Integrated Multi-Omics Analysis Reveals Stage-Specific Molecular Modules Regulating Uterine Function and Fecundity in Large White Pigs Across Reproductive Lifespan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. RNA Library Preparation and Transcriptome Sequencing
2.3. Proteome Detection and Analysis
2.4. LCMS Non-Targeted Metabolomics Analysis
2.5. Multi-Omics Integrated Analysis
2.6. Real-Time PCR Quantification of mRNAs
3. Results
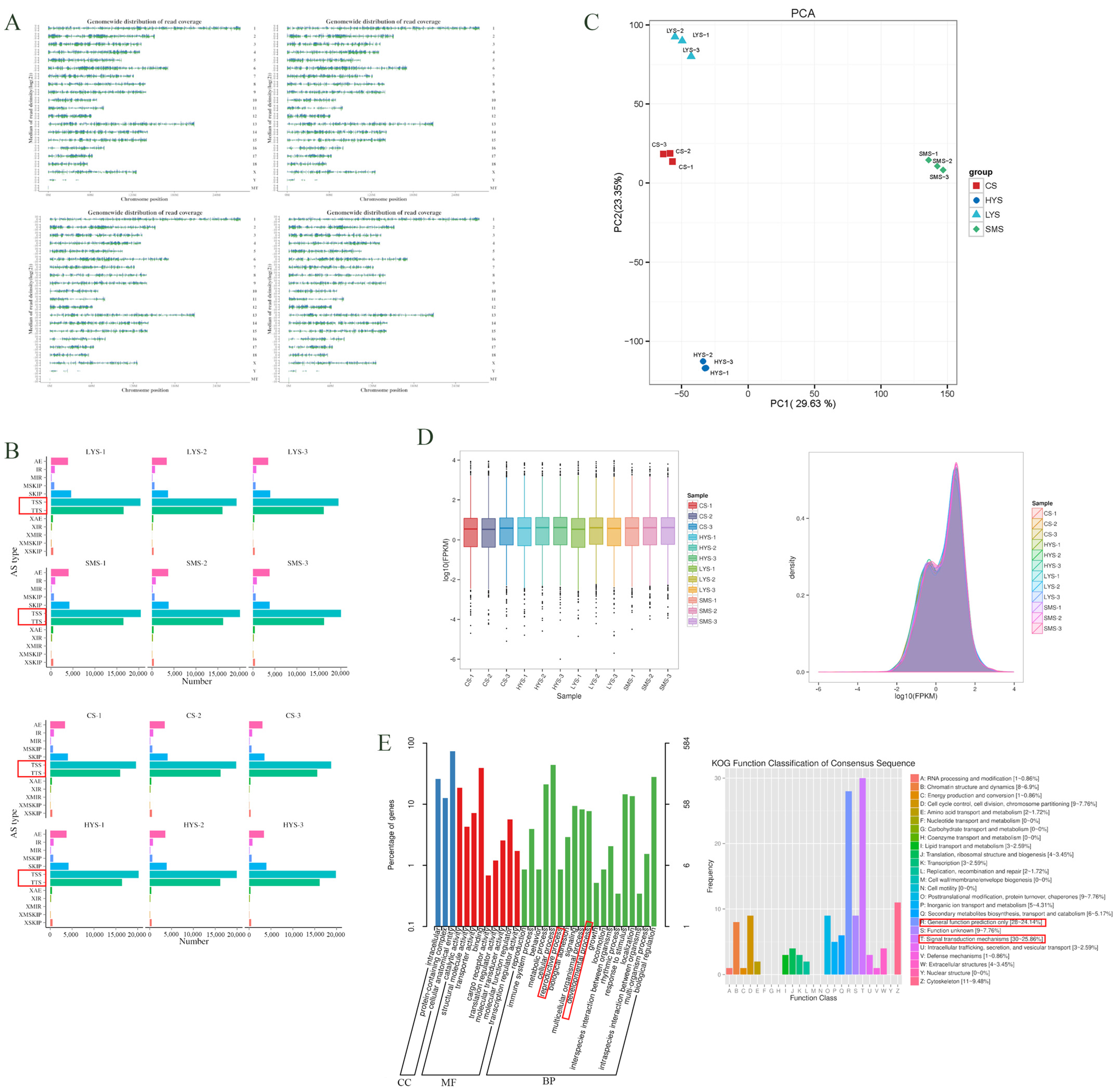
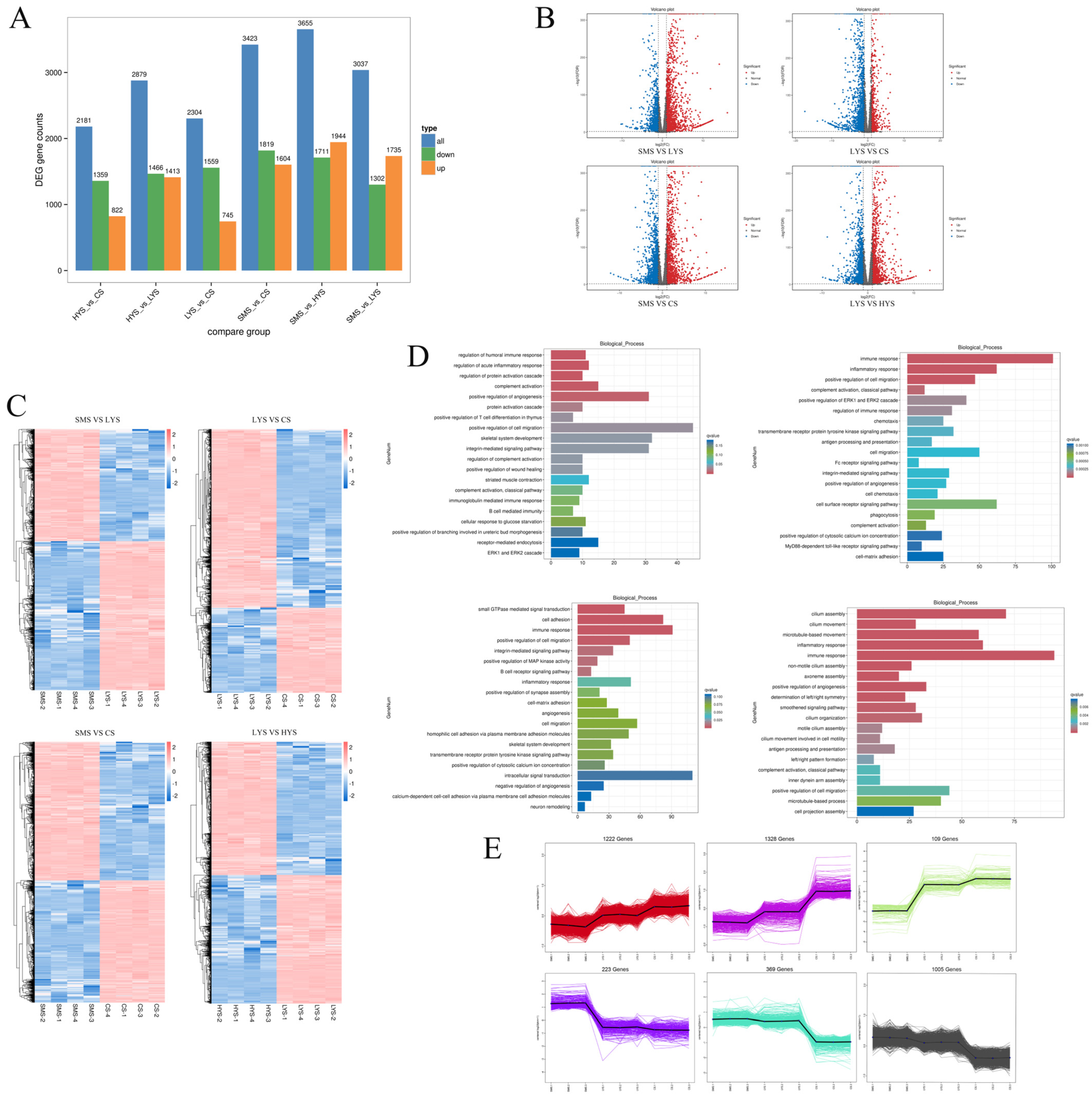
3.1. Analysis of Transcriptome Sequencing Data
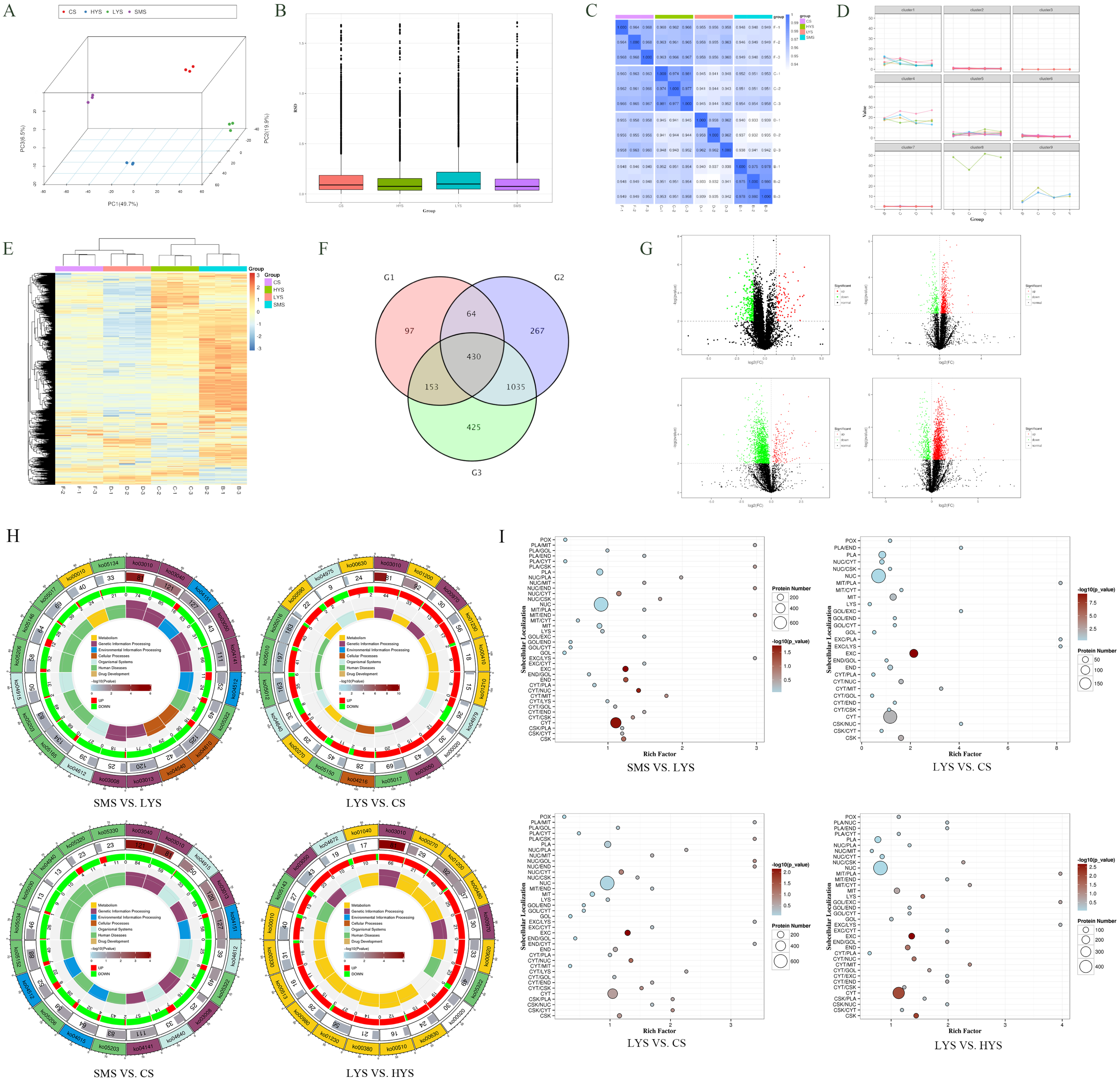
3.2. Analysis of Differentially Accumulated Proteins (DAPs)
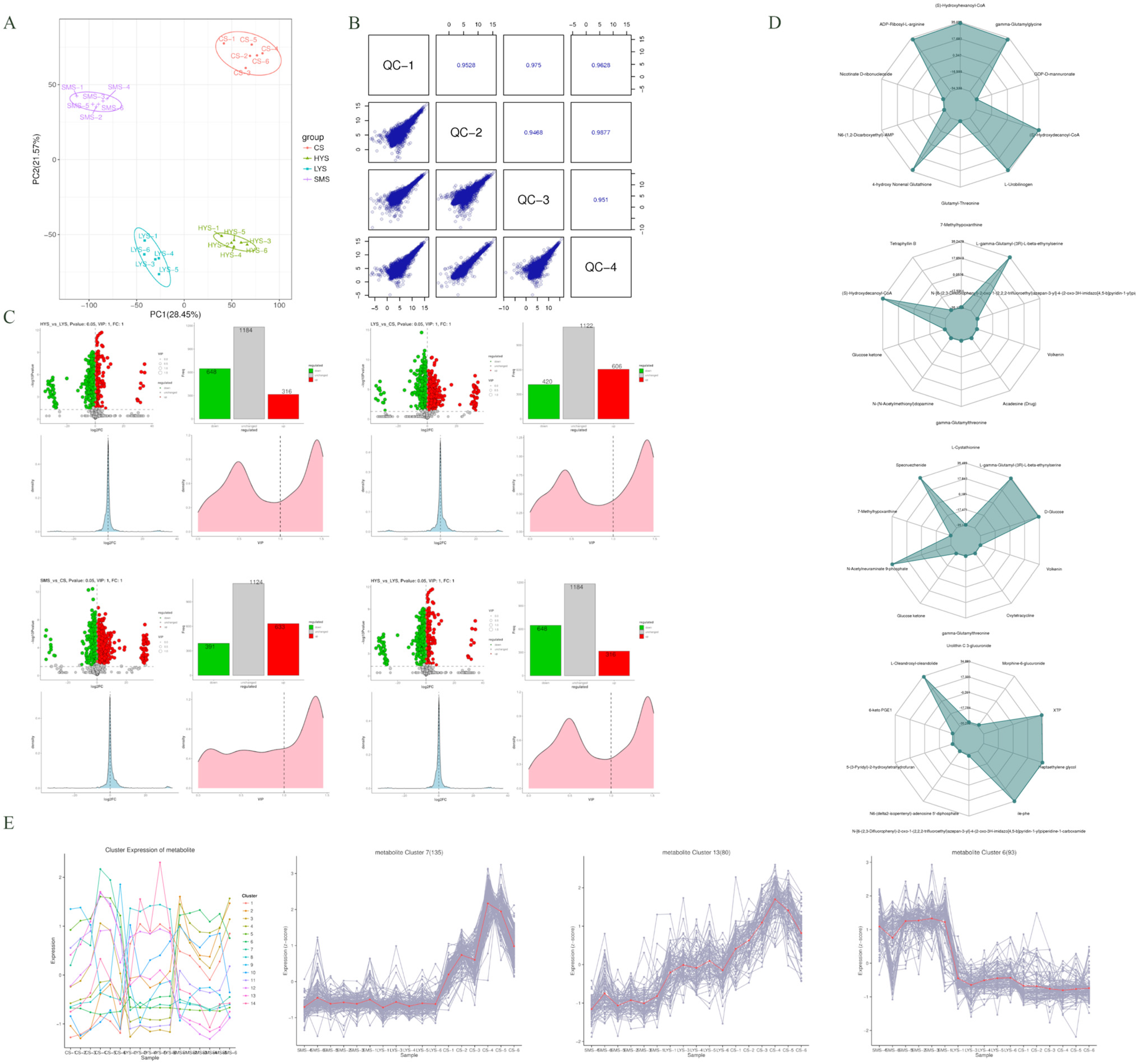
3.3. Analysis of Differential Metabolites
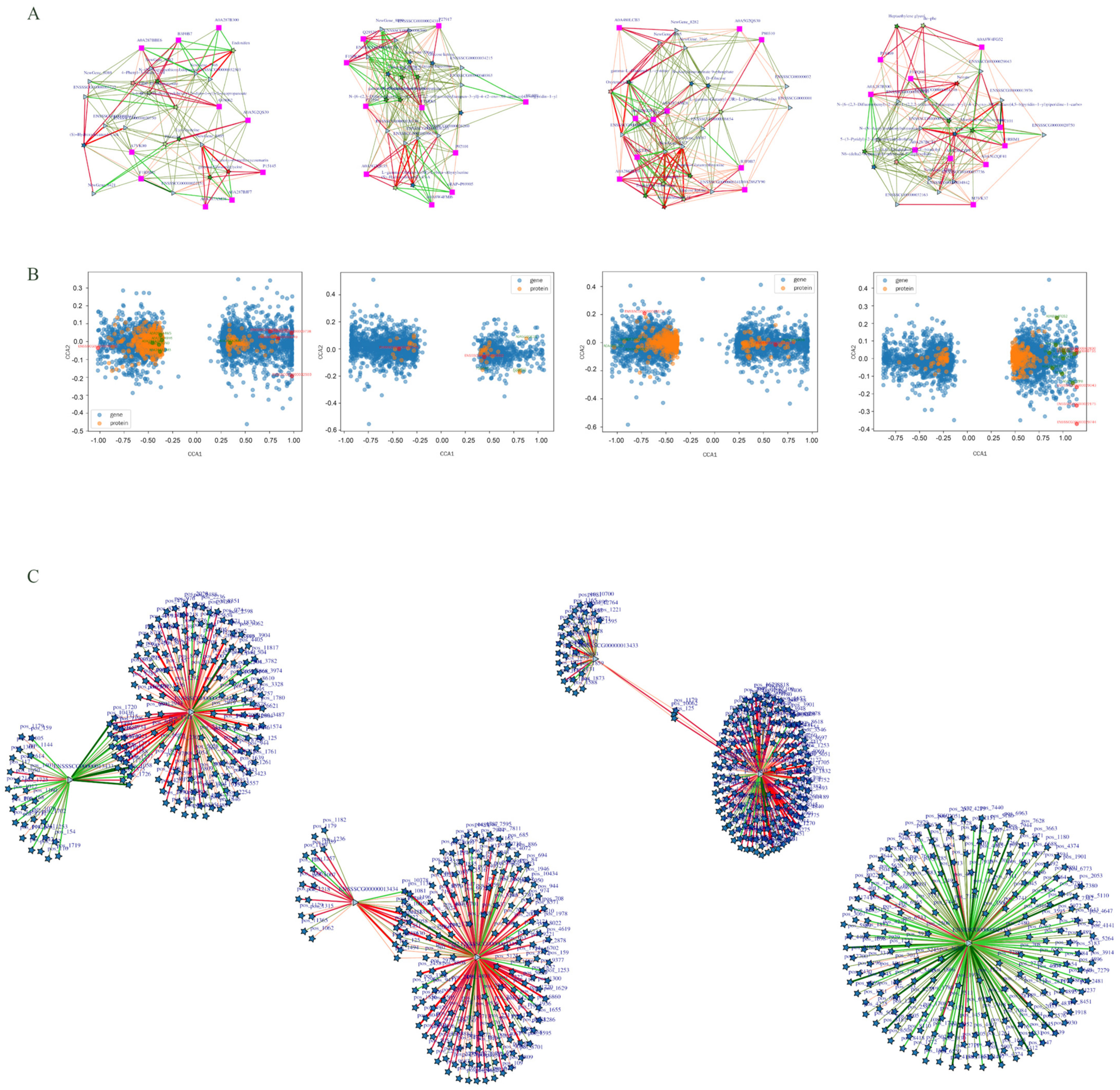
3.4. Multi-Omics Integrated Analysis
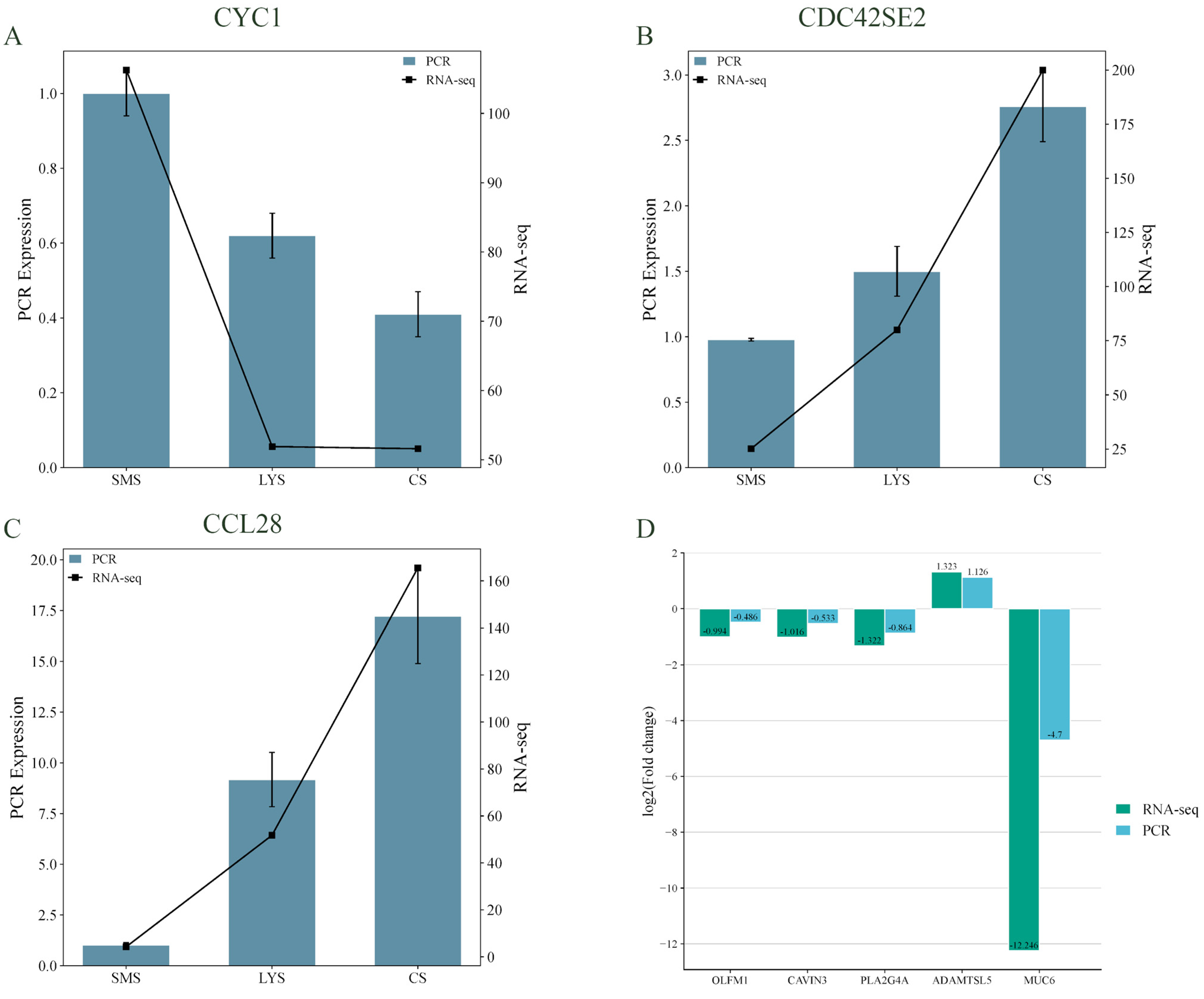
3.5. RT-qPCR Quantification of mRNAs
4. Discussion
4.1. Transcriptomic Profiles Reveal Core Pathways of Dynamic Uterine Function Regulation
4.2. Proteomic Differences Reflect the Structural and Functional Fit of the Uterine Functional State
4.3. Metabolomic Characteristics Reveal the Material Basis for Maintaining Uterine Function
4.4. Multi-Omics Joint Analysis of the Molecular Network Regulating Uterine Function
