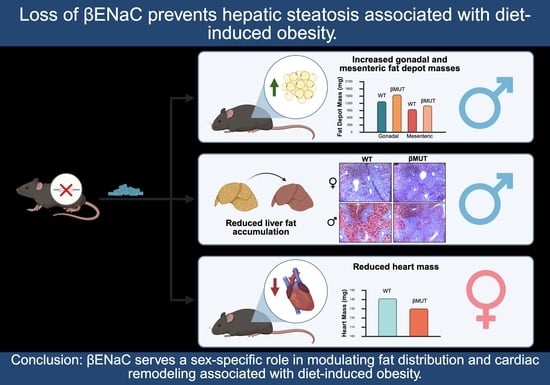
Loss of βENaC Prevents Hepatic Steatosis but Promotes Abdominal Fat Deposition Associated with a High-Fat Diet
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
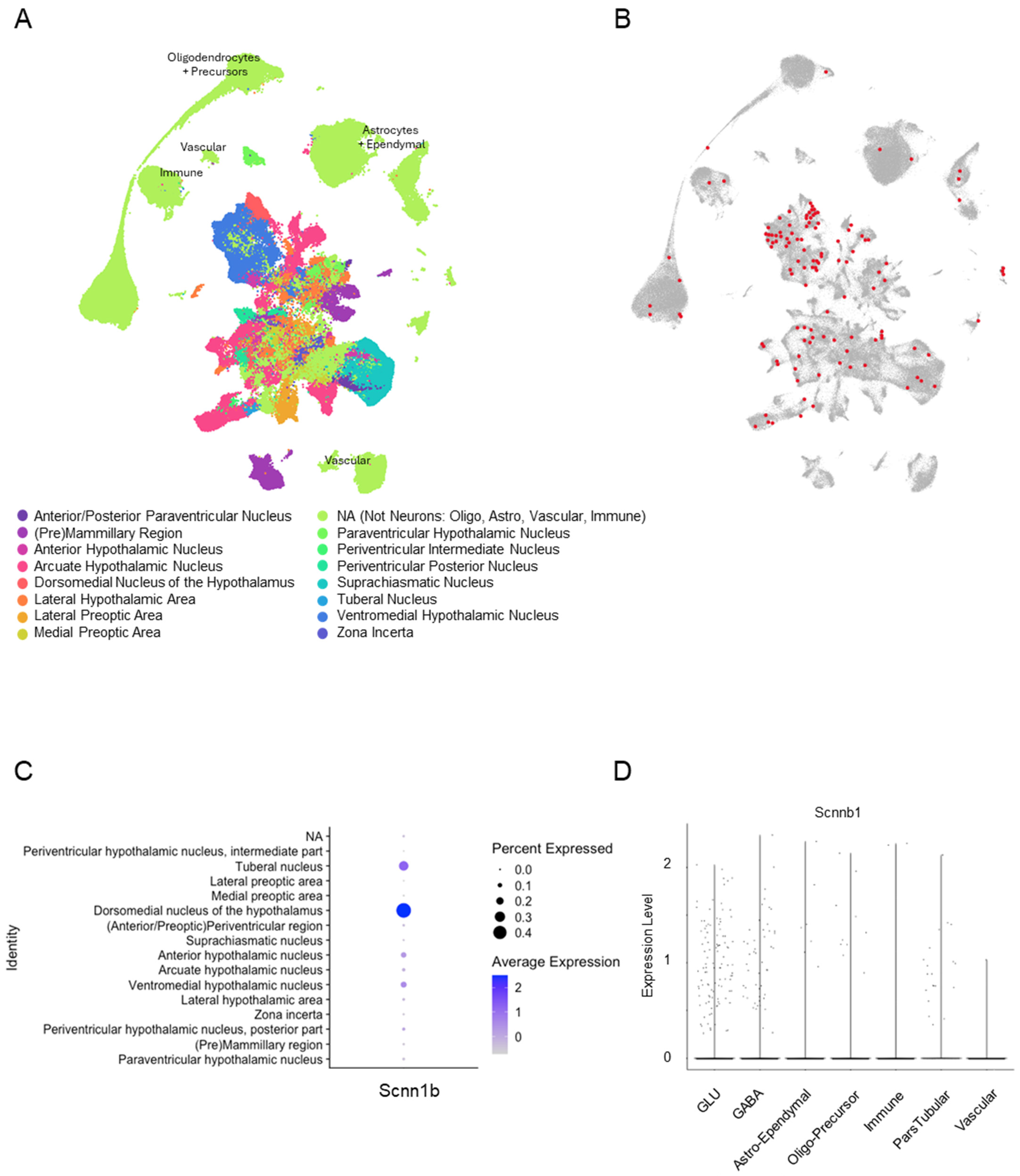
3.1. βENaC Is Expressed in the Murine Hypothalamus
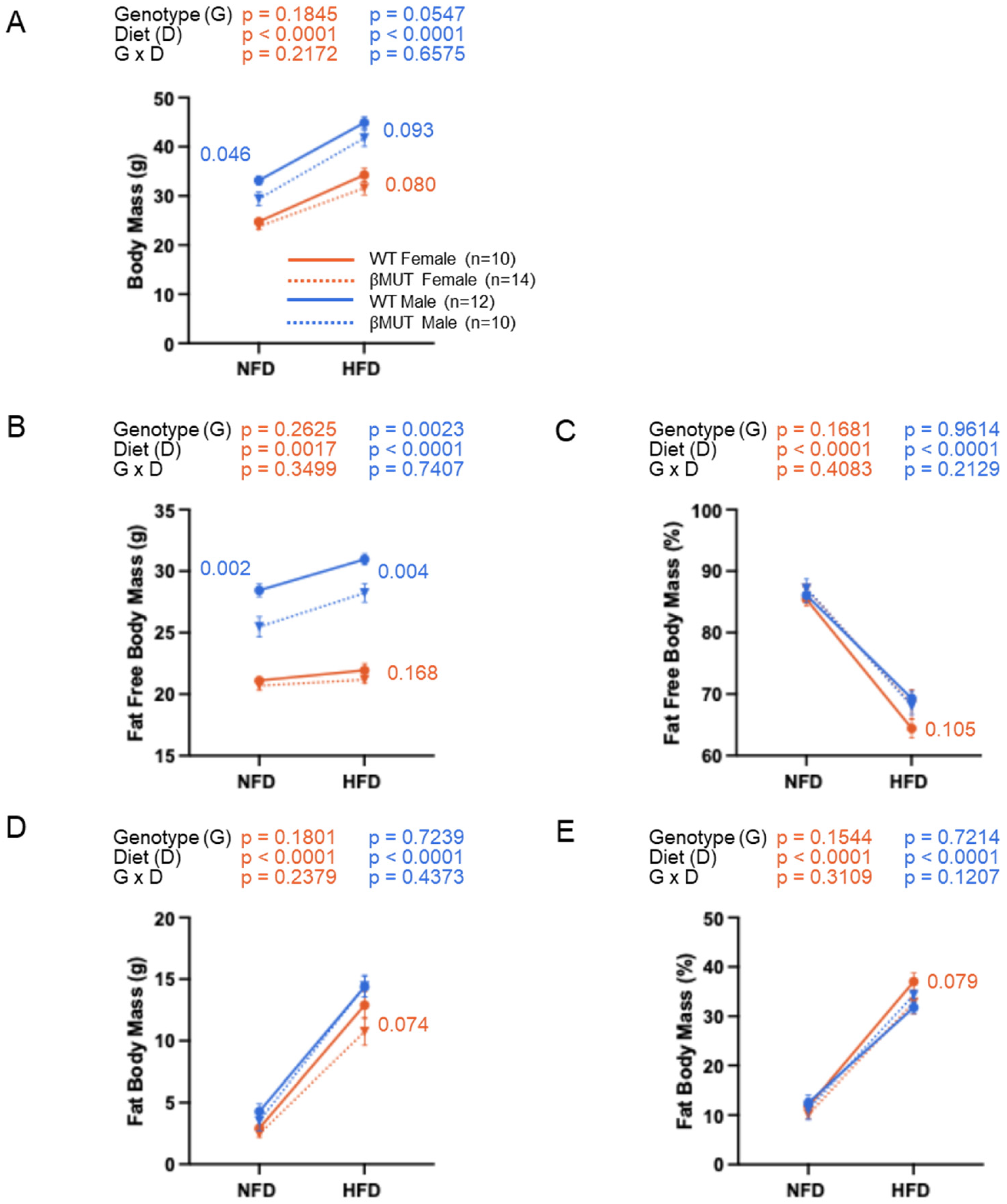
3.2. Male βMUT Mice Weigh Less than WT Controls
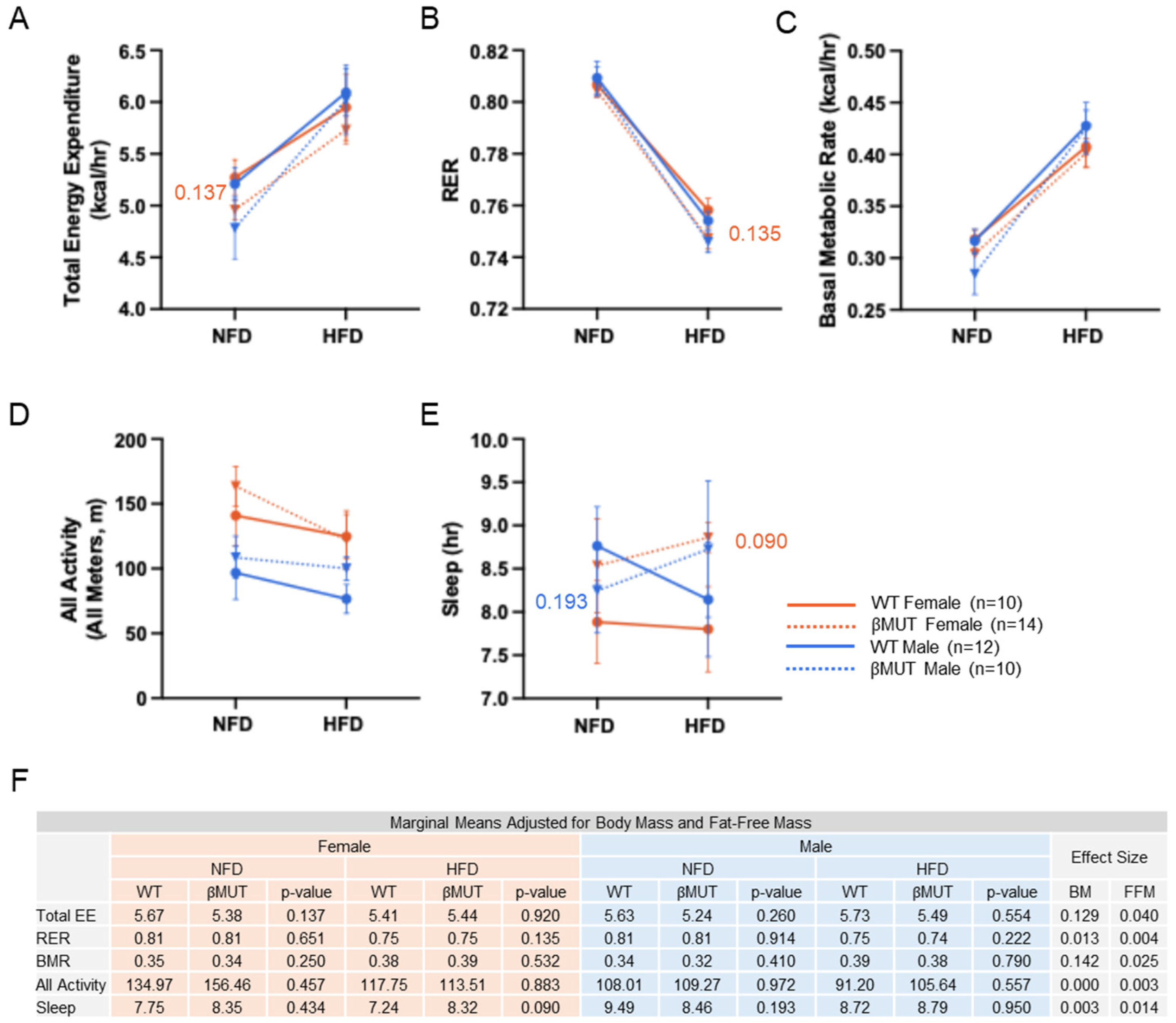
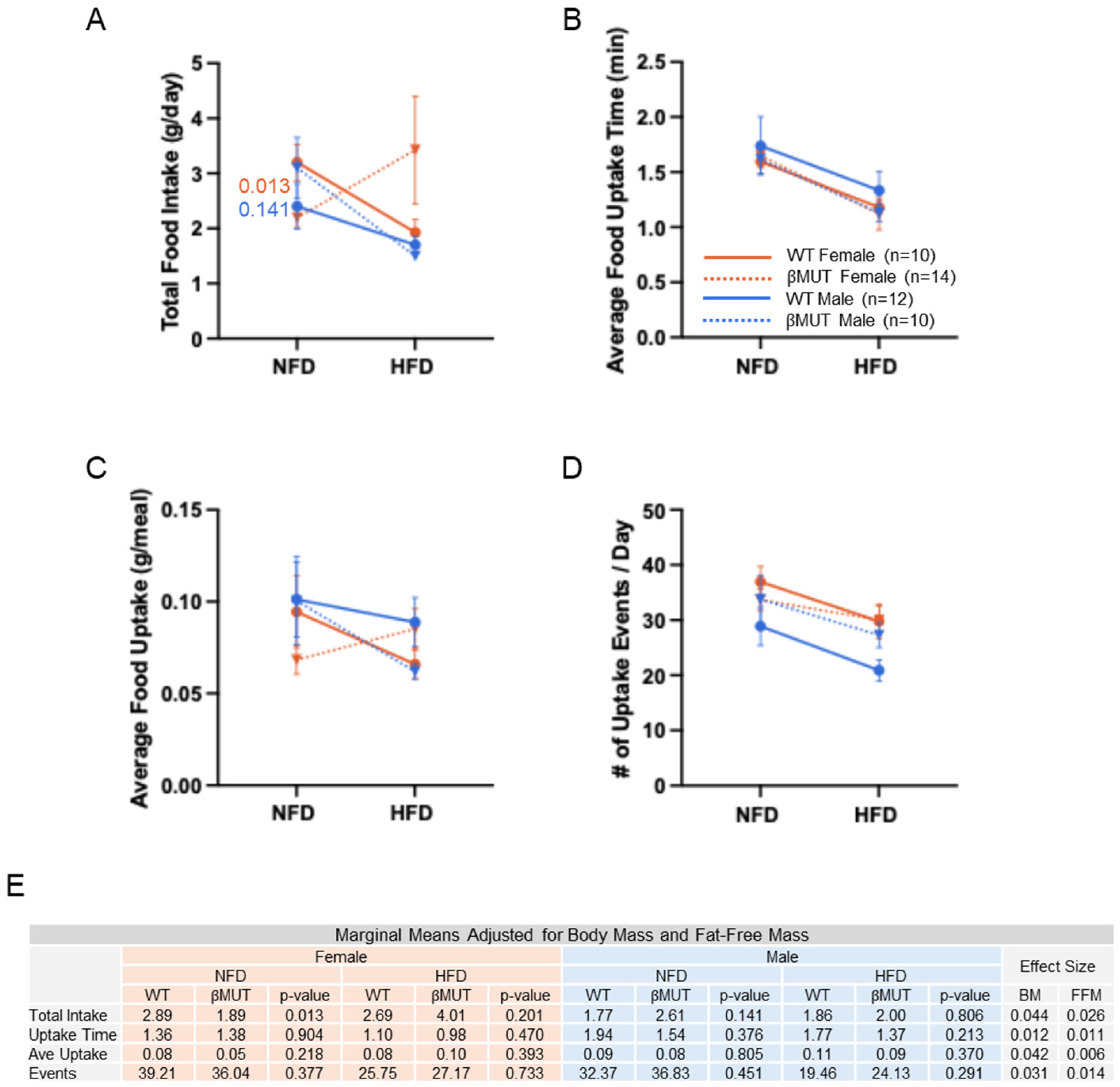
3.3. Metabolic Indices on Normal Chow and HFD Were Mostly Similar Between βMUT and WT Controls
3.4. Female βMUT Mice Tend to Eat Less Food on Normal Chow
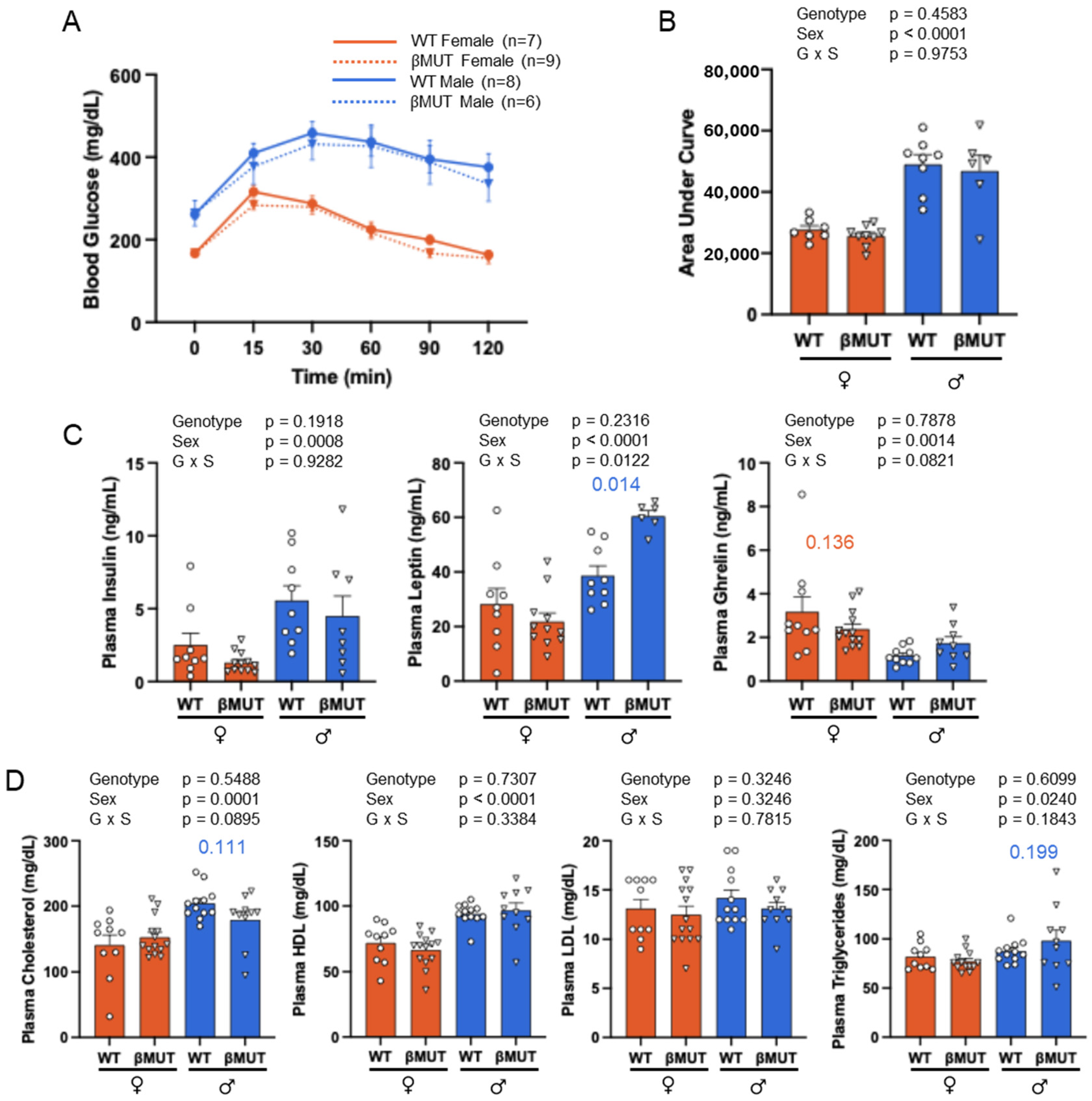
3.5. Glucose Tolerance and Plasma Hormones and Lipids Following HFD
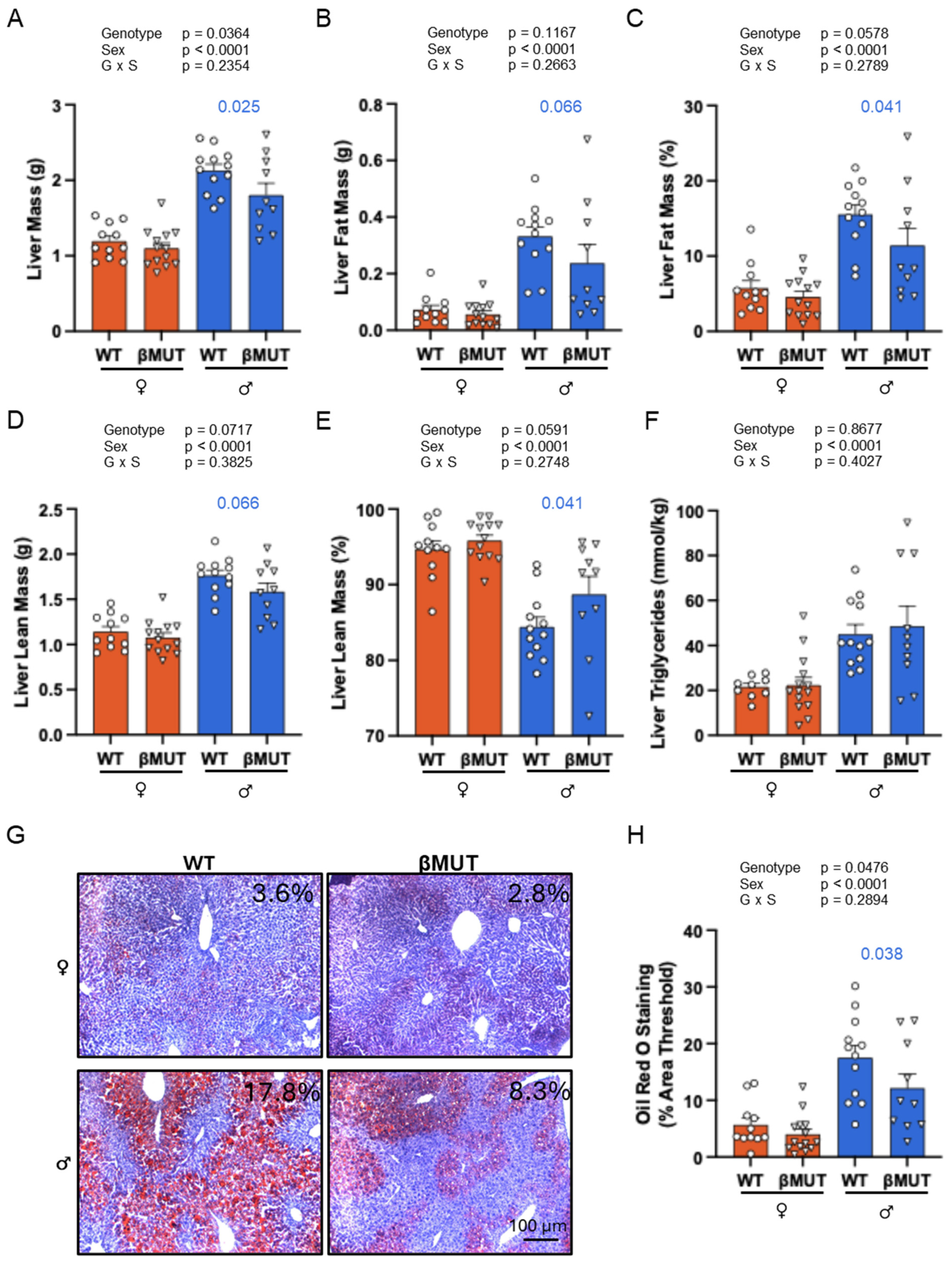
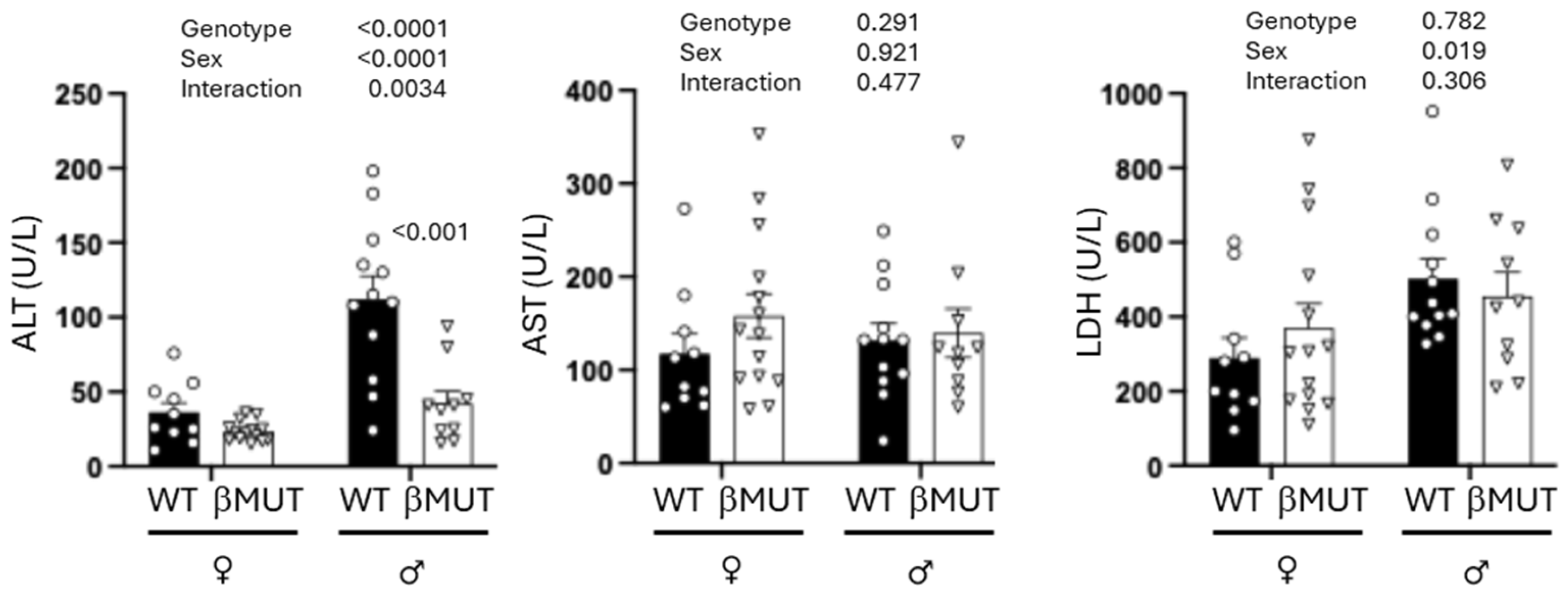
3.6. βMUT Males Tend to Develop Less Hepatic Steatosis Following a 5-Week HFD
3.7. βMUT Females Exhibit Sex-Dependent Differences in Select Organ Masses and Males Have Larger Abdominal Fat Depots Following a 5-Week HFD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| βENaC | Beta subunit of the Epithelial Sodium Channel |
| SCNN1a/SCNN1b/SCNN1g | Gene name for αENaC, βENaC, and γENaC |
| Asic2 | Acid-Sensing Ion Channel 2 |
| Accn1/Accn2/Accn3 | Gene name for Asic2, Asic1 and Asic3 |
