Melatonin Improves Intestinal Barrier Impairment in a Mouse Model of Autism Spectrum Disorder
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experimental Groups
2.2. Behavioral Tests
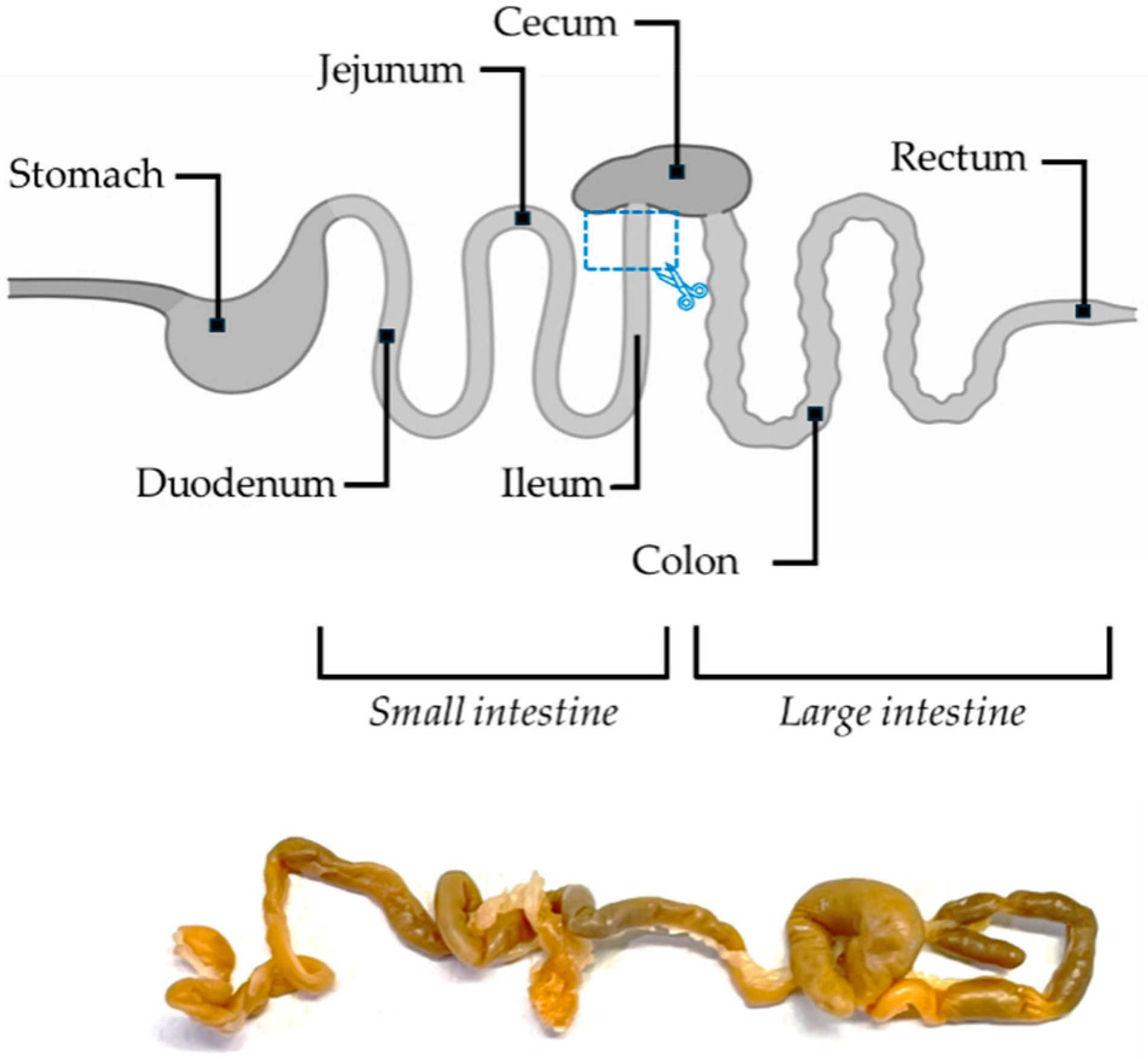
2.3. Morphological and Morphometrical Evaluations of the Ileum
2.4. Immunohistochemical and Immunofluorescence Evaluation of the Ileum
2.5. Statistical Analysis
3. Results
3.1. Repetitive and Stereotyped Behaviors
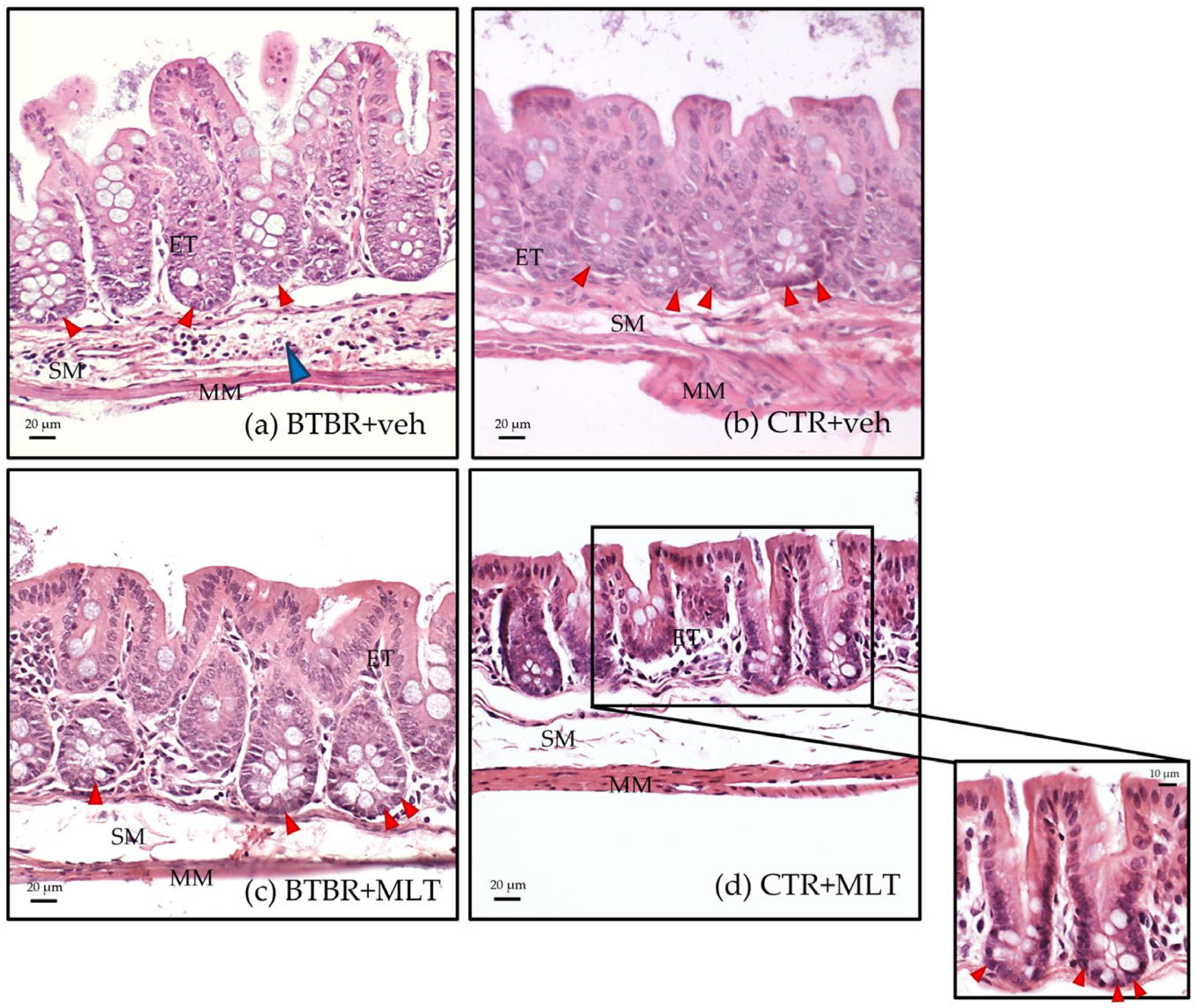
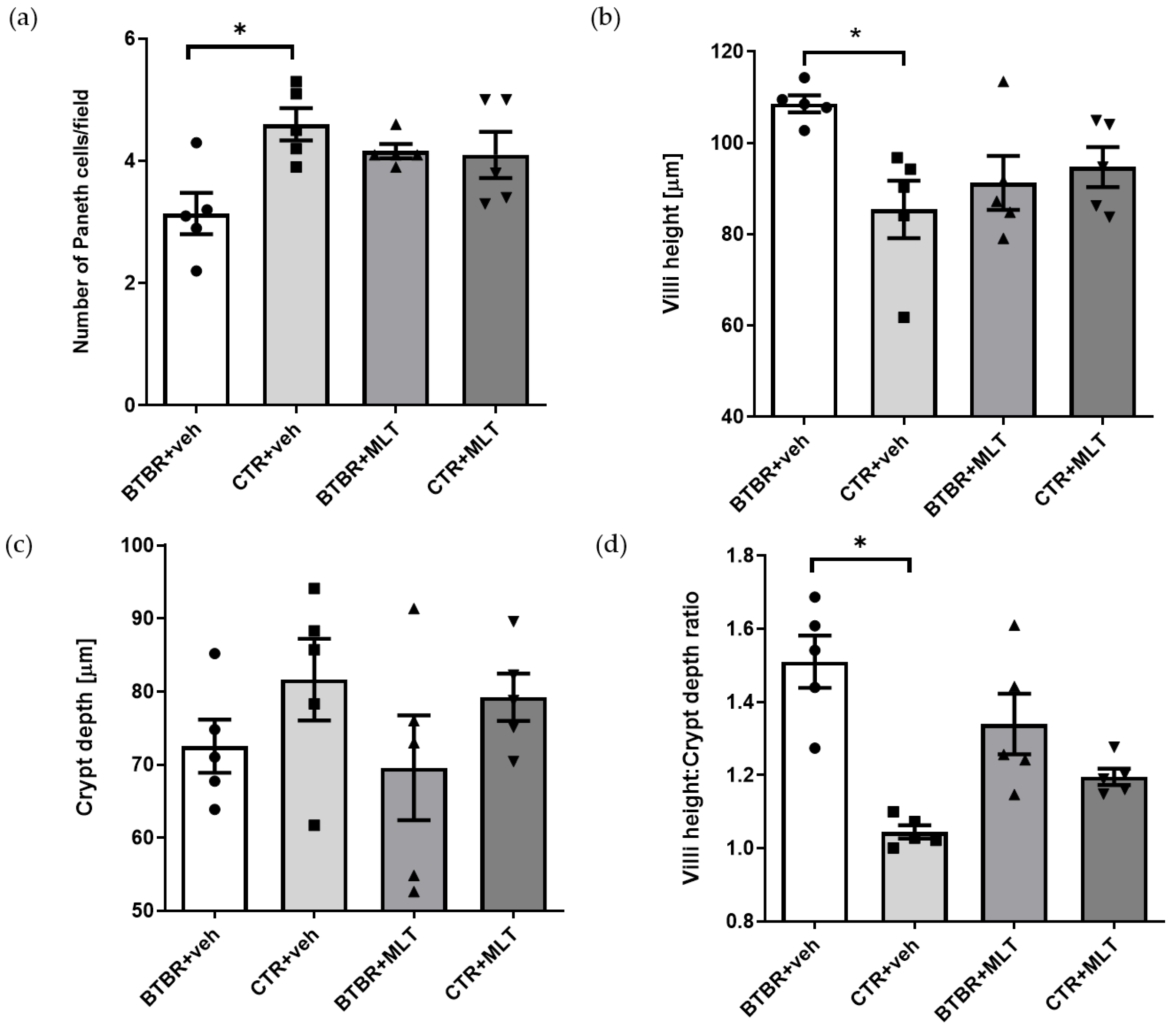
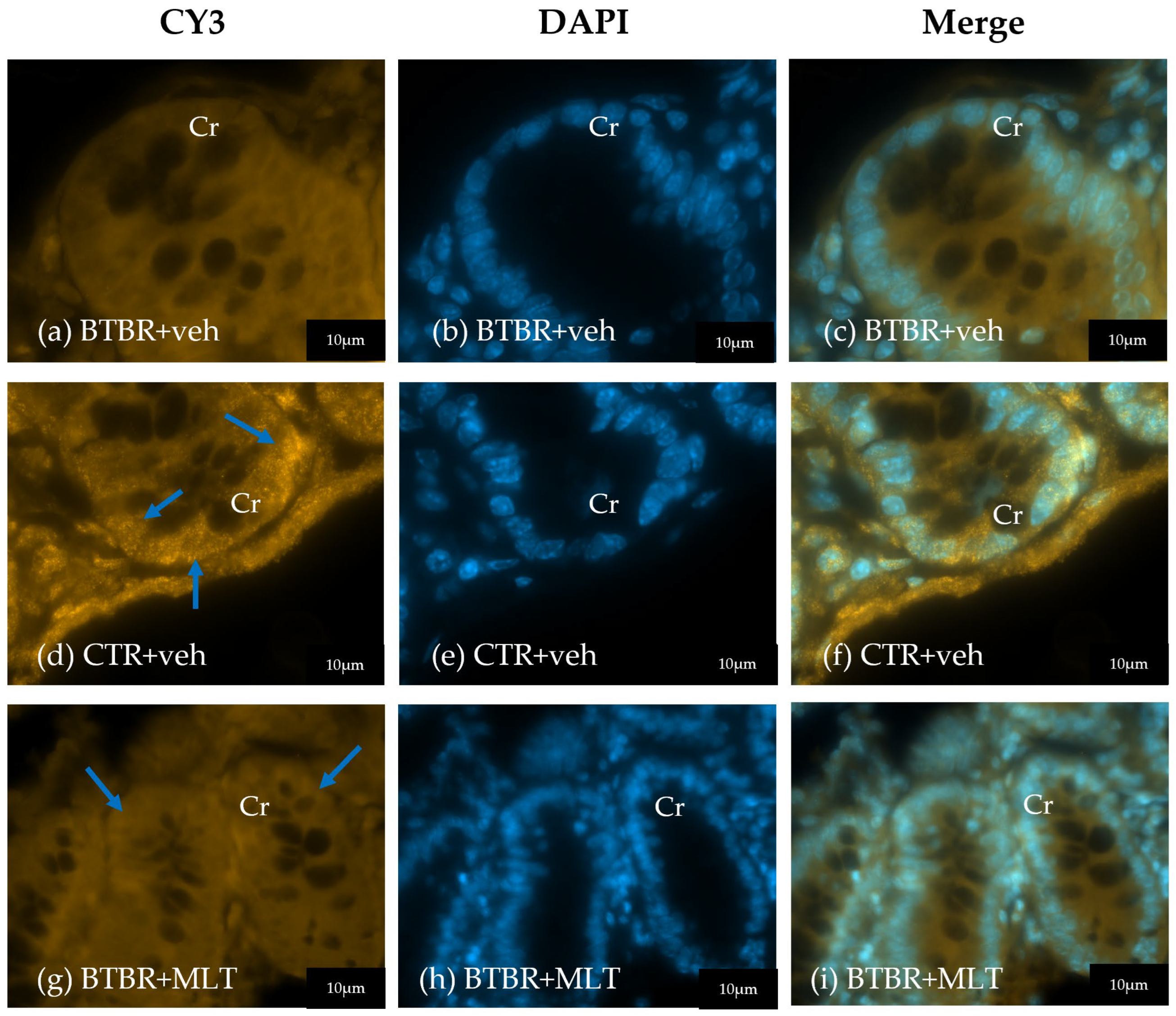
3.2. Morphological and Morphometrical Evaluation of the Ileum and α-Defensin 5 Analyses
3.3. Evaluation of Tight Junctions in the Ileum
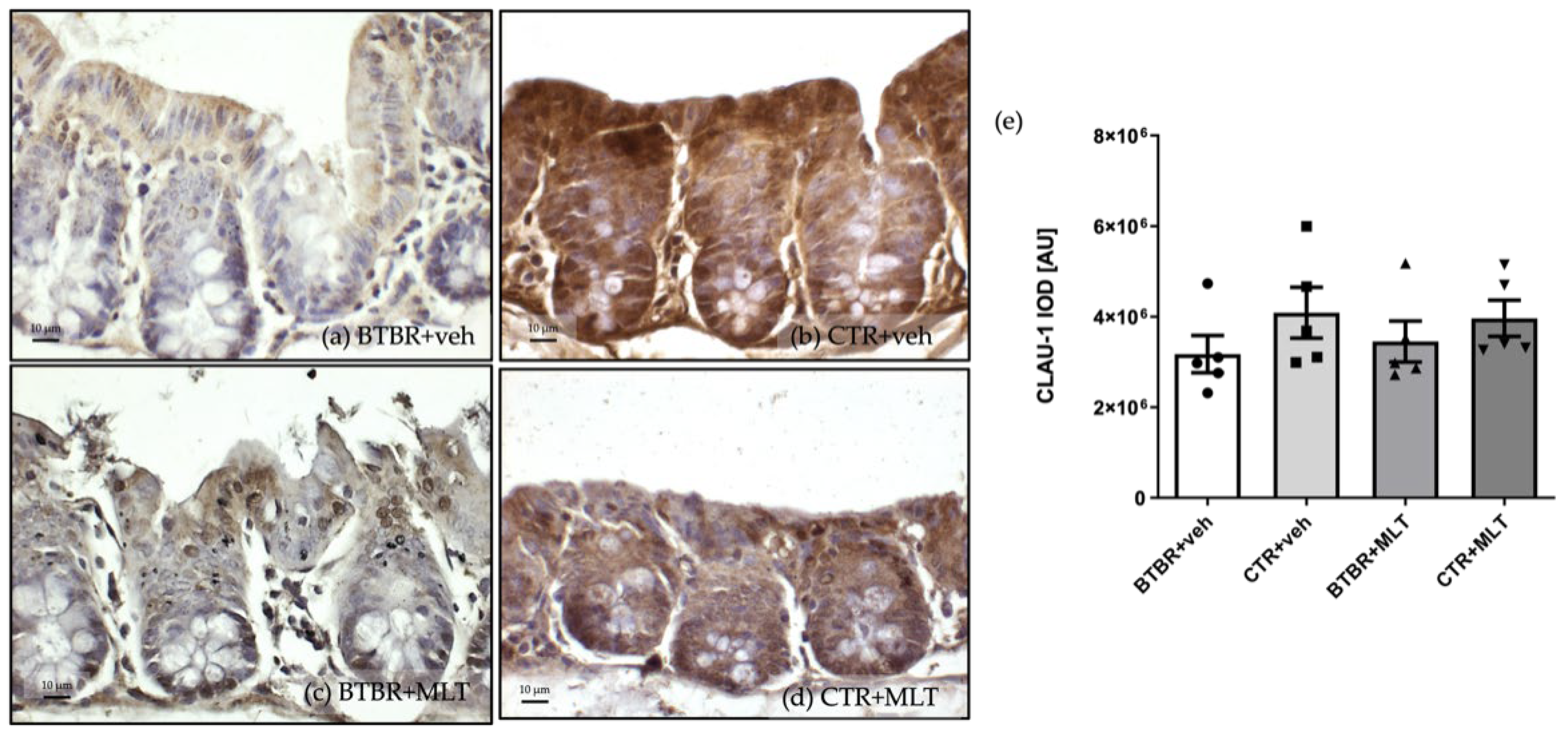
3.3.1. Immunohistochemical and Immunomorphometric Analyses of Claudin-1
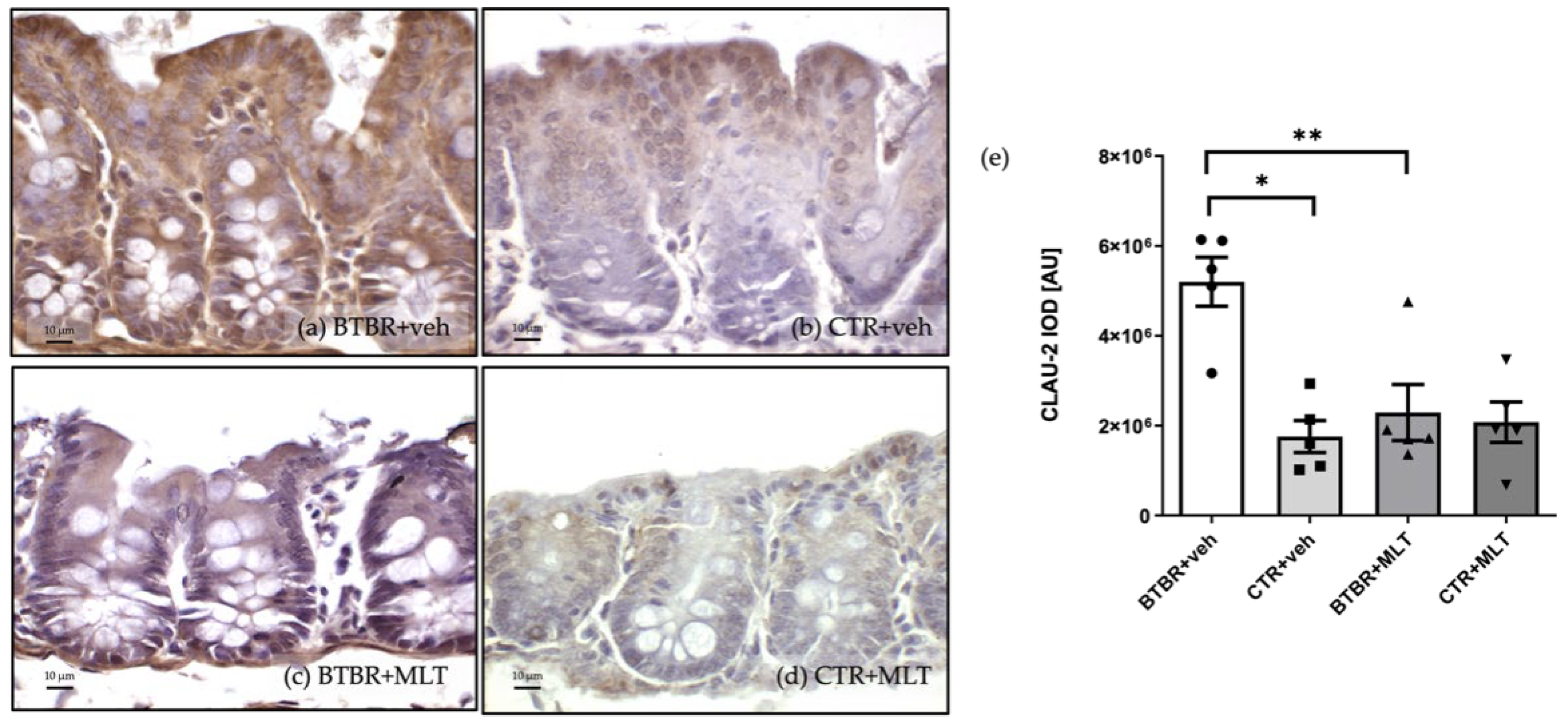
3.3.2. Immunohistochemical and Immunomorphometric Analyses of Claudin-2
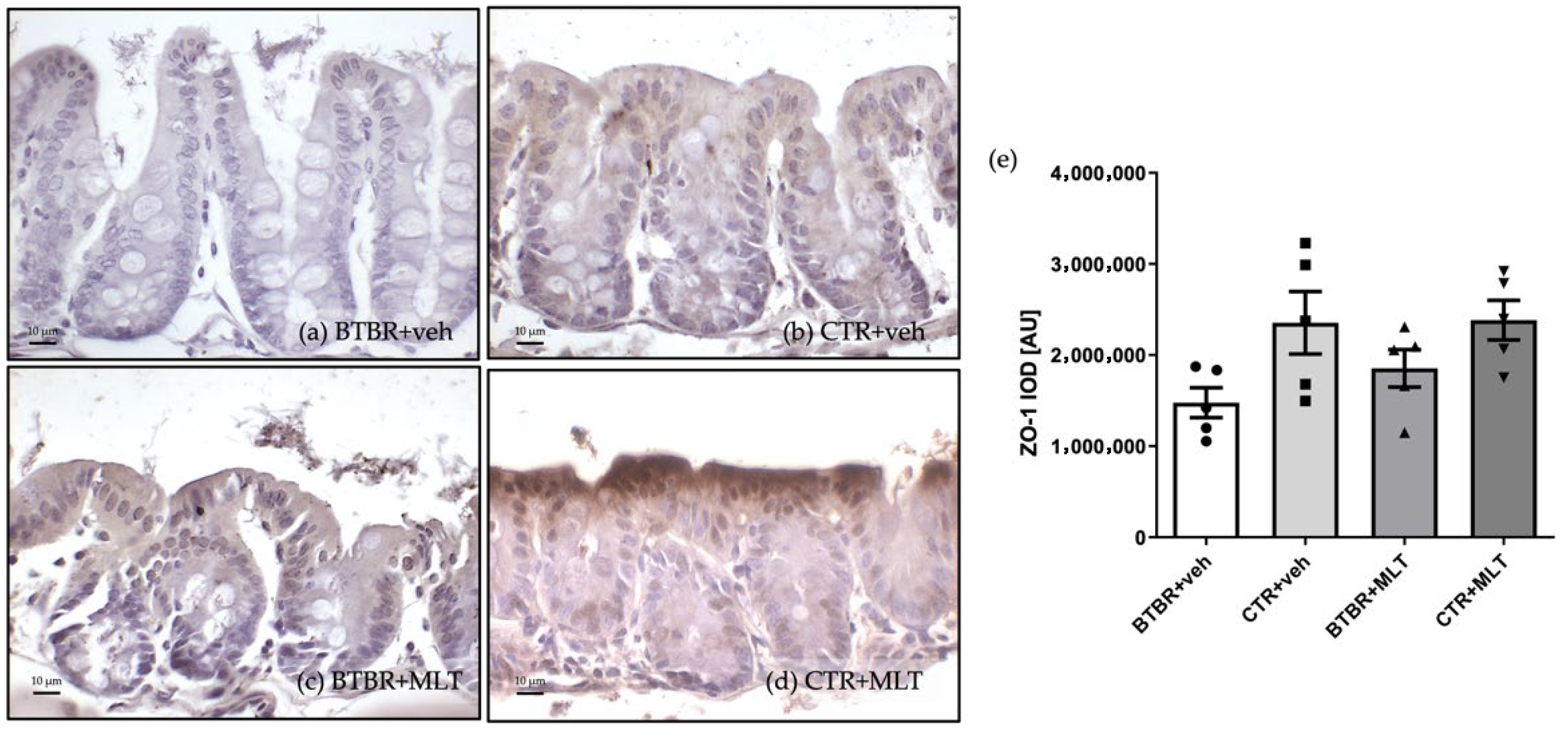
3.3.3. Immunohistochemical and Immunomorphometric Analyses of Zonula Occludens-1
4. Discussion
5. Conclusions
6. Limitations and Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortese, S.; Bellato, A.; Gabellone, A.; Marzulli, L.; Matera, E.; Parlatini, V.; Petruzzelli, M.G.; Persico, A.M.; Delorme, R.; Fusar-Poli, P.; et al. Latest Clinical Frontiers Related to Autism Diagnostic Strategies. Cell Rep. Med. 2025, 6, 101916. [Google Scholar] [CrossRef] [PubMed]
- Rowshan, N.; Anjomshoa, M.; Farahzad, A.; Bijad, E.; Amini-Khoei, H. Gut-Brain Barrier Dysfunction Bridge Autistic-like Behavior in Mouse Model of Maternal Separation Stress: A Behavioral, Histopathological, and Molecular Study. Int. J. Dev. Neurosci. 2024, 84, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.K.; Stanton, J.E.; Hans, S.; Grabrucker, A.M. Autism Spectrum Disorders: Etiology and Pathology. In Autism Spectrum Disorders; Grabrucker, A.M., Ed.; Exon Publications: Brisbane, Australia, 2021; ISBN 978-0-6450017-8-5. [Google Scholar]
- Longo, B.; Andriolo, I.R.L.; de Melo, D.M.; de Souza, M.M.; Prediger, R.D.; da Silva, L.M. Gastrointestinal Manifestations in Autism Spectrum and Attention-Deficit/Hyperactivity Disorders: Pathogenesis and Drug Targets. Curr. Dev. Disord. Rep. 2025, 12, 4. [Google Scholar] [CrossRef]
- Bi, D.; Huang, J.; Zhu, N.; Yao, L.; Wu, Y.; Peng, Y.; Chen, G.; Zhu, B.; Xu, X. Elucidation of Molecular Mechanisms of Sulfated Oligoguluronic Acid on Mitigating Intestinal Inflammation and Enhancing Epithelial Barrier Function. J. Agric. Food Chem. 2025, 73, 13605–13617. [Google Scholar] [CrossRef]
