Characterization of the Signaling Pathways Activated by KCl-Induced RTK Stimulation in Guinea Pig Airways
Simple Summary
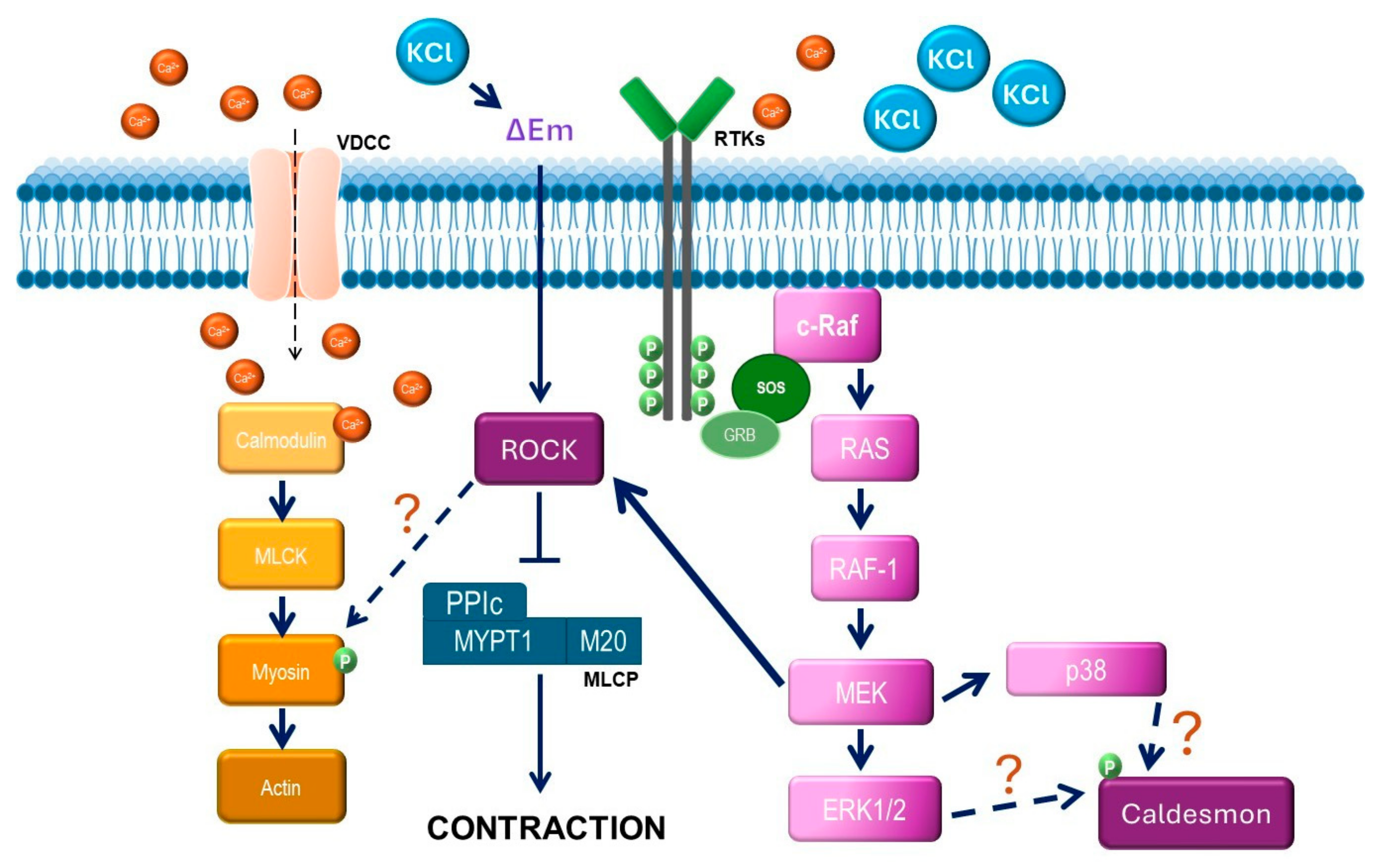
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Organ Bath Experiments
2.3. Western Blot Assays to Define ERK1/2 and MYPT1 Phosphorylation
2.4. Statistical Analysis
2.5. Densitometry Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASM | Airway smooth muscle |
| CaM | Calmodulin |
| CaMK | Calcium calmodulin kinase |
| EGFR | Epidermal growth factor receptor |
| ERK | Epidermal growth factor receptor |
| GAP | GTPase-activating proteins |
| GEF | Nucleotide exchange factor |
| IEG | Immediate early genes |
| L-VDCC | L-type voltage dependent Ca2+ channels |
| MAPK | Mitogen-activated kinase |
| MEK | Mitogen-activated kinase kinase |
| MLCK | Myosin light chain kinase |
| MLCP | Myosin light chain phosphatase |
| MYPT1 | Myosin phosphatase targeting subunit |
| ROCK | Rho kinase |
| RTK | Receptor tyrosine kinase |
References
- Amrani, Y.; Panettieri, R.A. Airway smooth muscle: Contraction and beyond. Int. J. Biochem. Cell Biol. 2003, 35, 272–276. [Google Scholar] [CrossRef]
- Roth, M.; Tamml, M. Airway smooth muscle cells respond directly to inhaled environmental factors. Swiss Med. Wkly. 2010, 140, w13066. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003, 83, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B.; Montano, L.M.; Chávez, J.; Carbajal, V.; García-Hernandez, L.M.; Irles, C.; Jiménez-Garduno, A.M.; Ortega, A. ROCK1 translocates from non-caveolar to caveolar regions upon KCl stimulation in airway smooth muscle. Physiol. Res. 2014, 63, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.J.; Tazzeo, T.; Zuo, J.; Pertens, E.; Keshavjee, S. KCl evokes contraction of airway smooth muscle via activation of RhoA and Rho-kinase. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L852–L858. [Google Scholar] [CrossRef]
- Semenov, I.; Brenner, R. Voltage effects on muscarinic acetylcholine receptor-mediated contractions of airway smooth muscle. Physiol. Rep. 2018, 6, e13856. [Google Scholar] [CrossRef]
- Woller, S.A.; Eddinger, K.A.; Corr, M.; Yaksh, T.L. An overview of pathways encoding nociception. Clin. Exp. Rheumatol. 2017, 35 (Suppl. 107), 40–46. [Google Scholar]
- Yocum, G.T.; Chen, J.; Choi, C.H.; Townsend, E.A.; Zhang, Y.; Xu, D.; Fu, X.W.; Sanderson, M.J.; Emala, C.W. Role of transient receptor potential vanilloid 1 in the modulation of airway smooth muscle tone and calcium handling. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L812–L821. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, H.Y.; Hur, J.; Kim, K.H.; Kang, J.Y.; Rhee, C.K.; Lee, S.Y. TRPV1 Blocking Alleviates Airway Inflammation and Remodeling in a Chronic Asthma Murine Model. Allergy Asthma Immunol. Res. 2018, 10, 216. [Google Scholar] [CrossRef]
- Mita, M.; Tanaka, H.; Yanagihara, H.; Nakagawa, J.; Hishinuma, S.; Sutherland, C.; Walsh, M.P.; Shoji, M. Membrane depolarization-induced RhoA/Rho-associated kinase activation and sustained contraction of rat caudal arterial smooth muscle involves genistein-sensitive tyrosine phosphorylation. J. Smooth Muscle Res. 2013, 49, 26–45. [Google Scholar] [CrossRef]