| NFD | Normal-fat diet |
| HFD | High-fat diet |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| WT | Wildtype |
| βMUT | βENaC hypomorph model |
| SEM | Standard error of the mean |
| ANOVA/ANCOVA | Analysis of variance/covariance |
References
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Or, B.; Tsoi, M.F.; Cheung, C.L.; Cheung, B.M.Y. Prevalence of metabolic syndrome in the United States National Health and Nutrition Examination Survey 2011-18. Postgrad. Med. J. 2023, 99, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, X.; Sabharwal, R.; Snitsarev, V.; Morgan, D.; Rahmouni, K.; Drummond, H.A.; Whiteis, C.A.; Costa, V.; Price, M.; et al. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 2009, 64, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, N.R.; Foss, J.D.; Kryshtal, D.O.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W.; et al. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017, 21, 1009–1020. [Google Scholar] [CrossRef]
- Ertuglu, L.A.; Mutchler, A.P.; Jamison, S.; Laffer, C.L.; Elijovich, F.; Saleem, M.; Blackwell, D.J.; Kryshtal, D.O.; Egly, C.L.; Sahinoz, M.; et al. Eicosanoid-Regulated Myeloid ENaC and Isolevuglandin Formation in Human Salt-Sensitive Hypertension. Hypertension 2024, 81, 516–529. [Google Scholar] [CrossRef]