- Li, B.; Hsieh, Y.-R.; Lai, W.-D.; Tung, T.-H.; Chen, Y.-X.; Yang, C.-H.; Fang, Y.-C.; Huang, S.-Y. Melatonin Ameliorates Neuropsychiatric Behaviors, Gut Microbiome, and Microbiota-Derived Metabolites in Rats with Chronic Sleep Deprivation. Int. J. Mol. Sci. 2023, 24, 16820. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Pathomthongtaweechai, N.; Steinhagen, P.R.; Chantawichitwong, P.; Satianrapapong, W.; Pongkorpsakol, P. Tight Junctions: From Molecules to Gastrointestinal Diseases. Tissue Barriers 2023, 11, 2077620. [Google Scholar] [CrossRef]
- Shindler, A.E.; Hill-Yardin, E.L.; Petrovski, S.; Cunningham, A.C.; Bishop, N.; Franks, A.E. Potential Determinants of Gastrointestinal Dysfunction in Autism Spectrum Disorders. Rev. J. Autism Dev. Disord. 2020, 7, 182–196. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of Intestinal Epithelial Permeability by Tight Junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Papageorgiou, I.; Charonis, A. Enterocytes’ Tight Junctions: From Molecules to Diseases. World J. Gastrointest. Pathophysiol. 2011, 2, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayadhi, L.; Zayed, N.; Bhat, R.S.; Moubayed, N.M.S.; Al-Muammar, M.N.; El-Ansary, A. The Use of Biomarkers Associated with Leaky Gut as a Diagnostic Tool for Early Intervention in Autism Spectrum Disorder: A Systematic Review. Gut Pathog. 2021, 13, 54. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Rosso, C.; Ribaldone, D.G.; Dughera, F.; Fagoonee, S.; Astegiano, M.; Pellicano, R. Physiopathology of Intestinal Barrier and the Role of Zonulin. Minerva Biotecnol. 2019, 31, 83–92. [Google Scholar] [CrossRef]
- Veres-Székely, A.; Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Vannay, Á. Zonulin as a Potential Therapeutic Target in Microbiota-Gut-Brain Axis Disorders: Encouraging Results and Emerging Questions. Int. J. Mol. Sci. 2023, 24, 7548. [Google Scholar] [CrossRef]
- Fasano, A. Physiological, Pathological, and Therapeutic Implications of Zonulin-Mediated Intestinal Barrier Modulation. Am. J. Pathol. 2008, 173, 1243–1252. [Google Scholar] [CrossRef]
- Tirelli, E.; Pucci, M.; Squillario, M.; Bignotti, G.; Messali, S.; Zini, S.; Bugatti, M.; Cadei, M.; Memo, M.; Caruso, A.; et al. Effects of Methylglyoxal on Intestine and Microbiome Composition in Aged Mice. Food Chem. Toxicol. 2025, 197, 115276. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, H.M.; Abdelmawgoud, A.S.; El-kady, N.M.; Abdelhay, E.S.; Abdel Tawab, H.E. Serum Zonulin Level in Autistic Children and Its Relation to Severity of Symptoms a Case-Control Study. Sci. Rep. 2025, 15, 27802. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, B.; Zhou, D.; Xing, J.; Li, H.; Li, J.; Zhang, Z.; Zhang, B.; Li, P. Supplementation of Diet with Different N-3/n-6 PUFA Ratios Ameliorates Autistic Behavior, Reduces Serotonin, and Improves Intestinal Barrier Impairments in a Valproic Acid Rat Model of Autism. Front. Psychiatry 2020, 11, 552345. [Google Scholar] [CrossRef]
- Luettig, J.; Rosenthal, R.; Barmeyer, C.; Schulzke, J.D. Claudin-2 as a Mediator of Leaky Gut Barrier during Intestinal Inflammation. Tissue Barriers 2015, 3, e977176. [Google Scholar] [CrossRef]