4.5. Research Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; He, Y.; Ke, J.; Zhang, X.; Fei, J.; Sun, B.; Sun, H.; Bai, C. Genomic Analysis of Reproductive Trait Divergence in Duroc and Yorkshire Pigs: A Comparison of Mixed Models and Selective Sweep Detection. Vet. Sci. 2025, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, N.N. FAO Animal Production and Health Paper. Available online: https://www.fao.org/statistics/data-dissemination/agrifood-systems/en (accessed on 8 June 2025).
- Wang, C.; Wu, Y.; Shu, D.; Wei, H.; Zhou, Y.; Peng, J. An Analysis of Culling Patterns during the Breeding Cycle and Lifetime Production from the Aspect of Culling Reasons for Gilts and Sows in Southwest China. Animals 2019, 9, 160. [Google Scholar] [CrossRef]
- Chelenga, M.; Sakaguchi, K.; Kawano, K.; Furukawa, E.; Yanagawa, Y.; Katagiri, S.; Nagano, M. Low Oxygen Environment and Astaxanthin Supplementation Promote the Developmental Competence of Bovine Oocytes Derived from Early Antral Follicles during 8 Days of in Vitro Growth in a Gas-Permeable Culture Device. Theriogenology 2022, 177, 116–126. [Google Scholar] [CrossRef]
- Huang, J.; Liu, R.; Su, L.; Xiao, Q.; Yu, M. Transcriptome Analysis Revealed the Embryo-Induced Gene Expression Patterns in the Endometrium from Meishan and Yorkshire Pigs. Int. J. Mol. Sci. 2015, 16, 22692–22710. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Wu, Q.; Gao, N.; Wang, Z.; Yang, Y.; Shan, A. Exopolysaccharides of Lactobacillus rhamnosus GG Ameliorate Salmonella typhimurium-Induced Intestinal Inflammation via the TLR4/NF-κB/MAPK Pathway. J. Anim. Sci. Biotechnol. 2023, 14, 23. [Google Scholar] [CrossRef]