- Kellenberger, S.; Schild, L. Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol. Rev. 2002, 82, 735–767. [Google Scholar] [CrossRef]
- Wasson, R.; Fleming, A.B.; McLin, J.L.; Hildebrandt, E.; Drummond, H.A. Bone marrow monocytes and macrophages from mice lacking betaENaC and ASIC2 have a reduced chemotactic migration response and polarization. Physiol. Rep. 2024, 12, e16139. [Google Scholar] [CrossRef]
- Nemeth, Z.; Hildebrandt, E.; Parsa, N.; Fleming, A.B.; Wasson, R.; Pittman, K.; Bell, X.; Granger, J.P.; Ryan, M.J.; Drummond, H.A. Epithelial sodium channels in macrophage migration and polarization: Role of proinflammatory cytokines TNFalpha and IFNgamma. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R763–R775. [Google Scholar] [CrossRef]
- Storozhuk, M.; Cherninskyi, A.; Maximyuk, O.; Isaev, D.; Krishtal, O. Acid-Sensing Ion Channels: Focus on Physiological and Some Pathological Roles in the Brain. Curr. Neuropharmacol. 2021, 19, 1570–1589. [Google Scholar]
- Tong, J.; Wu, W.-N.; Kong, X.; Wu, P.-F.; Tian, L.; Du, W.; Fang, M.; Zheng, F.; Chen, J.-G.; Tan, Z.; et al. Acid-sensing ion channels contribute to the effect of acidosis on the function of dendritic cells. J. Immunol. 2011, 186, 3686–3692. [Google Scholar] [CrossRef]