- Ehmann, D.; Wendler, J.; Koeninger, L.; Larsen, I.S.; Klag, T.; Berger, J.; Marette, A.; Schaller, M.; Stange, E.F.; Malek, N.P.; et al. Paneth Cell α-Defensins HD-5 and HD-6 Display Differential Degradation into Active Antimicrobial Fragments. Proc. Natl. Acad. Sci. USA 2019, 116, 3746–3751. [Google Scholar] [CrossRef]
- Nakamura, K.; Sakuragi, N.; Takakuwa, A.; Ayabe, T. Paneth Cell α-Defensins and Enteric Microbiota in Health and Disease. Biosci. Microbiota Food Health 2016, 35, 57–67. [Google Scholar] [CrossRef]
- Bevins, C.L.; Salzman, N.H. Paneth Cells, Antimicrobial Peptides and Maintenance of Intestinal Homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef]
- Petropoulos, A.; Stavropoulou, E.; Tsigalou, C.; Bezirtzoglou, E. Microbiota Gut-Brain Axis and Autism Spectrum Disorder: Mechanisms and Therapeutic Perspectives. Nutrients 2025, 17, 2984. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Gianò, M.; Favero, G.; Rezzani, R. Impairment in the Intestinal Morphology and in the Immunopositivity of Toll-like Receptor-4 and Other Proteins in an Autistic Mouse Model. Int. J. Mol. Sci. 2022, 23, 8731. [Google Scholar] [CrossRef]
- Meyza, K.Z.; Blanchard, D.C. The BTBR Mouse Model of Idiopathic Autism–Current View on Mechanisms. Neurosci. Biobehav. Rev. 2017, 76, 99–110. [Google Scholar] [CrossRef]
- Tan, D.X.; Xu, B.; Zhou, X.; Reiter, R.J. Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Molecules 2018, 23, 301. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Sutradhar, S.; Das, P.; Mukherjee, S. Gut Melatonin: A Potent Candidate in the Diversified Journey of Melatonin Research. Gen. Comp. Endocrinol. 2021, 303, 113693. [Google Scholar] [CrossRef]
- Pereira, N.; Naufel, M.F.; Ribeiro, E.B.; Tufik, S.; Hachul, H. Influence of Dietary Sources of Melatonin on Sleep Quality: A Review. J. Food Sci. 2020, 85, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, F.S.; Alghamdi, B.S.; Hakami, A.Y.; Alshehri, A.A.; Althobaiti, Y.S. Melatonin Attenuates Morphine-Induced Conditioned Place Preference in Wistar Rats. Brain Behav. 2021, 11, e2397. [Google Scholar] [CrossRef]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an Anti-Inflammatory Agent Modulating Inflammasome Activation. Int. J. Endocrinol. 2017, 2017, 1835195. [Google Scholar] [CrossRef]
- Guo, R.; Rao, P.; Liao, B.; Luo, X.; Yang, W.; Lei, X.; Ye, J. Melatonin Suppresses PD-L1 Expression and Exerts Antitumor Activity in Hepatocellular Carcinoma. Sci. Rep. 2025, 15, 8451. [Google Scholar] [CrossRef]