- Yan, G.R.; Xiao, C.L.; He, G.W.; Yin, X.F.; Chen, N.P.; Cao, Y.; He, Q.Y. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics 2010, 10, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Croisy-Delcey, M.; Croisy, A.; Mousset, S.; Letourneur, M.; Bisagni, E.; Jacquemin-Sablon, A.; Pierre, J. Genistein analogues: Effects on epidermal growth factor receptor tyrosine kinase and on stress-activated pathways. Biomed. Pharmacother. 1997, 51, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.V. RTK/Ras/MAPK signaling. WormBook 2006, 11, 1–19. [Google Scholar] [CrossRef]
- Sakai, H.; Watanabe, Y.; Honda, M.; Tsuiki, R.; Ueda, Y.; Nagai, Y.; Narita, M.; Misawa, M.; Chiba, Y. Involvement of the Tyr kinase/JNK pathway in carbachol-induced bronchial smooth muscle contraction in the rat. Anesthesiology 2013, 118, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Zubkov, A.Y.; Rollins, K.S.; Zhang, J.H. KCl activates mitogen-activated protein kinase in rabbit bailar artery. Biochem. Biophys. Res. Commun. 2002, 293, 660–664. [Google Scholar] [CrossRef]
- McKay, M.M.; Morrison, D.K. Integrating signals from RTK to ERK/MAPK. Oncogene 2007, 26, 3113–3121. [Google Scholar] [CrossRef]
- Jeong, K.J.; Cho, K.H.; Panupinthu, N.; Kim, H.; Kang, J.; Park, C.G.; Mills, G.B.; Lee, H.Y. EGFR mediates LPA-induced proteolytic enzyme expression and ovarian cancer invasion: Inhibition by resveratrol. Mol. Oncol. 2013, 7, 121–129. [Google Scholar] [CrossRef]
- Stewart, A.G. Airway wall remodelling and hyperresponsiveness: Modelling remodelling in vitro and in vivo. Pulm. Pharmacol. Ther. 2001, 14, 255–265. [Google Scholar] [CrossRef]
- Hallgren, O.; Rolandsson, S.; Andersson-Sjöland, A.; Nihlberg, K.; Wieslander, E.; Kvist-Reimer, M.; Dahlbäck, M.; Eriksson, L.; Bjermer, L.; Erjefält, J.S. Enhanced ROCK1 dependent contractility in fibroblast from chronic obstructive pulmonary disease patients. J. Transl. Med. 2012, 10, 171. [Google Scholar] [CrossRef]
- Shiraishi, T.; Domoto, T.; Imai, N.; Shimada, Y.; Watanabe, K. Specific inhibitors of tyrosine-specific protein kinase, synthetic 4-hydroxycinnamamide derivatives. Biochem. Biophys. Res. Commun. 1987, 147, 322–328. [Google Scholar] [CrossRef]
- Shiraishi, T.; Owada, M.K.; Tatsuka, M.; Fuse, Y.; Watanabe, K.; Kakunaga, T. A tyrosine-specific protein kinase inhibitor, alpha-cyano-3-ethoxy-4-hydroxy-5-phenylthiomethylcinnamamide, blocks the phosphorylation of tyrosine kinase substrate in intact cells. Jpn. J. Cancer Res. 1990, 81, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Chopra, L.C.; Hucks, D.; Twort, C.H.; Ward, J.P. Effects of protein tyrosine kinase inhibitors on contractility of isolated bronchioles of the rat. Am. J. Respir. Cell Mol. Biol. 1997, 16, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.J.; Jha, S.; Restaino, C.R.; Dayananth, P.; Zhu, H.; Cooper, A.; Carr, D.; Deng, Y.; Jin, W.; Black, S.; et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013, 3, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B.; Flores-Soto, E.; Reyes-García, J.; Díaz-Hernández, V.; Carbajal, V.; Montaño, L.M. Na+ permeates through L-type Ca2+ channel in bovine airway smooth muscle. Eur. J. Pharmacol. 2016, 782, 77–88. [Google Scholar] [CrossRef]