- Cushman, R.A.; Akbarinejad, V.; Perry, G.A.; Lents, C.A. Developmental Programming of the Ovarian Reserve in Livestock. Anim. Reprod. Sci. 2024, 264, 107458. [Google Scholar] [CrossRef]
- Yang, K.; Wang, J.; Wang, K.; Luo, Y.; Fang, M. Integrated Analysis of miRNA-mRNA Network Reveals Different Regulatory Patterns in the Endometrium of Meishan and Duroc Sows during Mid-Late Gestation. Animals 2020, 10, 420. [Google Scholar] [CrossRef]
- Blitek, A.; Kiewisz, J.; Waclawik, A.; Kaczmarek, K.M.; Ziecik, A.J. Effect of steroids on HOXA10 mRNA and protein expression and prostaglandin production in the porcine endometrium. J. Reprod. Dev. 2010, 56, 643–648. [Google Scholar] [CrossRef]
- Liu, B.; Duan, L.; Liu, X.; Wang, X. 43 Metabolomic Analysis of Uterine Luminal Fluid during the Peri-Implantation Period of Pregnancy in Pigs. J. Anim. Sci. 2023, 101, 40–41. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Hou, Y. BOARD-INVITED REVIEW: Arginine Nutrition and Metabolism in Growing, Gestating, and Lactating Swine. J. Anim. Sci. 2018, 96, 5035–5051. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Kumar, S. Whole-Genome Uterine Artery Transcriptome Profiling and Alternative Splicing Analysis in Rat Pregnancy. Int. J. Mol. Sci. 2020, 21, 2079. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, Y.; Egecioglu, E.; Li, X.; Shao, L.R.; Billig, H. Uterine Glycolytic Enzyme Expression Is Affected by Knockout of Different Estrogen Receptor Subtypes. Biomed. Rep. 2019, 11, 135–144. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.-P.; DeMayo, F.J. Hormone Dependent Uterine Epithelial-Stromal Communication for Pregnancy Support. Placenta 2017, 60, S20–S26. [Google Scholar] [CrossRef]
- Winkler, I.; Tolkachov, A.; Lammers, F.; Lacour, P.; Daugelaite, K.; Schneider, N.; Koch, M.-L.; Panten, J.; Grünschläger, F.; Poth, T.; et al. The Cycling and Aging Mouse Female Reproductive Tract at Single-Cell Resolution. Cell 2024, 187, 981–998.e25. [Google Scholar] [CrossRef]
- Weberling, A.; Zernicka-Goetz, M. Trophectoderm Mechanics Direct Epiblast Shape upon Embryo Implantation. Cell Rep. 2021, 34, 108655. [Google Scholar] [CrossRef]
- Baumann, M.U.; Schneider, H.; Malek, A.; Palta, V.; Surbek, D.V.; Sager, R.; Zamudio, S.; Illsley, N.P. Regulation of Human Trophoblast GLUT1 Glucose Transporter by Insulin-like Growth Factor I (IGF-I). PLoS ONE 2014, 9, e106037. [Google Scholar] [CrossRef]
- Kurusu, S.; Sapirstein, A.; Bonventre, J.V. Group IVA Phospholipase A2 Optimizes Ovulation and Fertilization in Rodents through Induction of and Metabolic Coupling with Prostaglandin Endoperoxide Synthase 2. FASEB J. 2012, 26, 3800–3810. [Google Scholar] [CrossRef]