- Mohammadi, N.; Alizadeh, M.; Akbarzadeh, S.; Rezaei, M.; Mahmoodi, M.; Netticadan, T.; Movahed, A. Melatonin Administered Postoperatively Lowers Oxidative Stress and Inflammation and Significantly Recovers Heart Function in Patients Undergoing CABG Surgery. Eur. J. Med. Res. 2025, 30, 585. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Z.; Cao, J.; Gao, T.; Dong, Y.; Chen, Y. Role of Melatonin in Intestinal Mucosal Injury Induced by Restraint Stress in Mice. Pharm. Biol. 2020, 58, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Sommansson, A.; Yamskova, O.; Schiöth, H.B.; Nylander, O.; Sjöblom, M. Long-Term Oral Melatonin Administration Reduces Ethanol-Induced Increases in Duodenal Mucosal Permeability and Motility in Rats. Acta Physiol. 2014, 212, 152–165. [Google Scholar] [CrossRef]
- Rezzani, R.; Gianò, M.; Pinto, D.; Rinaldi, F.; van Noorden, C.J.F.; Favero, G. Hepatic Alterations in a BTBR T + Itpr3tf/J Mouse Model of Autism and Improvement Using Melatonin via Mitigation Oxidative Stress, Inflammation and Ferroptosis. Int. J. Mol. Sci. 2024, 25, 1086. [Google Scholar] [CrossRef]
- Borsani, E.; Bonomini, F.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Giugno, L.; Mastinu, A.; Aria, F.; Memo, M.; Rezzani, R. Role of Melatonin in Autism Spectrum Disorders in a Male Murine Transgenic Model: Study in the Prefrontal Cortex. J. Neurosci. Res. 2022, 100, 780–797. [Google Scholar] [CrossRef]
- Adiguzel, C.; Karaboduk, H.; Uzunhisarcikli, M. Protective Role of Melatonin Against Abamectin-Induced Biochemical, Immunohistochemical, and Ultrastructural Alterations in the Testicular Tissues of Rats. Microanal 2024, 30, 962–977. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Yu, Z.; Wang, T.; Jiao, Y.; Wang, K.; Dou, W.; Yi, C.; Song, B. Effects of Melatonin on Fat Graft Retention Through Browning of Adipose Tissue and Alternative Macrophage Polarization. Aesthet. Plast. Surg. 2023, 47, 1578–1586. [Google Scholar] [CrossRef]
- Ghorbani, F.; Osatd-Rahimi, N.; Mansouritorghabeh, F.; Ebrahimzadeh-Bideskan, A.; Saburi, E.; Rajabian, A.; Hosseini, M. Methamphetamine Exposure during Gestation and Lactation Periods Impairs the Learning and Memory of Offspring Mice, Which Is Reversed by Melatonin: The Role of Oxidative Stress and Acetylcholinesterase. Res. Pharm. Sci. 2025, 20, 218–229. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of Oral and Intravenous Melatonin in Healthy Volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive Self-Grooming Behavior in the BTBR Mouse Model of Autism Is Blocked by the mGluR5 Antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Favero, G.; Gianò, M.; Franco, C.; Pinto, D.; van Noorden, C.J.F.; Rinaldi, F.; Rezzani, R. Relation Between Reactive Oxygen Species Production and Transient Receptor Potential Vanilloid1 Expression in Human Skin During Aging. J. Histochem. Cytochem. 2024, 72, 157–171. [Google Scholar] [CrossRef]