- Sommer, B.; Flores-Soto, E.; Gonzalez-Avila, G. Cellular Na+ handling mechanisms involved in airway smooth muscle contraction (Review). Int. J. Mol. Med. 2017, 40, 3–9. [Google Scholar] [CrossRef]
- Liu, C.; Zuo, J.; Pertens, E.; Helli, P.B.; Janssen, L.J. Regulation of Rho/ROCK signaling in airway smooth muscle by membrane potential and [Ca2+]i. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L574–L582. [Google Scholar] [CrossRef]
- Momin, A.A.; Mendes, T.; Barthe, P.; Faure, C.; Hong, S.; Yu, P.; Kadaré, G.; Jaremko, M.; Girault, J.A.; Jaremko, Ł.; et al. PYK2 senses calcium through a disordered dimerization and calmodulin-binding element. Commun. Biol. 2022, 5, 800. [Google Scholar] [CrossRef]
- Vetter, S.W.; Leclerc, E. Novel aspects of calmodulin target recognition and activation. Eur. J. Biochem. 2003, 270, 404–414. [Google Scholar] [CrossRef]
- Pelaia, G.; Renda, T.; Gallelli, L.; Vatrella, A.; Busceti, M.T.; Agati, S.; Caputi, M.; Cazzola, M.; Maselli, R.; Marsico, S.A. Molecular mechanisms underlying airway smooth muscle contraction and proliferation: Implications for asthma. Respir. Med. 2008, 102, 1173–1181. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Borrás, C.; Viña, J. Genistein, a tool for geroscience. Mech. Ageing Dev. 2022, 204, 111665. [Google Scholar] [CrossRef]
- Hossain, M.A. Targeting the RAS upstream and downstream signaling pathway for cancer treatment. Eur. J. Pharmacol. 2024, 979, 176727. [Google Scholar] [CrossRef]
- Ashton-Beaucage, D.; Therrien, M. How Genetics Has Helped Piece Together the MAPK Signaling Pathway. Methods Mol. Biol. 2017, 1487, 1–21. [Google Scholar]
- Scardaci, R.; Berlinska, E.; Scaparone, P.; Vietti Michelina, S.; Garbo, E.; Novello, S.; Santamaria, D.; Ambrogio, C. Novel RAF-directed approaches to overcome current clinical limits and block the RAS/RAF node. Mol. Oncol. 2024, 18, 1355–1377. [Google Scholar] [CrossRef]
- Ammon, H.P.; Heurich, R.O.; Kolb, H.A.; Lang, F.; Schaich, R.; Drews, G.; Leiers, T. The phosphatase inhibitor okadaic acid blocks KCl-depolarization-induced rise of cytosolic calcium of rat insulinoma cells (RINm5F). Naunyn Schmiedebergs Arch. Pharmacol. 1996, 354, 95–101. [Google Scholar] [CrossRef]
- Archana, G.M.; Arunkumar, R.C.; Omkumar, R.V. Assays for L-type voltage gated calcium channels. Anal. Biochem. 2022, 656, 114827. [Google Scholar] [CrossRef] [PubMed]
- Barbado, M.; Fablet, K.; Ronjat, M.; De Waard, M. Gene regulation by voltage-dependent calcium channels. Biochim. Biophys. Acta 2009, 1793, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Dolmetsch, R.E.; Pajvani, U.; Fife, K.; Spotts, J.M.; Greenberg, M.E. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 2001, 294, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M.; Wayman, G.A.; Nozaki, N.; Soderling, T.R. Calcium Activation of ERK Mediated by Calmodulin Kinase I. J. Biol. Chem. 2004, 279, 24064–24072. [Google Scholar] [CrossRef]