- Kalhan, S.C. Protein Metabolism in Pregnancy1234. Am. J. Clin. Nutr. 2000, 71, 1249S–1255S. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Feng, L. Disrupted Metabolic Signatures in Amniotic Fluid Associated with Increased Risk of Intestinal Inflammation in Cesarean Section Offspring. Front. Immunol. 2023, 14, 1067602. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, X.; Guo, X.; Pan, G.; Yan, L.; Ding, Z.; Li, R.; Wang, D.; Yan, Y.; Dong, Z.; et al. DAP3 Promotes Mitochondrial Activity and Tumour Progression in Hepatocellular Carcinoma by Regulating MT-ND5 Expression. Cell Death Dis. 2024, 15, 540. [Google Scholar] [CrossRef]
- Li, J.-W.; Hu, J.; Wei, M.; Guo, Y.-Y.; Yan, P.-S. The Effects of Maternal Obesity on Porcine Placental Efficiency and Proteome. Animals 2019, 9, 546. [Google Scholar] [CrossRef]
- Siavoshi, A.; Taghizadeh, M.; Dookhe, E.; Piran, M. Gene Expression Profiles and Pathway Enrichment Analysis to Identification of Differentially Expressed Gene and Signaling Pathways in Epithelial Ovarian Cancer Based on High-Throughput RNA-Seq Data. Genomics 2022, 114, 161–170. [Google Scholar] [CrossRef]
- Suzuki, N.; Zara, J.; Sato, T.; Ong, E.; Bakhiet, N.; Oshima, R.G.; Watson, K.L.; Fukuda, M.N. A Cytoplasmic Protein, Bystin, Interacts with Trophinin, Tastin, and Cytokeratin and May Be Involved in Trophinin-Mediated Cell Adhesion between Trophoblast and Endometrial Epithelial Cells. Proc. Natl. Acad. Sci. USA 1998, 95, 5027–5032. [Google Scholar] [CrossRef]
