verum fruit extracts to fumigate S. zeamais. Under LD50 treatment at 12, 24, 36, 48, 60, and 72 h, the extract exhibited an inhibitory effect on GST, with the inhibition strengthening over time [30]. Shang et al. demonstrated that after fumigating S. zeamais with Artemisia annua essential oil, the α-naphthyl acetate esterase (α-NACarE) activity at 2, 4, 8, 12, and 24 h showed a decreasing trend from 2 to 12 h. Although the activity decreased at 2 h and increased again at 4 h, it remained significantly lower than that of the control group [31]. Chaubey et al. reported the effects of α-pinene and β-caryophyllene on acetylcholinesterase (AchE) activity in S. zeamais, demonstrating that the enzyme activity in the treated groups decreased significantly. This reveals that α-pinene and β-caryophyllene significantly inhibit AchE activity in S. zeamais [32]. These findings are largely consistent with the experimental results obtained in this study. The inhibition of enzyme activity by essential oils may occur because certain small molecules in the oil are structurally similar to the enzyme’s natural substrate. These molecules bind to the enzyme’s active site but cannot be catalyzed by the enzyme. By occupying the active site, they prevent the genuine substrate from binding to the enzyme, thereby inhibiting its function. The insecticidal mechanism of essential oils may involve a combination of factors, including neurotoxic effects, interference with nerve signal transduction, modulation of GABA receptors, and cellular and physiological toxicity.
Huang et al. evaluated the effects of terpinen-4-ol fumigation on gene expression in S. zeamais through RNA-seq analysis, revealing 592 DEGs, among which 308 and 284 genes were upregulated and downregulated, respectively. The DEGs were subjected to GO and KEGG enrichment analyses [9]. KEGG enrichment primarily mapped to metabolic pathways, related to the respiration and metabolism of exogenous organisms [33]. Li et al. extracted total RNA from S. zeamais and conducted high-throughput sequencing, reporting GO functional classification and KEGG metabolic pathways [34]. These findings are largely consistent with the experimental results obtained in this study.
5. Conclusions
This study comprehensively evaluated the control efficacy of A. archangelica essential oil against S. zeamais through GC-MS analysis, fumigation toxicity assays, enzyme activity measurements, RNA-seq sequencing, and qRT-PCR. The research revealed 35 chemical components in the essential oil, its fumigation toxicity to S. zeamais, and its effects on the activities of three detoxification enzymes in the insect. A total of 3718 DEGs were obtained, and their expression patterns were validated. These findings will provide data support for developing novel, eco-friendly, plant-based pesticides.
Author Contributions
Conceptualization, G.W. and H.W.; methodology, G.W., D.L., Z.Z. and X.G.; software, G.W., L.W., S.S. and X.J.; validation, Z.Z., D.L. and X.G.; formal analysis, L.W., S.S. and X.J.; investigation, K.K. and S.D.; resources, B.Z., S.D. and H.W.; data curation, Z.Z., G.W., D.L. and X.G.; writing—original draft preparation, G.W. and D.L.; writing—review and editing, X.G. and H.W.; visualization, D.L.; supervision, B.Z., K.K. and S.D.; project administration, G.W. and X.G.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Scientific and Technological Research Project of Henan Province (No. 252102111108 and 242102110194), the Key Research and Development and Promotion Projects of Henan Province (No. 241111111700).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the associated data are available in the manuscript.