- Kim, I.; Je, H.D.; Gallant, C.; Zhan, Q.; Riper, D.V.; Badwey, J.A.; Singer, H.A.; Morgan, K.G. Ca2+-calmodulin-dependent protein kinase II-dependent activation of contractility in ferret aorta. J. Physiol. 2000, 526 Pt 2, 367–374. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Tyssowski, K.M.; DeStefino, N.R.; Cho, J.H.; Dunn, C.J.; Poston, R.G.; Carty, C.E.; Jones, R.D.; Chang, S.M.; Romeo, P.; Wurzelmann, M.K.; et al. Different Neuronal Activity Patterns Induce Different Gene Expression Programs. Neuron 2018, 98, 530–546.e11. [Google Scholar] [CrossRef] [PubMed]
- Pulver-Kaste, R.A.; Barlow, C.A.; Bond, J.; Watson, A.; Penar, P.L.; Tranmer, B.; Lounsbury, K.M. Ca2+ source-dependent transcription of CRE-containing genes in vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H97–H105. [Google Scholar] [CrossRef]
- McKay, S.; de Jongste, J.C.; Saxena, P.R.; Sharma, H.S. Angiotensin II induces hypertrophy of human airway smooth muscle cells: Expression of transcription factors and transforming growth factor-beta1. Am. J. Respir. Cell Mol. Biol. 1998, 18, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Hjoberg, J.; Le, L.; Imrich, A.; Subramaniam, V.; Mathew, S.I.; Vallone, J.; Haley, K.J.; Green, F.H.; Shore, S.A.; Silverman, E.S. Induction of early growth-response factor 1 by platelet-derived growth factor in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L817–L825. [Google Scholar] [CrossRef] [PubMed]
- Ressler, B.; Lee, R.T.; Randell, S.H.; Drazen, J.M.; Kamm, R.D. Molecular responses of rat tracheal epithelial cells to transmembrane pressure. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L1264–L1272. [Google Scholar] [CrossRef]
- Herschman, H.R. Primary response genes induced by growth factors and tumor promoters. Annu. Rev. Biochem. 1991, 60, 281–319. [Google Scholar] [CrossRef]
- O’Donnell, A.; Odrowaz, Z.; Sharrocks, A.D. Immediate-early gene activation by the MAPK pathways: What do and don’t we know? Biochem. Soc. Trans. 2012, 40, 58–66. [Google Scholar] [CrossRef]
- Lau, L.F.; Nathans, D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: Coordinate regulation with c-fos or c-myc. Proc. Natl. Acad. Sci. USA 1987, 84, 1182–1186. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Ziff, E.B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature 1984, 311, 433–438. [Google Scholar] [CrossRef]
- Cochran, B.H.; Zullo, J.; Verma, I.M.; Stiles, C.D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science 1984, 226, 1080–1082. [Google Scholar] [CrossRef]
- Gomard, T.; Jariel-Encontre, I.; Basbous, J.; Bossis, G.; Moquet-Torcy, G.; Piechaczyk, M. Fos family protein degradation by the proteasome. Biochem. Soc. Trans. 2008, 36 Pt 5, 858–863. [Google Scholar] [CrossRef]
- Di Paola, R.; Galuppo, M.; Mazzon, E.; Paterniti, I.; Bramanti, P.; Cuzzocrea, S. PD98059, a specific MAP kinase inhibitor, attenuates multiple organ dysfunction syndrome/failure (MODS) induced by zymosan in mice. Pharmacol. Res. 2010, 61, 175–187. [Google Scholar] [CrossRef]
- Gerthoffer, W.T. Regulation of the contractile element of airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 1991, 261, L15–L28. [Google Scholar] [CrossRef]
- Gerthoffer, W.T.; Yamboliev, I.A.; Pohl, J.; Haynes, R.; Dang, S.; McHugh, J. Activation of MAP kinases in airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 1997, 272, L244–L252. [Google Scholar] [CrossRef]
