Managing Spinal Muscular Atrophy: A Look at the Biology and Treatment Strategies
Simple Summary
Abstract
1. The History of SMA Research
2. Key Genetic Evidence for SMA
3. Key Phenotypic Evidence for SMA
- Clinically undetermined and genetically determined;
- Clinically and genetically determined infants;
- Clinically determined but genetically undetermined infants.
4. Therapeutic Approaches
4.1. Biotechnological Treatments
4.2. Antisense Oligonucleotides
4.3. Gene Therapy
4.4. Splicing Modifier
4.5. Ongoing Trials of Approved and New Molecules
4.6. Alternative Approaches
5. SMA Metabolism and Nutrients: Key Evidence
6. Rehabilitation
7. Psychological Interventions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, C.; Li, X.; Shi, Y.; Zhu, X.; Zhao, L.; Li, W.; Zhou, S.; Wang, Y. Comprehensive profile and natural history of pediatric patients with spinal muscular atrophy: A large retrospective study from China. Front. Neurol. 2022, 13, 1038012. [Google Scholar] [CrossRef] [PubMed]
- Adami, R.; Bottai, D. Spinal Muscular Atrophy Modeling and Treatment Advances by Induced Pluripotent Stem Cells Studies. Stem. Cell. Rev. Rep. 2019, 15, 795–813. [Google Scholar] [CrossRef] [PubMed]
- Wadman, R.I.; Jansen, M.D.; Stam, M.; Wijngaarde, C.A.; Curial, C.A.D.; Medic, J.; Sodaar, P.; Schouten, J.; Vijzelaar, R.; Lemmink, H.H.; et al. Intragenic and structural variation in the. Brain Commun. 2020, 2, fcaa075. [Google Scholar] [CrossRef]
- Melki, J.; Abdelhak, S.; Sheth, P.; Bachelot, M.F.; Burlet, P.; Marcadet, A.; Aicardi, J.; Barois, A.; Carriere, J.P.; Fardeau, M. Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature 1990, 344, 767–768. [Google Scholar] [CrossRef]
- Brzustowicz, L.M.; Lehner, T.; Castilla, L.H.; Penchaszadeh, G.K.; Wilhelmsen, K.C.; Daniels, R.; Davies, K.E.; Leppert, M.; Ziter, F.; Wood, D. Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2-13.3. Nature 1990, 344, 540–541. [Google Scholar] [CrossRef]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef]
- Lefebvre, S.; Burlet, P.; Liu, Q.; Bertrandy, S.; Clermont, O.; Munnich, A.; Dreyfuss, G.; Melki, J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997, 16, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.N.; Howell, M.D.; Ottesen, E.W.; Singh, N.N. Diverse role of survival motor neuron protein. Biochim. Biophys. Acta Gene Regul. Mech. 2017, 1860, 299–315. [Google Scholar] [CrossRef]
- Sugarman, E.A.; Nagan, N.; Zhu, H.; Akmaev, V.R.; Zhou, Z.; Rohlfs, E.M.; Flynn, K.; Hendrickson, B.C.; Scholl, T.; Sirko-Osadsa, D.A.; et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72,400 specimens. Eur. J. Hum. Genet. 2012, 20, 27–32. [Google Scholar] [CrossRef]
- Adam, M.P.; Feldman, J.; Mirzaa, G.M.; Pagon, R.A.; Wallace, S.E.; Amemiya, A. GeneReviews; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—A literature review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef]
- Boemer, F.; Caberg, J.H.; Beckers, P.; Dideberg, V.; di Fiore, S.; Bours, V.; Marie, S.; Dewulf, J.; Marcelis, L.; Deconinck, N.; et al. Three years pilot of spinal muscular atrophy newborn screening turned into official program in Southern Belgium. Sci. Rep. 2021, 11, 19922. [Google Scholar] [CrossRef] [PubMed]
- Dangouloff, T.; Servais, L. Clinical Evidence Supporting Early Treatment Of Patients With Spinal Muscular Atrophy: Current Perspectives. Ther. Clin. Risk Manag. 2019, 15, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.T.; Farrar, M.A.; Wiley, V.; Chambers, G. Newborn screening for spinal muscular atrophy with disease-modifying therapies: A cost-effectiveness analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1296–1304. [Google Scholar] [CrossRef]
- Zaidman, C.M.; Crockett, C.D.; Wedge, E.; Tabatabai, G.; Goedeker, N. Newborn Screening for Spinal Muscular Atrophy: Variations in Practice and Early Management of Infants with Spinal Muscular Atrophy in the United States. Int. J. Neonatal. Screen. 2024, 10, 58. [Google Scholar] [CrossRef]
- Adami, R.; Bottai, D. Curcumin and neurological diseases. Nutr. Neurosci. 2022, 25, 441–461. [Google Scholar] [CrossRef]