Acknowledgments
We thank the anonymous reviewers for valuable comments on the manuscript. We are grateful for the assistance of all staff members and students in the Laboratory of Entomology and Pesticide, Henan Institute of Science and Technology, Xinxiang, Henan, China.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kifle, F.; Girma, M.; Gebresilassie, A.; Woldehawariat, Y.; Ele, E. Chemical composition and insecticidal potential of botanical fractionation extracts for the management of Sitophilus zeamais Motschulsky, 1855 (Coleoptera: Curculionidae) in stored maize. Helicon 2025, 11, e42131. [Google Scholar] [CrossRef] [PubMed]
- Wanna, R.; Bunphan, D.; Kunlanit, B.; Khaengkhan, P.; Khaengkhan, P.; Bozdoğan, H. Chemical composition of essential oil from Apium graveolens L. and its biological activities against Sitophilus zeamais Motschulsky (Coleoptera: Dryophthoridae). Plants 2025, 14, 347. [Google Scholar] [CrossRef]
- Usseglio, V.; Dambolena, J.S.; Zunino, M.P. Insect-corn kernel interaction: Chemical signaling of the grain and host recognition by Sitophilus zeamais. J. Stored Prod. Res. 2018, 79, 66–72. [Google Scholar] [CrossRef]
- Boyer, S.; Zhang, H.; Lempérière, G. A review of control methods and resistance mechanisms in stored-product insects. Bull. Entomol. Res. 2012, 102, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Liu, Y.; Wu, X.W.; Hua, R.M.; Wang, G.R.; Cao, H.Q. Insecticidal activity of melaleuca alternifolia essential oil and RNA-Seq analysis of Sitophilus zeamais transcriptome in response to oil fumigation. PLoS ONE 2016, 11, e0167748. [Google Scholar] [CrossRef]
- Nagawa, C.B.; Kitiibwa, I.S.; Kizito, S.S.; Syofuna, A.; Kyarimpa, C.M.; Omara, T. Chemical composition and insecticidal potential of Eucalyptus essential oils against Sitophilus zeamais (Motschulsky, 1855) and Acanthoscelides obtectus (Say, 1831). S. Afr. J. Bot. 2025, 179, 48–55. [Google Scholar] [CrossRef]
- Wanna, R.; Kaewduangta, W. Fumigant activity of Tridax procumbens (Asterales: Asteraceae) essential oil against Sitophilus zeamais (Coleoptera: Curculionidae) and its effects on Thai rice seed germination. J. Entomol. Sci. 2022, 57, 561–572. [Google Scholar] [CrossRef]
- Liao, M.; Li, S.N.; Wu, H.L.; Gao, Q.; Shi, S.; Huang, Y.; Cao, H.Q. Transcriptomic analysis of Sitophilus zeamais in response to limonene fumigation. Pest Manag. Sci. 2022, 78, 4774–4782. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, M.; Yang, Q.Q.; Xiao, J.J.; Hu, Z.Y.; Zhou, L.J.; Cao, H.Q. Transcriptome profiling reveals differential gene expression of detoxification enzymes in Sitophilus zeamais responding to terpinen-4-ol fumigation. Pestic. Biochem. Physiol. 2018, 149, 44–53. [Google Scholar] [CrossRef]
- Bounouira, Y.; Benyelles, N.G.; Senouci, H.; Benazzouz, F.Z.; Chaieb, I. The insecticidal activity of a formulation of ammoides verticillata essential oil and diatomaceous earth on Sitophilus zeamais. Int. J. Trop. Insect. Sci. 2022, 42, 2979–2985. [Google Scholar] [CrossRef]
- Aryal, S.; Poudel, A.; Kafle, K.; Aryal, L.N. Insecticidal toxicity of essential oil of Nepalese Acorus calamus (Acorales: Acoraceae) against Sitophilus zeamais (Coleoptera: Curculionidae). Heliyon 2023, 9, e22130. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.A.; Câmara, C.A.G.; Moraes, M.M.; Tavares, W.S.; Legaspi, J.C.; Zanuncio, J.C. Insecticidal and repellent activities of four essential oils against Sitophilus zeamais (Coleoptera: Curculionidae). Dose Response 2023, 21, 15593258231210263. [Google Scholar] [CrossRef]
- Rodríguez, A.; Beato, M.; Usseglio, V.L.; Camina, J.L.; Zygadlo, J.A.; Dambolena, J.S.; Zunino, M.P. Phenolic compounds as controllers of Sitophilus zeamais: A look at the structure-activity relationship. J. Stored Prod. Res. 2022, 99, 102038. [Google Scholar] [CrossRef]
- Pathak, S.; Wanjari, M.M.; Jain, S.K.; Tripathi, M. Evaluation of antiseizure activity of essential oil from roots of Angelica archangelica Linn. in mice. Indian J. Pharm. Sci. 2010, 73, 371–375. [Google Scholar]