- Bajic, V.P.; Van Neste, C.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, 5028181. [Google Scholar] [CrossRef]
- Liu, H.; Liu, L.; Han, H.; Liu, K.; Wang, H. Panel of Suitable Reference Genes and Its Gender Differences of Fetal Rat Liver under Physiological Conditions and Exposure to Dexamethasone during Pregnancy. Reprod. Toxicol. 2021, 100, 74–82. [Google Scholar] [CrossRef]
- Ješeta, M.; Celá, A.; Žáková, J.; Mádr, A.; Crha, I.; Glatz, Z.; Kempisty, B.; Ventruba, P. Metabolic Activity of Human Embryos after Thawing Differs in Atmosphere with Different Oxygen Concentrations. J. Clin. Med. 2020, 9, 2609. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Z.; Sun, Y.; Yin, S.; Tang, M.; Zhang, F. Targeting the Key Enzymes of Abnormal Fatty Acid β-Oxidation as a Potential Strategy for Tumor Therapy. Front. Biosci. (Landmark Ed.) 2022, 27, 95. [Google Scholar] [CrossRef]
- Pagano, D.; Cutignano, A.; Manzo, E.; Tinto, F.; Fontana, A. Glycolipids Synthesis: Improved Hydrazinolysis Conditions for Preparation of 1,2-Polyunsaturated Fatty Acyl-β-Monogalactosyl-Glycerols. Carbohydr. Res. 2016, 424, 21–23. [Google Scholar] [CrossRef]
- Haghiac, M.; Yang, X.; Presley, L.; Smith, S.; Dettelback, S.; Minium, J.; Belury, M.A.; Catalano, P.M.; Mouzon, S.H. Dietary Omega-3 Fatty Acid Supplementation Reduces Inflammation in Obese Pregnant Women: A Randomized Double-Blind Controlled Clinical Trial. PLoS ONE 2015, 10, e0137309. [Google Scholar] [CrossRef]