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal Muscular Atrophy: Mutations, Testing, and Clinical Relevance. Appl. Clin. Genet. 2021, 14, 11–25. [Google Scholar] [CrossRef]
- Adami, R.; Bottai, D. NSC Physiological Features in Spinal Muscular Atrophy: SMN Deficiency Effects on Neurogenesis. Int. J. Mol. Sci. 2022, 23, 15209. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Thacker, A.K.; Maloo, J.C. A clinical, epidemiological and genetic study of hereditary motor neuropathies in Benghazi, Libya. J. Neurol. 1988, 235, 422–424. [Google Scholar] [CrossRef]
- Tsai, C.H.; Jong, Y.J.; Hu, C.J.; Chen, C.M.; Shih, M.C.; Chang, C.P.; Chang, J.G. Molecular analysis of SMN, NAIP and P44 genes of SMA patients and their families. J. Neurol. Sci. 2001, 190, 35–40. [Google Scholar] [CrossRef]
- Ruhno, C.; McGovern, V.L.; Avenarius, M.R.; Snyder, P.J.; Prior, T.W.; Nery, F.C.; Muhtaseb, A.; Roggenbuck, J.S.; Kissel, J.T.; Sansone, V.A.; et al. Complete sequencing of the SMN2 gene in SMA patients detects SMN gene deletion junctions and variants in SMN2 that modify the SMA phenotype. Hum. Genet. 2019, 138, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Zat’ková, A.; Hahnen, E.; Wirth, B.; Kádasi, L. Analysis of the SMN and NAIP genes in slovak spinal muscular atrophy patients. Hum. Hered. 2000, 50, 171–174. [Google Scholar] [CrossRef]
- Liu, Q.; Dreyfuss, G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 1996, 15, 3555–3565. [Google Scholar] [CrossRef]
- Liu, Q.; Fischer, U.; Wang, F.; Dreyfuss, G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 1997, 90, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Fallini, C.; Bassell, G.J.; Rossoll, W. Spinal muscular atrophy: The role of SMN in axonal mRNA regulation. Brain Res. 2012, 1462, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Rossoll, W.; Kröning, A.K.; Ohndorf, U.M.; Steegborn, C.; Jablonka, S.; Sendtner, M. Specific interaction of Smn, the spinal muscular atrophy determining gene product, with hnRNP-R and gry-rbp/hnRNP-Q: A role for Smn in RNA processing in motor axons? Hum. Mol. Genet. 2002, 11, 93–105. [Google Scholar] [CrossRef]
- Bowerman, M.; Shafey, D.; Kothary, R. Smn depletion alters profilin II expression and leads to upregulation of the RhoA/ROCK pathway and defects in neuronal integrity. J. Mol. Neurosci. 2007, 32, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Schüning, T.; Zeug, A.; Strienke, K.; Franz, P.; Tsiavaliaris, G.; Hensel, N.; Viero, G.; Ponimaskin, E.; Claus, P. The spinal muscular atrophy gene product regulates actin dynamics. FASEB J. 2024, 38, e70055. [Google Scholar] [CrossRef]
- Faravelli, I.; Riboldi, G.M.; Rinchetti, P.; Lotti, F. The SMN Complex at the Crossroad between RNA Metabolism and Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 2247. [Google Scholar] [CrossRef]
- Gubitz, A.K.; Feng, W.; Dreyfuss, G. The SMN complex. Exp. Cell Res. 2004, 296, 51–56. [Google Scholar] [CrossRef]
- Gavrilov, D.K.; Shi, X.; Das, K.; Gilliam, T.C.; Wang, C.H. Differential SMN2 expression associated with SMA severity. Nat. Genet. 1998, 20, 230–231. [Google Scholar] [CrossRef]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef]
- O’Hern, P.; Garcia, E.L.; Hao, L.T.; ùHart, A.C.; Matera, A.G.; Beattie, C.E. Nonmammalian Animal Models of Spinal Muscular Atrophy; Academic Press: Cambridge, MA, USA, 2017; pp. 221–239. [Google Scholar]
- Germain-Desprez, D.; Brun, T.; Rochette, C.; Semionov, A.; Rouget, R.; Simard, L.R. The SMN genes are subject to transcriptional regulation during cellular differentiation. Gene 2001, 279, 109–117. [Google Scholar] [CrossRef]
- Wirth, B.; Karakaya, M.; Kye, M.J.; Mendoza-Ferreira, N. Twenty-Five Years of Spinal Muscular Atrophy Research: From Phenotype to Genotype to Therapy, and What Comes Next. Annu. Rev. Genom. Hum. Genet. 2020, 21, 231–261. [Google Scholar] [CrossRef]
- Farrar, M.A.; Kiernan, M.C. The Genetics of Spinal Muscular Atrophy: Progress and Challenges. Neurotherapeutics 2015, 12, 290–302. [Google Scholar] [CrossRef]