- Chieco, P.; Jonker, A.; De Boer, B.A.; Ruijter, J.M.; Van Noorden, C.J.F. Image Cytometry: Protocols for 2D and 3D Quantification in Microscopic Images. Prog. Histochem. Cytochem. 2013, 47, 211–333. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, E.J.; Moss, J.I.; Atkinson, J.; Baakza, H.; Hayes, E.; Willis, S.E.; Waring, P.M.; Rodriguez Canales, J.; Jones, G.N. Epitope Lability of Phosphorylated Biomarkers of the DNA Damage Response Pathway Results in Increased Vulnerability to Effects of Delayed or Incomplete Formalin Fixation. J. Histochem. Cytochem. 2023, 71, 237–257. [Google Scholar] [CrossRef]
- Franco, C.; Bonomini, F.; Borsani, E.; Castrezzati, S.; Franceschetti, L.; Rezzani, R. Involvement of Intestinal Goblet Cells and Changes in Sodium Glucose Transporters Expression: Possible Therapeutic Targets in Autistic BTBR T+Itpr3tf/J Mice. Int. J. Environ. Res. Public Health 2021, 18, 11328. [Google Scholar] [CrossRef]
- Sturgeon, C.; Fasano, A. Zonulin, a Regulator of Epithelial and Endothelial Barrier Functions, and Its Involvement in Chronic Inflammatory Diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Nalbant, K.; Erden, S.; Yazar, A.; Kılınç, İ. Investigation of the Relation between Epithelial Barrier Function and Autism Symptom Severity in Children with Autism Spectrum Disorder. J. Mol. Neurosci. 2022, 72, 741–747. [Google Scholar] [CrossRef]
- Dargenio, V.N.; Dargenio, C.; Castellaneta, S.; De Giacomo, A.; Laguardia, M.; Schettini, F.; Francavilla, R.; Cristofori, F. Intestinal Barrier Dysfunction and Microbiota–Gut–Brain Axis: Possible Implications in the Pathogenesis and Treatment of Autism Spectrum Disorder. Nutrients 2023, 15, 1620. [Google Scholar] [CrossRef]
- Teskey, G.; Anagnostou, E.; Mankad, D.; Smile, S.; Roberts, W.; Brian, J.; Bowdish, D.M.E.; Foster, J.A. Intestinal Permeability Correlates with Behavioural Severity in Very Young Children with ASD: A Preliminary Study. J. Neuroimmunol. 2021, 357, 577607. [Google Scholar] [CrossRef]
- Gerbe, F.; Legraverend, C.; Jay, P. The Intestinal Epithelium Tuft Cells: Specification and Function. Cell. Mol. Life Sci. 2012, 69, 2907–2917. [Google Scholar] [CrossRef] [PubMed]
- Seyyedin, S.; Nazem, M.N. Histomorphometric Study of the Effect of Methionine on Small Intestine Parameters in Rat: An Applied Histologic Study. Folia Morphol. 2017, 76, 620–629. [Google Scholar] [CrossRef]
- Daveson, A.J.M.; Popp, A.; Taavela, J.; Goldstein, K.E.; Isola, J.; Truitt, K.E.; Mäki, M.; Anderson, R.P.; The RESET CeD Study Group; Adams, A.; et al. Baseline Quantitative Histology in Therapeutics Trials Reveals Villus Atrophy in Most Patients with Coeliac Disease Who Appear Well Controlled on Gluten-free Diet. GastroHep 2020, 2, 22–30. [Google Scholar] [CrossRef]
- Adolph, T.E.; Tomczak, M.F.; Niederreiter, L.; Ko, H.-J.; Böck, J.; Martinez-Naves, E.; Glickman, J.N.; Tschurtschenthaler, M.; Hartwig, J.; Hosomi, S.; et al. Paneth Cells as a Site of Origin for Intestinal Inflammation. Nature 2013, 503, 272–276. [Google Scholar] [CrossRef]