- Aćimović, M.G.; Pavlović, S.D.; Varga, A.O.; Filipović, V.M.; Cvetković, M.T.; Stanković, J.M.; Čabarkapa, I.S. Chemical composition and antibacterial activity of Angelica archangelica root essential oil. Nat. Prod. Commun. 2017, 12, 205–206. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition and antimicrobial activity of Angelica archangelica L. (Apiaceae) roots. J. Med. Food 2014, 17, 1043–1047. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Li, D.M.; Zand, M.S.; Dye, T.D.; Goniewicz, M.L.; Rahman, I.; Xie, Z.D. An evaluation of RNA-seq differential analysis methods. PLoS ONE 2022, 17, e0264246. [Google Scholar] [CrossRef] [PubMed]
- Lü, D.B.; Yan, Z.Z.; Hu, D.; Zhao, A.P.; Wei, S.J.; Wang, P.; Yuan, X.Q.; Li, Y.P. RNA Sequencing reveals the potential adaptation mechanism to different hosts of Grapholita molesta. Insects 2022, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Prentice, K.; Pertry, I.; Christiaens, O.; Bauters, L.; Bailey, A.; Niblett, C.; Ghislain, M.; Gheysen, G.; Smagghe, G. Transcriptome analysis and systemic RNAi response in the African sweetpotato weevil (Cylas puncticollis, Coleoptera, Brentidae). PLoS ONE 2015, 10, e0115336. [Google Scholar] [CrossRef]
- Wedge, D.E.; Klun, J.A.; Tabanca, N.; Demirci, B.; Ozek, T.; Baser, K.H.C.; Liu, Z.; Zhang, S.; Cantrell, C.L.; Zhang, J. Bioactivity-guided fractionation and GC/MS fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. J. Agric. Food Chem. 2009, 57, 464–470. [Google Scholar] [CrossRef]
- Zheng, R.R.; Zhao, J.Y.; Ma, L.; Qie, X.T.; Yan, X.Z.; Hao, C. Behavioral, electrophysiological, and toxicological responses of Plutella xylostella to extracts from Angelica pubescens. Insects 2023, 14, 613. [Google Scholar] [CrossRef]
- Champakaew, D.; Junkum, A.; Chaithong, U.; Jitpakdi, A.; Riyong, D.; Sanghong, R.; Pitasawat, B. Angelica sinensis (Umbelliferae) with proven repellent properties against Aedes aegypti, the primary dengue fever vector in Thailand. Parasitol. Res. 2015, 114, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Tabanca, N.; Gao, Z.; Demirci, B.; Techen, N.; Wedge, D.E.; Ali, A.; Baser, K.H.C. Molecular and phytochemical investigation of Angelica dahurica and Angelica pubescentis essential oils and their biological activity against Aedes aegypti, Stephanitis pyrioides, and Colletotrichum species. J. Agric. Food Chem. 2014, 62, 8848–8857. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, E.H.; Lee, J.H.; Lee, Y.C.; Moon, H.I. Immunotoxicity activity from various essential oils of Angelica genus from South Korea against Aedes aegypti L. Immunopharm. Immunot. 2012, 34, 42–45. [Google Scholar]
- Pavela, R.; Vrchotová, N. Insecticidal effect of furanocoumarins from fruits of Angelica archangelica L. against larvae Spodoptera littoralis Boisd. Ind. Crop Prod. 2013, 43, 33–39. [Google Scholar] [CrossRef]
- Galeano, L.J.N.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Synergistic insecticidal activity of plant volatile compounds: Impact on neurotransmission and detoxification enzymes in Sitophilus zeamais. Insects 2025, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Brito, R.; de Moura Pádua, L.E.; da Silva, L.R.; Briozo, M.E.O.; Silva, P.R.R.; de Carvalho, L.F.; de França, S.M. Use of essential oils and α-pinene as insecticides against Sitophilus zeamais and their effects on maize seed germination. Agronomy 2024, 14, 2282. [Google Scholar] [CrossRef]
- Chaubey, M.K. Insecticidal property of terpenes against maize weevil, Sitophilus zeamais (Motschulsky). J. Biopestic. 2022, 15, 92. [Google Scholar] [CrossRef]
- Li, S.G.; Li, M.Y.; Huang, Y.Z.; Hua, R.M.; Lin, H.F.; He, Y.J.; Wei, L.L.; Liu, Z.Q. Fumigant activity of Illicium verum fruit extracts and their effects on the acetylcholinesterase and glutathione S-transferase activities in adult Sitophilus zeamais. J. Pest Sci. 2013, 86, 677–683. [Google Scholar] [CrossRef]
- Shang, L.N.; Yuan, H.B.; Wei, C.Y.; Ren, B.Z. Effect of essential oil extracted from Artemisia annua L. on activities of several enzymes in Sitophihus zeamais. J. Jilin Agr. Univ. 2010, 32, 616–621. [Google Scholar]
- Chaubey, M.K. Essential oils as green pesticides of stored grain insects. Eur. J. Bio. Res. 2019, 4, 202–244. [Google Scholar]
- Bens, M.; Sahm, A.; Groth, M.; Jahn, N.; Morhart, M.; Holtze, S.; Hildebrandt, T.B.; Platzer, M.; Szafranski, K. FRAMA: From RNA-seq data to annotated mRNA assemblies. BMC Genom. 2016, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Zhao, J.H. Transcriptome analysis of Sitophilus zeamais by RNA-Seq. J. Environ. Entomol. 2021, 43, 1485–1492. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).