- Alías, L.; Bernal, S.; Fuentes-Prior, P.; Barceló, M.J.; Also, E.; Martínez-Hernández, R.; Rodríguez-Alvarez, F.J.; Martín, Y.; Aller, E.; Grau, E.; et al. Mutation update of spinal muscular atrophy in Spain: Molecular characterization of 745 unrelated patients and identification of four novel mutations in the SMN1 gene. Hum. Genet. 2009, 125, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Bussaglia, E.; Clermont, O.; Tizzano, E.; Lefebvre, S.; Bürglen, L.; Cruaud, C.; Urtizberea, J.A.; Colomer, J.; Munnich, A.; Baiget, M. A frame-shift deletion in the survival motor neuron gene in Spanish spinal muscular atrophy patients. Nat. Genet. 1995, 11, 335–337. [Google Scholar] [CrossRef]
- Jędrzejowska, M.; Gos, M.; Zimowski, J.G.; Kostera-Pruszczyk, A.; Ryniewicz, B.; Hausmanowa-Petrusewicz, I. Novel point mutations in survival motor neuron 1 gene expand the spectrum of phenotypes observed in spinal muscular atrophy patients. Neuromuscul. Disord. 2014, 24, 617–623. [Google Scholar] [CrossRef]
- Wirth, B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum. Mutat. 2000, 15, 228–237. [Google Scholar] [CrossRef]
- Feldkötter, M.; Schwarzer, V.; Wirth, R.; Wienker, T.F.; Wirth, B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: Fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 2002, 70, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B.; Schmidt, T.; Hahnen, E.; Rudnik-Schöneborn, S.; Krawczak, M.; Müller-Myhsok, B.; Schönling, J.; Zerres, K. De novo rearrangements found in 2% of index patients with spinal muscular atrophy: Mutational mechanisms, parental origin, mutation rate, and implications for genetic counseling. Am. J. Hum. Genet. 1997, 61, 1102–1111. [Google Scholar] [CrossRef]
- Aasdev, A.; Sreelekshmi, R.S.; Iyer, V.R.; Moharir, S.C. Spinal muscular atrophy: Molecular mechanism of pathogenesis, diagnosis, therapeutics, and clinical trials in the Indian context. J. Biosci. 2024, 49, 36. [Google Scholar] [CrossRef]
- Carlson, L.M.; Vora, N.L. Prenatal Diagnosis: Screening and Diagnostic Tools. Obstet. Gynecol. Clin. North Am. 2017, 44, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, Z.; Navaratnam, K.; Mujezinovic, F. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst. Rev. 2017, 9, CD003252. [Google Scholar] [CrossRef]
- Chaytow, H.; Huang, Y.T.; Gillingwater, T.H.; Faller, K.M.E. The role of survival motor neuron protein (SMN) in protein homeostasis. Cell. Mol. Life Sci. 2018, 75, 3877–3894. [Google Scholar] [CrossRef] [PubMed]
- Bottai, D.; Adami, R. Spinal muscular atrophy: New findings for an old pathology. Brain Pathol. 2013, 23, 613–622. [Google Scholar] [CrossRef]
- Shi, T.; Zhou, Z.; Xiang, T.; Suo, Y.; Shi, X.; Li, Y.; Zhang, P.; Dai, J.; Sheng, L. Cytoskeleton dysfunction of motor neuron in spinal muscular atrophy. J. Neurol. 2024, 272, 19. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, Z.; Risher, N.; Mollin, A.; Sheedy, J.; Ling, K.K.Y.; Narasimhan, J.; Dakka, A.; Baird, J.D.; Ratni, H.; et al. SMN protein is required throughout life to prevent spinal muscular atrophy disease progression. Hum. Mol. Genet. 2021, 31, 82–96. [Google Scholar] [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. [Google Scholar] [CrossRef]
- Talbot, K.; Tizzano, E.F. The clinical landscape for SMA in a new therapeutic era. Gene. Ther. 2017, 24, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, J.; Bernert, G.; Butoianu, N.; De Waele, L.; Fattal-Valevski, A.; Haberlova, J.; Moreno, T.; Klein, A.; Kostera-Pruszczyk, A.; Mercuri, E.; et al. 2024 update: European consensus statement on gene therapy for spinal muscular atrophy. Eur. J. Paediatr. Neurol. 2024, 51, 73–78. [Google Scholar] [CrossRef]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef]
- D’Amico, A.; Mercuri, E.; Tiziano, F.D.; Bertini, E. Spinal muscular atrophy. Orphanet J. Rare Dis. 2011, 6, 71. [Google Scholar] [CrossRef]
- Dubowitz, V. Very severe spinal muscular atrophy (SMA type 0): An expanding clinical phenotype. Eur. J. Paediatr. Neurol. 1999, 3, 49–51. [Google Scholar] [CrossRef]
- Arnold, W.D.; Kassar, D.; Kissel, J.T. Spinal muscular atrophy: Diagnosis and management in a new therapeutic era. Muscle Nerve 2015, 51, 157–167. [Google Scholar] [CrossRef]