- Zha, X.M. Acid-sensing ion channels: Trafficking and synaptic function. Mol. Brain 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Hamby, M.; Stec, D.E.; Hildebrandt, E.; Stec, D.F.; Drummond, H.A. Mice lacking ASIC2 and betaENaC are protected from high-fat-diet-induced metabolic syndrome. Front. Endocrinol. 2024, 15, 1449344. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Weissman, J.L.; Farley, J.; Drummond, H.A. betaENaC is required for whole cell mechanically gated currents in renal vascular smooth muscle cells. Am. J. Physiol. Ren. Physiol. 2013, 304, F1428–F1437. [Google Scholar] [CrossRef] [PubMed]
- Steuernagel, L.; Lam, B.Y.H.; Klemm, P.; Dowsett, G.K.C.; Bauder, C.A.; Tadross, J.A.; Hitschfeld, T.S.; Martin, A.d.R.; Chen, W.; de Solis, A.J.; et al. HypoMap-a unified single-cell gene expression atlas of the murine hypothalamus. Nat. Metab. 2022, 4, 1402–1419. [Google Scholar] [CrossRef]
- Pradervand, S.; Barker, P.M.; Wang, Q.; Ernst, S.A.; Beermann, F.; Grubb, B.R.; Burnier, M.; Schmidt, A.; Bindels, R.J.M.; Gatzy, J.T.; et al. Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc. Natl. Acad. Sci. USA 1999, 96, 1732–1737. [Google Scholar] [CrossRef]