- Jiang, W.; Kumar, J.M.; Matters, G.L.; Bond, J.S. Structure of the Mouse Metalloprotease Meprin β Gene (Mep1b): Alternative Splicing in Cancer Cells. Gene 2000, 248, 77–87. [Google Scholar] [CrossRef]
- Wang, Y.; Hussein, A.M.; Somasundaram, L.; Sankar, R.; Detraux, D.; Mathieu, J.; Ruohola-Baker, H. microRNAs Regulating Human and Mouse Naïve Pluripotency. Int. J. Mol. Sci. 2019, 20, 5864. [Google Scholar] [CrossRef]
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 Proteins as an Important Regulator of Macrophage Inflammation. Front. Immunol. 2018, 8, 1908. [Google Scholar] [CrossRef]
| Genes | Primer | Length (bp) |
|---|---|---|
| CYC1 | F: GGCTGAGGAGGTGGAGGTTCAA | 163 |
| R: TCGCACGATGTAGCTGAGGTCA | ||
| CDC42SE2 | F: TCGGTCAGCTCCATCCAGAACC | 100 |
| R: ATCCTGCCTTCGTGTCCACAAG | ||
| CCL28 | F: GCTGCTGCACTGAGGTTTCACA | 80 |
| R: ATCCGCTCTCTGAAGGCGACAT | ||
| OLFM1 | F: ACCTGAAGACCGAGAGCATCCT | 150 |
| R: CGTTCTGGTTGGTGGCGTAGAC | ||
| CAVIN3 | F: CCACGACACGACGAGCAACA | 186 |
| R: TCAGCCTCCTCCTTGAAGAGCA | ||
| PLA2G4A | F: TGGTGGACAGTGGCCTCACATT | 121 |
| R: AACGGAGGACTGGAGTCGCTTG | ||
| ADAMTSL5 | F: CCACTATTGCGGCAGCGACTT | 118 |
| R: CAGAGGCGAGCGGTTCTTGTAG | ||
| MUC6 | F: ACTCCGCACTGGACCGAAGAA | 130 |
| R: CCGTTCCGCTGGTGGTTGTT | ||