- Corsello, A.; Scatigno, L.; Pascuzzi, M.C.; Calcaterra, V.; Dilillo, D.; Vizzuso, S.; Pelizzo, G.; Zoia, E.; Mandelli, A.; Govoni, A.; et al. Nutritional, Gastrointestinal and Endo-Metabolic Challenges in the Management of Children with Spinal Muscular Atrophy Type 1. Nutrients 2021, 13, 2400. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, J.N.; Gillingwater, T.H.; Talbot, K. The contribution of mouse models to understanding the pathogenesis of spinal muscular atrophy. Dis. Model. Mech. 2011, 4, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Palladino, A.; Passamano, L.; Taglia, A.; D’Ambrosio, P.; Scutifero, M.; Cecio, M.R.; Picillo, E.; Viggiano, E.; Torre, V.; De Luca, F.; et al. Cardiac involvement in patients with spinal muscular atrophies. Acta Myol. 2011, 30, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.; Araujo, M.; Swoboda, K.J. Vascular perfusion abnormalities in infants with spinal muscular atrophy. J. Pediatr. 2009, 155, 292–294. [Google Scholar] [CrossRef]
- Rudnik-Schöneborn, S.; Vogelgesang, S.; Armbrust, S.; Graul-Neumann, L.; Fusch, C.; Zerres, K. Digital necroses and vascular thrombosis in severe spinal muscular atrophy. Muscle Nerve 2010, 42, 144–147. [Google Scholar] [CrossRef]
- Bowerman, M.; Swoboda, K.J.; Michalski, J.P.; Wang, G.S.; Reeks, C.; Beauvais, A.; Murphy, K.; Woulfe, J.; Screaton, R.A.; Scott, F.W.; et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann Neurol 2012, 72, 256–268. [Google Scholar] [CrossRef]
- Kölbel, H.; Hauffa, B.P.; Wudy, S.A.; Bouikidis, A.; Della Marina, A.; Schara, U. Hyperleptinemia in children with autosomal recessive spinal muscular atrophy type I-III. PLoS ONE 2017, 12, e0173144. [Google Scholar] [CrossRef]
- Butchbach, M.E.R. Genomic Variability in the Survival Motor Neuron Genes (SMN1 and SMN2): Implications for Spinal Muscular Atrophy Phenotype and Therapeutics Development. Int. J. Mol. Sci. 2021, 22, 7896. [Google Scholar] [CrossRef]
- Agyenkwa, S.K.; Cibooğlu, P.; Jhar, N.; Abo Orabi, A.; Mustafaoglu, R. Rehabilitation in Spinal Muscular Atrophy; A Narrative Review. Eur. J. Ther. 2023, 29, 656–666. [Google Scholar] [CrossRef]
- Lin, C.W.; Kalb, S.J.; Yeh, W.S. Delay in Diagnosis of Spinal Muscular Atrophy: A Systematic Literature Review. Pediatr. Neurol. 2015, 53, 293–300. [Google Scholar] [CrossRef]
- Varone, A.; Esposito, G.; Bitetti, I. Spinal muscular atrophy in the era of newborn screening: How the classification could change. Front. Neurol. 2025, 16, 1542396. [Google Scholar] [CrossRef] [PubMed]
- Cantara, S.; Simoncelli, G.; Ricci, C. Antisense Oligonucleotides (ASOs) in Motor Neuron Diseases: A Road to Cure in Light and Shade. Int. J. Mol. Sci. 2024, 25, 4809. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K. RNA therapy: Rich history, various applications and unlimited future prospects. Exp. Mol. Med. 2022, 54, 455–465. [Google Scholar] [CrossRef]
- Messina, S.; Sframeli, M. New Treatments in Spinal Muscular Atrophy: Positive Results and New Challenges. J. Clin. Med. 2020, 9, 2222. [Google Scholar] [CrossRef]
- Corey, D.R. Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nat. Neurosci. 2017, 20, 497–499. [Google Scholar] [CrossRef]
- López-Cortés, A.; Echeverría-Garcés, G.; Ramos-Medina, M.J. Molecular Pathogenesis and New Therapeutic Dimensions for Spinal Muscular Atrophy. Biology 2022, 11, 894. [Google Scholar] [CrossRef]
- Yao, X.; Peng, J.; Luo, R.; Wang, X.; Lu, X.; Wu, L.; Jin, R.; Zhong, J.; Liang, J.; Hong, S.; et al. Nusinersen effectiveness and safety in pediatric patients with 5q-spinal muscular atrophy: A multi-center disease registry in China. J. Neurol. 2024, 271, 5378–5391. [Google Scholar] [CrossRef]
- Zingariello, C.D.; Brandsema, J.; Drum, E.; Henderson, A.A.; Dubow, S.; Glanzman, A.M.; Mayer, O.; Yum, S.W.; Kichula, E.A. A multidisciplinary approach to dosing nusinersen for spinal muscular atrophy. Neurol. Clin. Pract. 2019, 9, 424–432. [Google Scholar] [CrossRef]
- Neil, E.E.; Bisaccia, E.K. Nusinersen: A Novel Antisense Oligonucleotide for the Treatment of Spinal Muscular Atrophy. J. Pediatr. Pharmacol. Ther. 2019, 24, 194–203. [Google Scholar] [CrossRef]