- VanLandingham, L.G.; Gannon, K.P.; Drummond, H.A.; Warrington, J.P.; Coleman, K.; Skaggs, C.; Hosick, P.A.; George, E.M.; Stec, D.E.; Ryan, M.J.; et al. Pressure-induced constriction is inhibited in a mouse model of reduced betaENaC. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R723–R728. [Google Scholar] [CrossRef]
- Grifoni, S.C.; Chiposi, R.; McKey, S.E.; Ryan, M.J.; Drummond, H.A. Altered whole kidney blood flow autoregulation in a mouse model of reduced beta-ENaC. Am. J. Physiol. Ren. Physiol. 2010, 298, F285–F292. [Google Scholar] [CrossRef]
- Drummond, H.A.; Grifoni, S.C.; Abu-Zaid, A.; Gousset, M.; Chiposi, R.; Barnard, J.M.; Murphey, B.; Stec, D.E. Renal inflammation and elevated blood pressure in a mouse model of reduced beta-ENaC. Am. J. Physiol. Ren. Physiol. 2011, 301, F443–F449. [Google Scholar] [CrossRef]
- Ge, Y.; Gannon, K.; Gousset, M.; Liu, R.; Murphey, B.; Drummond, H.A. Impaired myogenic constriction of the renal afferent arteriole in a mouse model of reduced betaENaC expression. Am. J. Physiol. Ren. Physiol. 2012, 302, F1486–F1493. [Google Scholar] [CrossRef]
- Lu, Y.; Stec, D.E.; Liu, R.; Ryan, M.; Drummond, H.A. betaENaC and ASIC2 associate in VSMCs to mediate pressure-induced constriction in the renal afferent arteriole. Am. J. Physiol. Ren. Physiol. 2022, 322, F498–F511. [Google Scholar] [CrossRef]