| GAPDH | F: GGTGAAGGTCGGAGTGAACG | 195 |
| R: CCTGGAAGATGGTGATGGGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Yang, F.; Liu, J.; Yi, L.; Liufu, S.; Wang, K.; Gong, Y.; Li, Z.; Ma, H. Integrated Multi-Omics Analysis Reveals Stage-Specific Molecular Modules Regulating Uterine Function and Fecundity in Large White Pigs Across Reproductive Lifespan. Biology 2025, 14, 1589. https://doi.org/10.3390/biology14111589
Chen W, Yang F, Liu J, Yi L, Liufu S, Wang K, Gong Y, Li Z, Ma H. Integrated Multi-Omics Analysis Reveals Stage-Specific Molecular Modules Regulating Uterine Function and Fecundity in Large White Pigs Across Reproductive Lifespan. Biology. 2025; 14(11):1589. https://doi.org/10.3390/biology14111589
Chicago/Turabian StyleChen, Wenwu, Fang Yang, Jingwen Liu, Lei Yi, Sui Liufu, Kaiming Wang, Yan Gong, Zhi Li, and Haiming Ma. 2025. "Integrated Multi-Omics Analysis Reveals Stage-Specific Molecular Modules Regulating Uterine Function and Fecundity in Large White Pigs Across Reproductive Lifespan" Biology 14, no. 11: 1589. https://doi.org/10.3390/biology14111589
APA StyleChen, W., Yang, F., Liu, J., Yi, L., Liufu, S., Wang, K., Gong, Y., Li, Z., & Ma, H. (2025). Integrated Multi-Omics Analysis Reveals Stage-Specific Molecular Modules Regulating Uterine Function and Fecundity in Large White Pigs Across Reproductive Lifespan. Biology, 14(11), 1589. https://doi.org/10.3390/biology14111589