- Acsadi, G.; Crawford, T.O.; Müller-Felber, W.; Shieh, P.B.; Richardson, R.; Natarajan, N.; Castro, D.; Ramirez-Schrempp, D.; Gambino, G.; Sun, P.; et al. Safety and efficacy of nusinersen in spinal muscular atrophy: The EMBRACE study. Muscle Nerve 2021, 63, 668–677. [Google Scholar] [CrossRef]
- Crawford, T.O.; Swoboda, K.J.; De Vivo, D.C.; Bertini, E.; Hwu, W.L.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Nazario, A.N.; Parsons, J.A.; et al. Continued benefit of nusinersen initiated in the presymptomatic stage of spinal muscular atrophy: 5-year update of the NURTURE study. Muscle Nerve 2023, 68, 157–170. [Google Scholar] [CrossRef]
- Günther, R.; Wurster, C.D.; Brakemeier, S.; Osmanovic, A.; Schreiber-Katz, O.; Petri, S.; Uzelac, Z.; Hiebeler, M.; Thiele, S.; Walter, M.C.; et al. Long-term efficacy and safety of nusinersen in adults with 5q spinal muscular atrophy: A prospective European multinational observational study. Lancet Reg. Health Eur. 2024, 39, 100862. [Google Scholar] [CrossRef]
- Gonçalves, G.A.R.; Paiva, R.M.A. Gene therapy: Advances, challenges and perspectives. Einstein 2017, 15, 369–375. [Google Scholar] [CrossRef]
- Ogbonmide, T.; Rathore, R.; Rangrej, S.B.; Hutchinson, S.; Lewis, M.; Ojilere, S.; Carvalho, V.; Kelly, I. Gene Therapy for Spinal Muscular Atrophy (SMA): A Review of Current Challenges and Safety Considerations for Onasemnogene Abeparvovec (Zolgensma). Cureus 2023, 15, e36197. [Google Scholar] [CrossRef]
- Gowda, V.; Atherton, M.; Murugan, A.; Servais, L.; Sheehan, J.; Standing, E.; Manzur, A.; Scoto, M.; Baranello, G.; Munot, P.; et al. Efficacy and safety of onasemnogene abeparvovec in children with spinal muscular atrophy type 1: Real-world evidence from 6 infusion centres in the United Kingdom. Lancet Reg. Health Eur. 2024, 37, 100817. [Google Scholar] [CrossRef]
- Markati, T.; Fisher, G.; Ramdas, S.; Servais, L. Risdiplam: An investigational survival motor neuron 2 (SMN2) splicing modifier for spinal muscular atrophy (SMA). Expert Opin. Investig. Drugs 2022, 31, 451–461. [Google Scholar] [CrossRef]
- Chaytow, H.; Faller, K.M.E.; Huang, Y.T.; Gillingwater, T.H. Spinal muscular atrophy: From approved therapies to future therapeutic targets for personalized medicine. Cell Rep. Med. 2021, 2, 100346. [Google Scholar] [CrossRef]
- Ratni, H.; Scalco, R.S.; Stephan, A.H. Risdiplam, the First Approved Small Molecule Splicing Modifier Drug as a Blueprint for Future Transformative Medicines. ACS Med. Chem. Lett. 2021, 12, 874–877. [Google Scholar] [CrossRef]
- Masson, R.; Mazurkiewicz-Bełdzińska, M.; Rose, K.; Servais, L.; Xiong, H.; Zanoteli, E.; Baranello, G.; Bruno, C.; Day, J.W.; Deconinck, N.; et al. Safety and efficacy of risdiplam in patients with type 1 spinal muscular atrophy (FIREFISH part 2): Secondary analyses from an open-label trial. Lancet Neurol. 2022, 21, 1110–1119. [Google Scholar] [CrossRef]
- Ribero, V.A.; Daigl, M.; Martí, Y.; Gorni, K.; Evans, R.; Scott, D.A.; Mahajan, A.; Abrams, K.R.; Hawkins, N. How does risdiplam compare with other treatments for Types 1–3 spinal muscular atrophy: A systematic literature review and indirect treatment comparison. J. Comp. Eff. Res. 2022, 11, 347–370. [Google Scholar] [CrossRef]
- Belančić, A.; Strbad, T.; Kučan Štiglić, M.; Vitezić, D. Switching from Nusinersen to Risdiplam: A Croatian Real-World Experience on Effectiveness and Safety. J. Pers. Med. 2024, 14, 244. [Google Scholar] [CrossRef]
- Finkel, R.S.; Hughes, S.H.; Parker, J.; Civitello, M.; Lavado, A.; Mefford, H.C.; Mueller, L.; Kletzl, H. Risdiplam for Prenatal Therapy of Spinal Muscular Atrophy. N. Engl. J. Med. 2025, 392, 1138–1140. [Google Scholar] [CrossRef]
- Borges, B.; Brown, S.M.; Chen, W.J.; Clarke, M.T.; Herzeg, A.; Park, J.H.; Ross, J.; Kong, L.; Denton, M.; Smith, A.K.; et al. Intra-amniotic antisense oligonucleotide treatment improves phenotypes in preclinical models of spinal muscular atrophy. Sci. Transl. Med. 2025, 17, eadv4656. [Google Scholar] [CrossRef]