- Pack, A.I.; Galante, R.J.; Maislin, G.; Cater, J.; Metaxas, D.; Lu, S.; Zhang, L.; Von Smith, R.; Kay, T.; Lian, J.; et al. Novel method for high-throughput phenotyping of sleep in mice. Physiol. Genom. 2007, 28, 232–238. [Google Scholar] [CrossRef]
- Badmus, O.O.; Kipp, Z.A.; Bates, E.A.; da Silva, A.A.; Taylor, L.C.; Martinez, G.J.; Lee, W.-H.; Creeden, J.F.; Hinds, T.D.; Stec, D.E. Loss of hepatic PPARalpha in mice causes hypertension and cardiovascular disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 325, R81–R95. [Google Scholar] [PubMed]
- Stec, D.E.; Gordon, D.M.; Hipp, J.A.; Hong, S.; Mitchell, Z.L.; Franco, N.R.; Robison, J.W.; Anderson, C.D.; Stec, D.F.; Hinds, T.D., Jr. Loss of hepatic PPARalpha promotes inflammation and serum hyperlipidemia in diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R733–R745. [Google Scholar]
- Ehret, E.; Hummler, E. Lessons learned about epithelial sodium channels from transgenic mouse models. Curr. Opin. Nephrol. Hypertens. 2022, 31, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Zhang, C.; Méar, L.; Zhong, W.; Digre, A.; Katona, B.; Sjöstedt, E.; Butler, L.; Odeberg, J.; Dusart, P.; et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 2021, 7, eabh2169. [Google Scholar] [CrossRef] [PubMed]