- Stappenbeck, T.S.; McGovern, D.P.B. Paneth Cell Alterations in the Development and Phenotype of Crohn’s Disease. Gastroenterology 2017, 152, 322–326. [Google Scholar] [CrossRef]
- Wehkamp, J.; Stange, E.F. An Update Review on the Paneth Cell as Key to Ileal Crohn’s Disease. Front. Immunol. 2020, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight Junction Proteins in Gastrointestinal and Liver Disease. Gut 2019, 68, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Karim, A.; Iqbal, M.S.; Ahmad, F.; Shaikh, A.; Kamli, H.; Khamjan, N.A. A Leaky Gut Contributes to Postural Dysfunction in Patients with Alzheimer’s Disease. Heliyon 2023, 9, e19485. [Google Scholar] [CrossRef]
- Ding, W.; Xu, Y.; Ding, W.; Tang, Q.; Zhang, B.; Yuan, Y.; Jin, J. Research Progress on Melatonin, 5-HT, and Orexin in Sleep Disorders of Children with Autism Spectrum Disorder. Biomol. Biomed. 2025, 25, 525–533. [Google Scholar] [CrossRef]
- Lalanne, S.; Fougerou-Leurent, C.; Anderson, G.M.; Schroder, C.M.; Nir, T.; Chokron, S.; Delorme, R.; Claustrat, B.; Bellissant, E.; Kermarrec, S.; et al. Melatonin: From Pharmacokinetics to Clinical Use in Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 1490. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, M.; Giugno, L.; Borsani, E.; Bonomini, F. Potential Neuroprotective Effect of Melatonin in the Hippocampus of Male BTBR Mice. Nutrients 2024, 16, 1652. [Google Scholar] [CrossRef]
- Hussein, E.M.; Ghanem, N.F.; Bakr, S.M.; Kasem, S.M.; Dkhil, M.A.; Thagfan, F.A.; Essawy, A.E. Microscopic and Ultrastructural Insights into the Protective Role of Melatonin against Tartrazine-Induced Hepatotoxicity. Biotech. Histochem. 2025, 100, 415–429. [Google Scholar] [CrossRef]
- Joo, S.S.; Yoo, Y.-M. Protective Effect of Melatonin Against Bisphenol A Toxicity. Int. J. Mol. Sci. 2025, 26, 7526. [Google Scholar] [CrossRef]
- Moretti, R.; Zanin, A.; Pansiot, J.; Spiri, D.; Manganozzi, L.; Kratzer, I.; Favero, G.; Vasiljevic, A.; Rinaldi, V.E.; Pic, I.; et al. Melatonin Reduces Excitotoxic Blood–Brain Barrier Breakdown in Neonatal Rats. Neuroscience 2015, 311, 382–397. [Google Scholar] [CrossRef]
- Sun, Z.; Chai, L.; Li, D.; Yang, Y.; Yao, W.; Li, H.; Shan, C.; Wen, X.; Lin, R. Role of Melatonin in Intestinal Mucosal Injury Induced by Chronic Restraint Stress in Mice. Neuroendocrinology 2025, 115, 741–756. [Google Scholar] [CrossRef]
- Dokoohaki, S.; Ghareghani, M.; Ghanbari, A.; Farhadi, N.; Zibara, K.; Sadeghi, H. Corticosteroid Therapy Exacerbates the Reduction of Melatonin in Multiple Sclerosis. Steroids 2017, 128, 32–36. [Google Scholar] [CrossRef]