- Crawford, T.O.; Day, J.W.; De Vivo, D.C.; Krueger, J.M.; Mercuri, E.; Nascimento, A.; Pasternak, A.; Mazzone, E.S.; Duong, T.; Song, G.; et al. Long-term efficacy, safety, and patient-reported outcomes of apitegromab in patients with spinal muscular atrophy: Results from the 36-month TOPAZ study. Front. Neurol. 2024, 15, 1419791. [Google Scholar] [CrossRef]
- Crawford, T.O.; Darras, B.T.; Day, J.W.; Dunaway Young, S.; Duong, T.; Nelson, L.L.; Barrett, D.; Song, G.; Bilic, S.; Cote, S.; et al. Safety and Efficacy of Apitegromab in Patients With Spinal Muscular Atrophy Types 2 and 3: The Phase 2 TOPAZ Study. Neurology 2024, 102, e209151. [Google Scholar] [CrossRef]
- Welsh, B.T.; Cote, S.M.; Meshulam, D.; Jackson, J.; Pal, A.; Lansita, J.; Kalra, A. Preclinical Safety Assessment and Toxicokinetics of Apitegromab, an Antibody Targeting Proforms of Myostatin for the Treatment of Muscle-Atrophying Disease. Int. J. Toxicol. 2021, 40, 322–336. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Singh, N.N.; Seo, J.; Rahn, S.J.; Singh, R.N. A multi-exon-skipping detection assay reveals surprising diversity of splice isoforms of spinal muscular atrophy genes. PLoS ONE 2012, 7, e49595. [Google Scholar] [CrossRef][Green Version]
- Seo, J.; Singh, N.N.; Ottesen, E.W.; Sivanesan, S.; Shishimorova, M.; Singh, R.N. Oxidative Stress Triggers Body-Wide Skipping of Multiple Exons of the Spinal Muscular Atrophy Gene. PLoS ONE 2016, 11, e0154390. [Google Scholar] [CrossRef]
- Adami, R.; Pezzotta, M.; Cadile, F.; Cuniolo, B.; Rovati, G.; Canepari, M.; Bottai, D. Physiological Features of the Neural Stem Cells Obtained from an Animal Model of Spinal Muscular Atrophy and Their Response to Antioxidant Curcumin. Int. J. Mol. Sci. 2024, 25, 8364. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhao, Y.P.; Zhao, Y.; Deng, S.L.; Yu, K. Regulation of Myostatin on the Growth and Development of Skeletal Muscle. Front. Cell Dev. Biol. 2021, 9, 785712. [Google Scholar] [CrossRef]
- Abati, E.; Manini, A.; Comi, G.P.; Corti, S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell. Mol. Life Sci. 2022, 79, 374. [Google Scholar] [CrossRef] [PubMed]
- Servais, L.; Lair, L.L.; Connolly, A.M.; Byrne, B.J.; Chen, K.S.; Coric, V.; Qureshi, I.; Durham, S.; Campbell, D.J.; Maclaine, G.; et al. Taldefgrobep Alfa and the Phase 3 RESILIENT Trial in Spinal Muscular Atrophy. Int. J. Mol. Sci. 2024, 25, 10273. [Google Scholar] [CrossRef]
- Chen, T.H. New and Developing Therapies in Spinal Muscular Atrophy: From Genotype to Phenotype to Treatment and Where Do We Stand? Int. J. Mol. Sci. 2020, 21, 3297. [Google Scholar] [CrossRef]
- Liu, M.; Hammers, D.W.; Barton, E.R.; Sweeney, H.L. Activin Receptor Type IIB Inhibition Improves Muscle Phenotype and Function in a Mouse Model of Spinal Muscular Atrophy. PLoS ONE 2016, 11, e0166803. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Caine, C.; Awano, T.; Herbst, R.; Monani, U.R. Motor neuronal repletion of the NMJ organizer, Agrin, modulates the severity of the spinal muscular atrophy disease phenotype in model mice. Hum. Mol. Genet. 2017, 26, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Antonaci, L.; Pera, M.C.; Mercuri, E. New therapies for spinal muscular atrophy: Where we stand and what is next. Eur. J. Pediatr. 2023, 182, 2935–2942. [Google Scholar] [CrossRef]
- Tejero, R.; Balk, S.; Franco-Espin, J.; Ojeda, J.; Hennlein, L.; Drexl, H.; Dombert, B.; Clausen, J.D.; Torres-Benito, L.; Saal-Bauernschubert, L.; et al. R-Roscovitine Improves Motoneuron Function in Mouse Models for Spinal Muscular Atrophy. iScience 2020, 23, 100826. [Google Scholar] [CrossRef]
- Alrafiah, A.; Karyka, E.; Coldicott, I.; Iremonger, K.; Lewis, K.E.; Ning, K.; Azzouz, M. Plastin 3 Promotes Motor Neuron Axonal Growth and Extends Survival in a Mouse Model of Spinal Muscular Atrophy. Mol. Ther. Methods Clin. Dev. 2018, 9, 81–89. [Google Scholar] [CrossRef]
- Hosseinibarkooie, S.; Schneider, S.; Wirth, B. Advances in understanding the role of disease-associated proteins in spinal muscular atrophy. Expert Rev. Proteom. 2017, 14, 581–592. [Google Scholar] [CrossRef]