- Boric, M.; Varas, R.; Sepulvida-Kattan, E.; Venegas, F.; Perez, F.; Michea, L. Functional assessment of the epithelial sodium channel (ENaC) in the heart. In Proceedings of the APS Conference: 7th International Symposium on Aldosterone on the ENaC/Degenerin Family of Ion Channels: Molecular Mechanisms and Pathophysiology, Pacific Grove, CA, USA, 18–22 September 2011; Available online: https://www.physiology.org/docs/default-source/archive_tphys/the-physiologist-newsletter-2011-december.pdf?sfvrsn=aeb038da_protein (accessed on 16 September 2025).
- Habibi, J.; Aroor, A.R.; Sowers, J.R.; Jia, G.; Hayden, M.R.; Garro, M.; Barron, B.; Mayoux, E.; Rector, R.S.; Whaley-Connell, A.; et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc. Diabetol. 2017, 16, 9. [Google Scholar] [CrossRef]
- Shi, S.; Carattino, M.D.; Hughey, R.P.; Kleyman, T.R. ENaC regulation by proteases and shear stress. Curr. Mol. Pharmacol. 2013, 6, 28–34. [Google Scholar] [CrossRef]
- Tavernarakis, N.; Driscoll, M. Mechanotransduction in Caenorhabditis elegans: The role of DEG/ENaC ion channels. Cell Biochem. Biophys. 2001, 35, 1–18. [Google Scholar] [CrossRef]

| Organ Mass (mg) | Female-HFD | Male-HFD | ||||
|---|---|---|---|---|---|---|
| WT | βMUT | p-Value | WT | βMUT | p-Value | |
| Heart | 141 | 130 | 0.014 | 155 | 157 | 0.628 |
| Kidney | 304 | 284 | 0.105 | 375 | 388 | 0.398 |