- Frye, R.E.; James, S.J. Metabolic Pathology of Autism in Relation to Redox Metabolism. Biomark. Med. 2014, 8, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Mantani, Y.; Sakata, N.; Kubota, N.; Shimada, A.; Nakanishi, S.; Yokoyama, T.; Hoshi, N. Diurnal Changes in Bacterial Settlement on the Peyer’s Patch and Surrounding Mucosa in the Rat Ileum and Its Effect against the Intestinal Immune System. Cell Tissue Res. 2023, 393, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of Melatonin in Sleep Deprivation-Induced Intestinal Barrier Dysfunction in Mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef] [PubMed]
- Kromm, F.; Baumann, A.; Sánchez, V.; Brandt, A.; Staltner, R.; Bergheim, I. Oral Supplementation of Melatonin Attenuates the Onset of Alcohol-Related Liver Disease. J. Mol. Med. 2025, 103, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- El-Hakim, Y.; Mani, K.K.; Eldouh, A.; Pandey, S.; Grimaldo, M.T.; Dabney, A.; Pilla, R.; Sohrabji, F. Sex Differences in Stroke Outcome Correspond to Rapid and Severe Changes in Gut Permeability in Adult Sprague-Dawley Rats. Biol. Sex Differ. 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kapur, S.; Kancharla, P.; Yang, T. Sex Differences in Gut Microbiota, Hypertension, and Cardiovascular Risk. Eur. J. Pharmacol. 2025, 987, 177183. [Google Scholar] [CrossRef]
- Rosser, E.C.; De Gruijter, N.M.; Matei, D.E. Mini-Review: Gut-Microbiota and the Sex-Bias in Autoimmunity–Lessons Learnt from Animal Models. Front. Med. 2022, 9, 910561. [Google Scholar] [CrossRef] [PubMed]
- Elderman, M.; Sovran, B.; Hugenholtz, F.; Graversen, K.; Huijskes, M.; Houtsma, E.; Belzer, C.; Boekschoten, M.; de Vos, P.; Dekker, J.; et al. The Effect of Age on the Intestinal Mucus Thickness, Microbiota Composition and Immunity in Relation to Sex in Mice. PLoS ONE 2017, 12, e0184274. [Google Scholar] [CrossRef]
- Volynets, V.; Reichold, A.; Bárdos, G.; Rings, A.; Bleich, A.; Bischoff, S.C. Assessment of the Intestinal Barrier with Five Different Permeability Tests in Healthy C57BL/6J and BALB/cJ Mice. Dig. Dis. Sci. 2016, 61, 737–746. [Google Scholar] [CrossRef]
- Gunn, P.J.; Middleton, B.; Davies, S.K.; Revell, V.L.; Skene, D.J. Sex Differences in the Circadian Profiles of Melatonin and Cortisol in Plasma and Urine Matrices under Constant Routine Conditions. Chronobiol. Int. 2016, 33, 39–50. [Google Scholar] [CrossRef]
- Lok, R.; Qian, J.; Chellappa, S.L. Sex Differences in Sleep, Circadian Rhythms, and Metabolism: Implications for Precision Medicine. Sleep Med. Rev. 2024, 75, 101926. [Google Scholar] [CrossRef]
- Cain, S.W.; Dennison, C.F.; Zeitzer, J.M.; Guzik, A.M.; Khalsa, S.B.S.; Santhi, N.; Schoen, M.W.; Czeisler, C.A.; Duffy, J.F. Sex Differences in Phase Angle of Entrainment and Melatonin Amplitude in Humans. J. Biol. Rhythm. 2010, 25, 288–296. [Google Scholar] [CrossRef]
- Santhi, N.; Lazar, A.S.; McCabe, P.J.; Lo, J.C.; Groeger, J.A.; Dijk, D.-J. Sex Differences in the Circadian Regulation of Sleep and Waking Cognition in Humans. Proc. Natl. Acad. Sci. USA 2016, 113, E2730–E2739. [Google Scholar] [CrossRef]