- Boido, M.; Gesmundo, I.; Caretto, A.; Pedrolli, F.; Schellino, R.; Leone, S.; Cai, R.; Sha, W.; Ghigo, E.; Schally, A.V.; et al. Agonist of growth hormone-releasing hormone improves the disease features of spinal muscular atrophy mice. Proc. Natl. Acad. Sci. USA 2023, 120, e2216814120. [Google Scholar] [CrossRef]
- Frongia, A.L.; Natera-de Benito, D.; Ortez, C.; Alarcón, M.; Borrás, A.; Medina, J.; Vigo, M.; Padrós, N.; Moya, O.; Armas, J.; et al. Salbutamol tolerability and efficacy in patients with spinal muscular atrophy type II. Neuromuscul. Disord. 2019, 29, 517–524. [Google Scholar] [CrossRef]
- Baranello, G.; De Amicis, R.; Arnoldi, M.T.; Zanin, R.; Mastella, C.; Masson, R.; Leone, A.; Alberti, K.; Foppiani, A.; Battezzati, A.; et al. Evaluation of body composition as a potential biomarker in spinal muscular atrophy. Muscle Nerve 2020, 61, 530–534. [Google Scholar] [CrossRef]
- Deguise, M.O.; Chehade, L.; Kothary, R. Metabolic Dysfunction in Spinal Muscular Atrophy. Int. J. Mol. Sci. 2021, 22, 5913. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, T.H.; Wu, Y.Z.; Tseng, Y.H. Metabolic and Nutritional Issues Associated with Spinal Muscular Atrophy. Nutrients 2020, 12, 3842. [Google Scholar] [CrossRef]
- Durkin, E.T.; Schroth, M.K.; Helin, M.; Shaaban, A.F. Early laparoscopic fundoplication and gastrostomy in infants with spinal muscular atrophy type I. J. Pediatr. Surg. 2008, 43, 2031–2037. [Google Scholar] [CrossRef]
- Vetlesen, H.S.; Wik-Klokk, M.; Wallace, S.; Rasmusse, M.; Hjartåker, A.; Nordstrøm, M. Nutritional status and dietary intake in children and adolescents with spinal muscular atrophy types II and III on treatment with nusinersen. Clin. Nutr. Open Sci. 2024, 53, 57–67. [Google Scholar] [CrossRef]
- Kant-Smits, K.; Bartels, B.; Asselman, F.L.; Veldhoen, E.S.; van Eijk, R.P.A.; van der Pol, W.L.; Hulzebos, E.H.J. The RESISTANT study (Respiratory Muscle Training in Patients with Spinal Muscular Atrophy): Study protocol for a randomized controlled trial. BMC Neurol. 2023, 23, 118. [Google Scholar] [CrossRef]
- Bartels, B.; Montes, J.; van der Pol, W.L.; de Groot, J.F. Physical exercise training for type 3 spinal muscular atrophy. Cochrane Database Syst. Rev. 2019, 3, CD012120. [Google Scholar] [CrossRef]
- Lewelt, A.; Krosschell, K.J.; Stoddard, G.J.; Weng, C.; Xue, M.; Marcus, R.L.; Gappmaier, E.; Viollet, L.; Johnson, B.A.; White, A.T.; et al. Resistance strength training exercise in children with spinal muscular atrophy. Muscle Nerve 2015, 52, 559–567. [Google Scholar] [CrossRef]
- Belli, S.; Prince, I.; Savio, G.; Paracchini, E.; Cattaneo, D.; Bianchi, M.; Masocco, F.; Bellanti, M.T.; Balbi, B. Airway Clearance Techniques: The Right Choice for the Right Patient. Front. Med. 2021, 8, 544826. [Google Scholar] [CrossRef]
- Chatwin, M.; Toussaint, M.; Gonçalves, M.R.; Sheers, N.; Mellies, U.; Gonzales-Bermejo, J.; Sancho, J.; Fauroux, B.; Andersen, T.; Hov, B.; et al. Airway clearance techniques in neuromuscular disorders: A state of the art review. Respir. Med. 2018, 136, 98–110. [Google Scholar] [CrossRef]
- Panda, P.K.; Ramachandran, A.; Verma, P.K.; Sharawat, I.K. Behavioral problems in infants and young children with spinal muscular atrophy and their siblings: A cross-sectional study. Eur. J. Paediatr. Neurol. 2023, 42, 47–52. [Google Scholar] [CrossRef]
- Yao, M.; Xia, Y.; Feng, Y.; Ma, Y.; Hong, Y.; Zhang, Y.; Chen, J.; Yuan, C.; Mao, S. Anxiety and depression in school-age patients with spinal muscular atrophy: A cross-sectional study. Orphanet J. Rare Dis. 2021, 16, 385. [Google Scholar] [CrossRef]
- Evkaya Acar, A.; Karadağ Saygı, E.; İmamoğlu, S.; Öztürk, G.; Ünver, O.; Ergenekon, P.; Gökdemir, Y.; Özel, G.; Türkdoğan, D. The Burden of Primary Caregivers of Spinal Muscular Atrophy Patients and Their Needs. Turk. Arch. Pediatr. 2021, 56, 366–373. [Google Scholar] [CrossRef]
- Inhestern, L.; Brandt, M.; Driemeyer, J.; Denecke, J.; Johannsen, J.; Bergelt, C. Experiences of Health Care and Psychosocial Needs in Parents of Children with Spinal Muscular Atrophy. Int. J. Environ. Res. Public Health 2023, 20, 5360. [Google Scholar] [CrossRef]