| Spleen | 133 | 148 | 0.479 | 79 | 80 | 0.897 |
| Brown Fat | 244 | 147 | 0.271 | 152 | 163 | 0.67 |
| Gonadal Fat | 3441 | 3095 | 0.547 | 1274 | 1555 | 0.014 |
| Visceral Fat | 2550 | 1580 | 0.7 | 943 | 1103 | 0.034 |
| Pancreas | 209 | 199 | 0.629 | 205 | 191 | 0.491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamby, M.; Barr, E.; Lirette, S.; Drummond, H.A. Loss of βENaC Prevents Hepatic Steatosis but Promotes Abdominal Fat Deposition Associated with a High-Fat Diet. Biology 2025, 14, 1558. https://doi.org/10.3390/biology14111558
Hamby M, Barr E, Lirette S, Drummond HA. Loss of βENaC Prevents Hepatic Steatosis but Promotes Abdominal Fat Deposition Associated with a High-Fat Diet. Biology. 2025; 14(11):1558. https://doi.org/10.3390/biology14111558
Chicago/Turabian StyleHamby, Madison, Elizabeth Barr, Seth Lirette, and Heather A. Drummond. 2025. "Loss of βENaC Prevents Hepatic Steatosis but Promotes Abdominal Fat Deposition Associated with a High-Fat Diet" Biology 14, no. 11: 1558. https://doi.org/10.3390/biology14111558
APA StyleHamby, M., Barr, E., Lirette, S., & Drummond, H. A. (2025). Loss of βENaC Prevents Hepatic Steatosis but Promotes Abdominal Fat Deposition Associated with a High-Fat Diet. Biology, 14(11), 1558. https://doi.org/10.3390/biology14111558