- Nathan, P.J.; Burrows, G.D.; Norman, T.R. The Effect of Dim Light on Suppression of Nocturnal Melatonin in Healthy Women and Men. J. Neural Transm. 1997, 104, 643–648. [Google Scholar] [CrossRef]
- Nathan, P.J.; Wyndham, E.L.; Burrows, G.D.; Norman, T.R. The Effect of Gender on the Melatonin Suppression by Light: A Dose Response Relationship. J. Neural Transm. 2000, 107, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Dahlgren, D.; Lennernäs, H.; Sjöblom, M. Melatonin-Activated Receptor Signaling Pathways Mediate Protective Effects on Surfactant-Induced Increase in Jejunal Mucosal Permeability in Rats. Int. J. Mol. Sci. 2021, 22, 10762. [Google Scholar] [CrossRef] [PubMed]
- Rezzani, R.; Rodella, L.F.; Bonomini, F.; Tengattini, S.; Bianchi, R.; Reiter, R.J. Beneficial Effects of Melatonin in Protecting against Cyclosporine A-induced Cardiotoxicity Are Receptor Mediated. J. Pineal Res. 2006, 41, 288–295. [Google Scholar] [CrossRef] [PubMed]

| Composition | Calories from Protein | Calories from Fat | Calories from Carbohydrate | Analytical Constituents | % |
|---|---|---|---|---|---|
| Wheat, maize, extracted and toasted soybean meal, corn gluten feed, wheat straw, fish meal, lucerne meal, mineral dicalcium phosphate, calcium carbonate, sodium chloride, whey powder, soybean oil, yeasts. | 24% | 18% | 58% | Moisture | 12.00 |
| Crude protein | 18.50 | ||||
| Crude oils and fats | 3.00 | ||||
| Crude fibers | 6.00 | ||||
| Crude ash | 7.00 |
| Experimental Group | BTBR + veh | CTR + veh | BTBR + MLT | CTR + MLT |
|---|---|---|---|---|
| Semi-quantitative evaluation of inflammatory cells | ++ | +/++ | +/++ | +/−/+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulas, F.; Favero, G.; Bonini, S.A.; Lonati, C.; Pinto, D.; Memo, M.; Rinaldi, F.; Rezzani, R. Melatonin Improves Intestinal Barrier Impairment in a Mouse Model of Autism Spectrum Disorder. Biology 2025, 14, 1594. https://doi.org/10.3390/biology14111594
Sulas F, Favero G, Bonini SA, Lonati C, Pinto D, Memo M, Rinaldi F, Rezzani R. Melatonin Improves Intestinal Barrier Impairment in a Mouse Model of Autism Spectrum Disorder. Biology. 2025; 14(11):1594. https://doi.org/10.3390/biology14111594
Chicago/Turabian StyleSulas, Francesca, Gaia Favero, Sara Anna Bonini, Claudio Lonati, Daniela Pinto, Maurizio Memo, Fabio Rinaldi, and Rita Rezzani. 2025. "Melatonin Improves Intestinal Barrier Impairment in a Mouse Model of Autism Spectrum Disorder" Biology 14, no. 11: 1594. https://doi.org/10.3390/biology14111594
APA StyleSulas, F., Favero, G., Bonini, S. A., Lonati, C., Pinto, D., Memo, M., Rinaldi, F., & Rezzani, R. (2025). Melatonin Improves Intestinal Barrier Impairment in a Mouse Model of Autism Spectrum Disorder. Biology, 14(11), 1594. https://doi.org/10.3390/biology14111594