- Grotto, S.; Cuisset, J.M.; Marret, S.; Drunat, S.; Faure, P.; Audebert-Bellanger, S.; Desguerre, I.; Flurin, V.; Grebille, A.G.; Guerrot, A.M.; et al. Type 0 Spinal Muscular Atrophy: Further Delineation of Prenatal and Postnatal Features in 16 Patients. J. Neuromuscul. Dis. 2016, 3, 487–495. [Google Scholar] [CrossRef]
- De Sanctis, R.; Pane, M.; Coratti, G.; Palermo, C.; Leone, D.; Pera, M.C.; Abiusi, E.; Fiori, S.; Forcina, N.; Fanelli, L.; et al. Clinical phenotypes and trajectories of disease progression in type 1 spinal muscular atrophy. Neuromuscul. Disord. 2018, 28, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Laufersweiler-Plass, C.; Rudnik-Schöneborn, S.; Zerres, K.; Backes, M.; Lehmkuhl, G.; von Gontard, A. Behavioural problems in children and adolescents with spinal muscular atrophy and their siblings. Dev. Med. Child Neurol. 2003, 45, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kriakous, S.A.; Elliott, K.A.; Lamers, C.; Owen, R. The Effectiveness of Mindfulness-Based Stress Reduction on the Psychological Functioning of Healthcare Professionals: A Systematic Review. Mindfulness 2021, 12, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ch’ng, G.S.; Koh, K.; Ahmad-Annuar, A.; Taib, F.; Koh, C.L.; Lim, E.S.C. A mixed method study on the impact of living with spinal muscular atrophy in Malaysia from patients’ and caregivers’ perspectives. Orphanet J. Rare Dis. 2022, 17, 200. [Google Scholar] [CrossRef]
- Schroth, M.; Deans, J.; Arya, K.; Castro, D.; De Vivo, D.C.; Gibbons, M.A.; Ionita, C.; Kuntz, N.L.; Lakhotia, A.; Neil Knierbein, E.; et al. Spinal Muscular Atrophy Update in Best Practices: Recommendations for Diagnosis Considerations. Neurol. Clin. Pract. 2024, 14, e200310. [Google Scholar] [CrossRef]

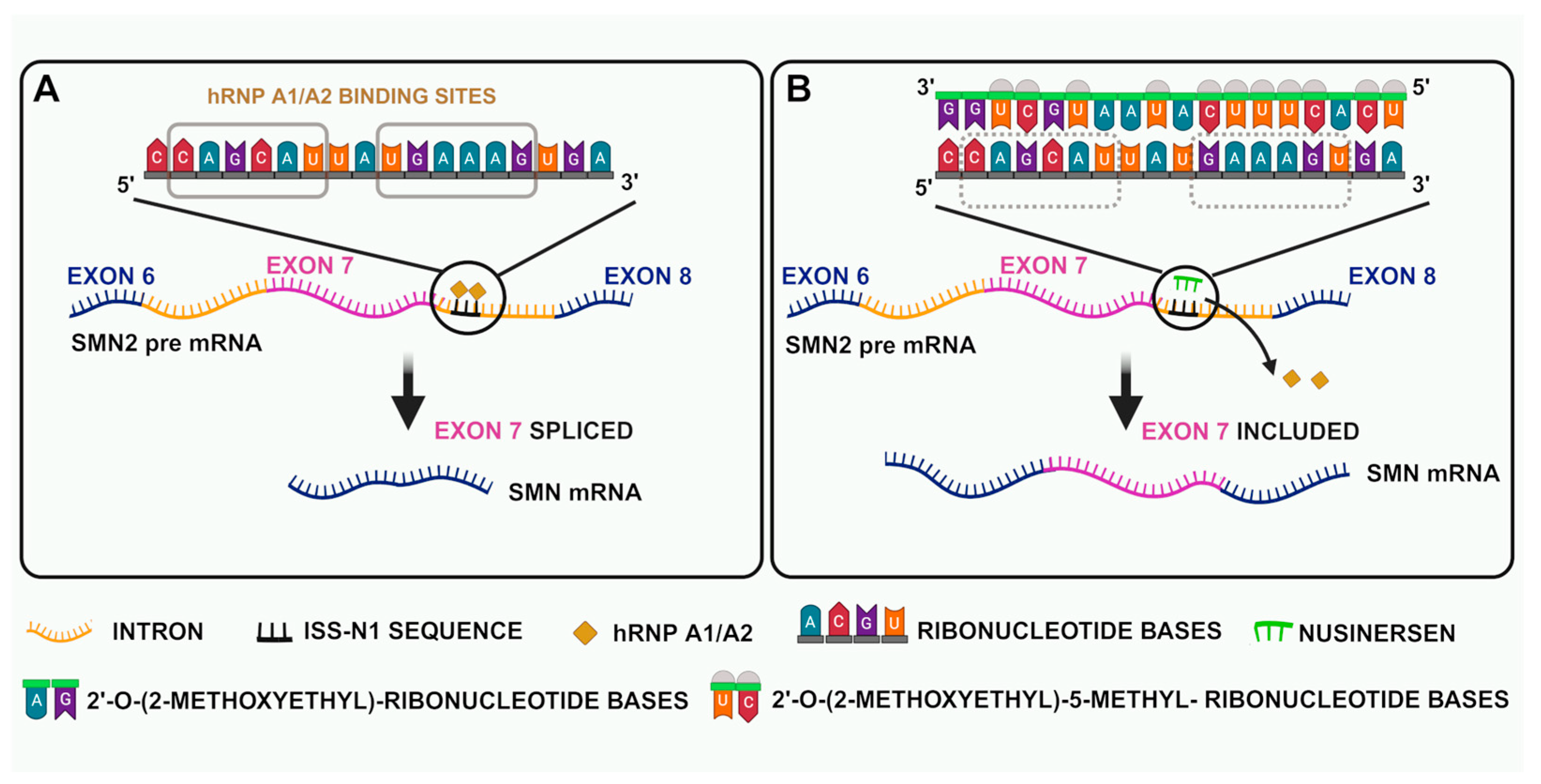
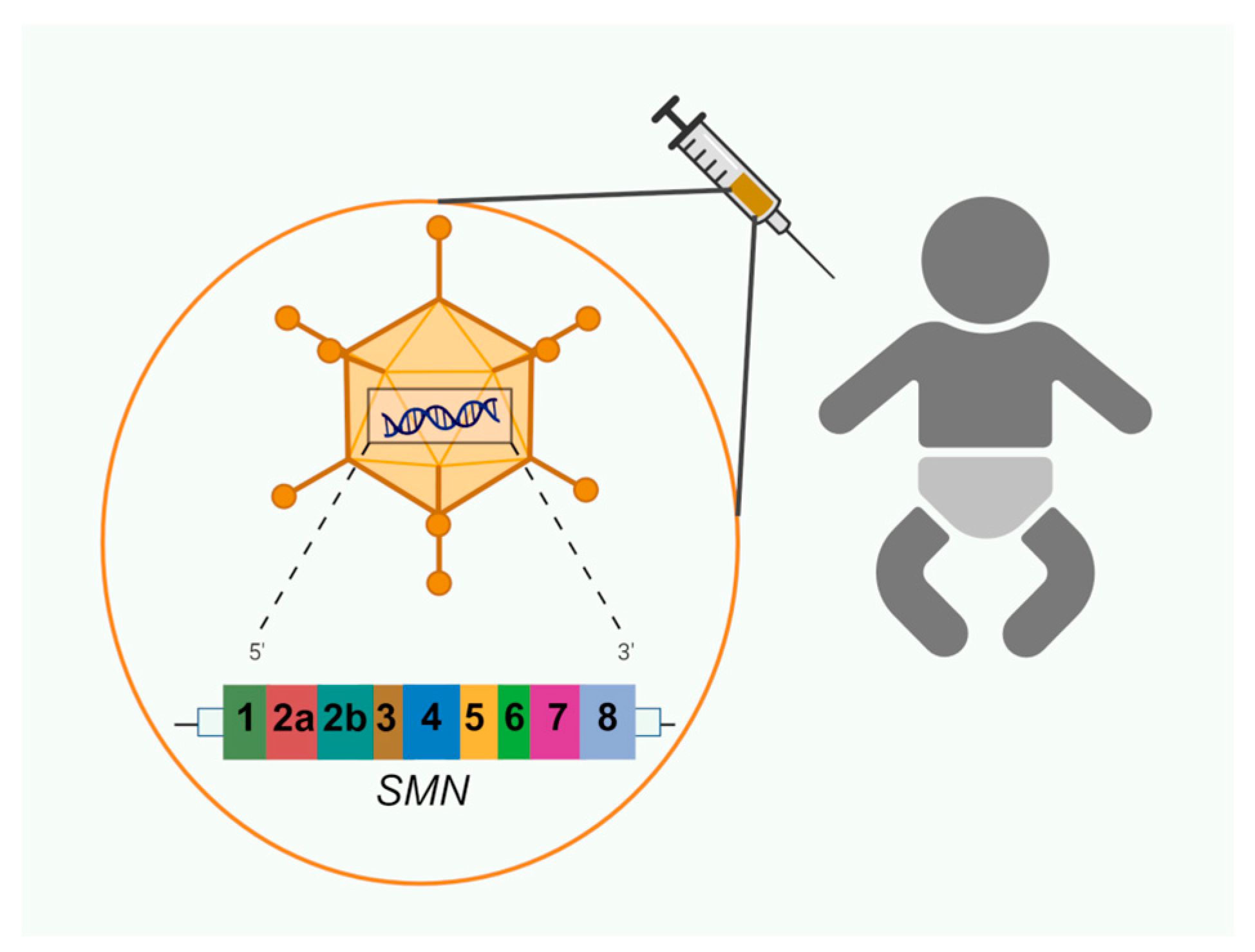
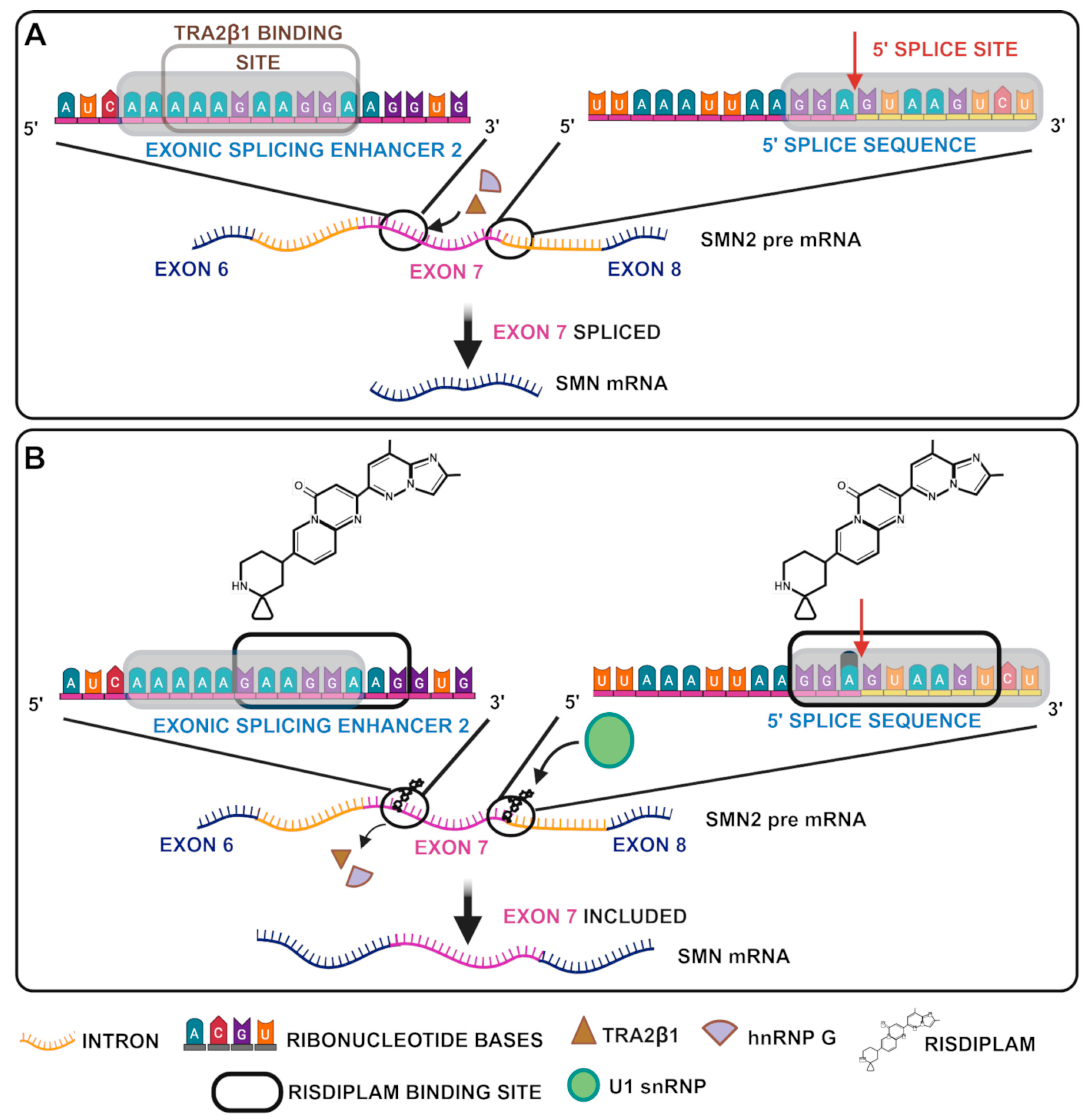
| Type | Age of Onset | Max Motor Milestone | Other Motor Features | Prognosis |
|---|---|---|---|---|
| SMA 1A SMA 1B SMA 1C | From before birth until 2 weeks after birth 3 months 6 months | None | Severe hypotonia, 1C may reach head control | Respiratory paucity at birth, death in the first 2 weeks for 1A and by 2 years for 1B and 1C; necessary ventilation |
| SMA 2A SMA 2B | 5 to 18 months | Sitting | Proximal weakness | Survival to adulthood |
| SMA 3A SMA 3B | >30 months | Walking | 3A may lose the ability to walk | Normal life span |
| SMA 4 | >30 years | Normal | Mild motor impairment | Normal life span |
| Treatment | Nusinersen | Onasemnogene Abeparvovec | Risdiplam |
|---|---|---|---|
| Synthetic description | Antisense oligonucleotide | Single-stranded SMN1 DNA inserted into an adeno-associated virus vector | Small molecule |
| Molecular action | Modifies the splicing in the SMN2 gene | Replace the SMN1 non-functioning gene | Modifies the splicing in the SMN2 gene |
| Approved for | SMA pediatric and adult | SMA 1 or up to 3 SMN2 gene copies, up to 21 kg | SMA 1, 2, or 3, or up to 4 SMN2 gene copies, pediatric and adult |
| Administration | Intrathecal | Slow intravenous infusion over 60 min | Enteral liquid (oral or feeding tube) |
| Frequency of administration | Four loading doses over 2 months, then every 4 months | Once | Daily |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vezzoli, A.; Bottai, D.; Adami, R. Managing Spinal Muscular Atrophy: A Look at the Biology and Treatment Strategies. Biology 2025, 14, 977. https://doi.org/10.3390/biology14080977
Vezzoli A, Bottai D, Adami R. Managing Spinal Muscular Atrophy: A Look at the Biology and Treatment Strategies. Biology. 2025; 14(8):977. https://doi.org/10.3390/biology14080977
Chicago/Turabian StyleVezzoli, Arianna, Daniele Bottai, and Raffaella Adami. 2025. "Managing Spinal Muscular Atrophy: A Look at the Biology and Treatment Strategies" Biology 14, no. 8: 977. https://doi.org/10.3390/biology14080977
APA StyleVezzoli, A., Bottai, D., & Adami, R. (2025). Managing Spinal Muscular Atrophy: A Look at the Biology and Treatment Strategies. Biology, 14(8), 977. https://doi.org/10.3390/biology14080977