- Goncharova, E.A.; Vorotnikov, A.V.; Gracheva, E.O.; Wang, C.L.; Panettieri, R.A., Jr.; Stepanova, V.V.; Tkachuk, V.A. Activation of p38 MAP-kinase and caldesmon phosphorylation are essential for urokinase-induced human smooth muscle cell migration. Biol. Chem. 2002, 383, 115–126. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.A.; Walsh, M.P. Regulation of Smooth Muscle Myosin Light Chain Phosphatase by Multisite Phosphorylation of the Myosin Targeting Subunit, MYPT1. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Tsai, M.H.; Chang, A.N.; He, W.; Chen, C.P.; Zhu, M.; Kamm, K.E.; Stull, J.T. Physiological vs. pharmacological signalling to myosin phosphorylation in airway smooth muscle. J. Physiol. 2017, 595, 6231–6247. [Google Scholar] [CrossRef]
- Saponara, S.; Fusi, F.; Sgaragli, G.; Cavalli, M.; Hopkins, B.; Bova, S. Effects of commonly used protein kinase inhibitors on vascular contraction and L-type Ca2+ current. Biochem. Pharmacol. 2012, 84, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Ito, M.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar] [CrossRef]
- Kureishi, Y.; Kobayashi, S.; Amano, M.; Kimura, K.; Kanaide, H.; Nakano, T.; Kaibuchi, K.; Ito, M. Rho-associated Kinase Directly Induces Smooth Muscle Contraction through Myosin Light Chain Phosphorylation. J. Biol. Chem. 1997, 272, 12257–12260. [Google Scholar] [CrossRef]
- Ueda, K.; Murata-Hori, M.; Tatsuka, M.; Hosoya, H. Rho-kinase contributes to diphosphorylation of myosin II regulatory light chain in nonmuscle cells. Oncogene 2002, 21, 5852–5860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, X.; Luo, T.; Luo, X.; Tang, Z. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens. Res. 2014, 37, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.B.; Tsunoda, Y.; Pawar, M.D.; Bitar, K.N. Translocation and association of ROCK-II with RhoA and HSP27 during contraction of rabbit colon smooth muscle cells. Biochem. Biophys. Res. Commun. 2004, 319, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Reiner, D.J.; Lundquist, E.A. Small GTPases. WormBook 2018, 2018, 1–65. [Google Scholar] [CrossRef]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO Family GTPases: Mechanisms of Regulation and Signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef]
- Cherfils, J.; Zeghouf, M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol. Rev. 2013, 93, 269–309. [Google Scholar] [CrossRef]
- Bos, J.L.; Rehmann, H.; Wittinghofer, A. GEFs and GAPs: Critical Elements in the Control of Small G Proteins. Cell 2007, 129, 865–877. [Google Scholar] [CrossRef]
- Zhang, W.; Bhetwal, B.P.; Gunst, S.J. Rho kinase collaborates with p21-activated kinase to regulate actin polymerization and contraction in airway smooth muscle. J. Physiol. 2018, 596, 3617–3635. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.; Gunst, S.J. The Small GTPase RhoA Regulates the Contraction of Smooth Muscle Tissues by Catalyzing the Assembly of Cytoskeletal Signaling Complexes at Membrane Adhesion Sites. J. Biol. Chem. 2012, 287, 33996–34008. [Google Scholar] [CrossRef]
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992, 70, 401–410. [Google Scholar] [CrossRef]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]

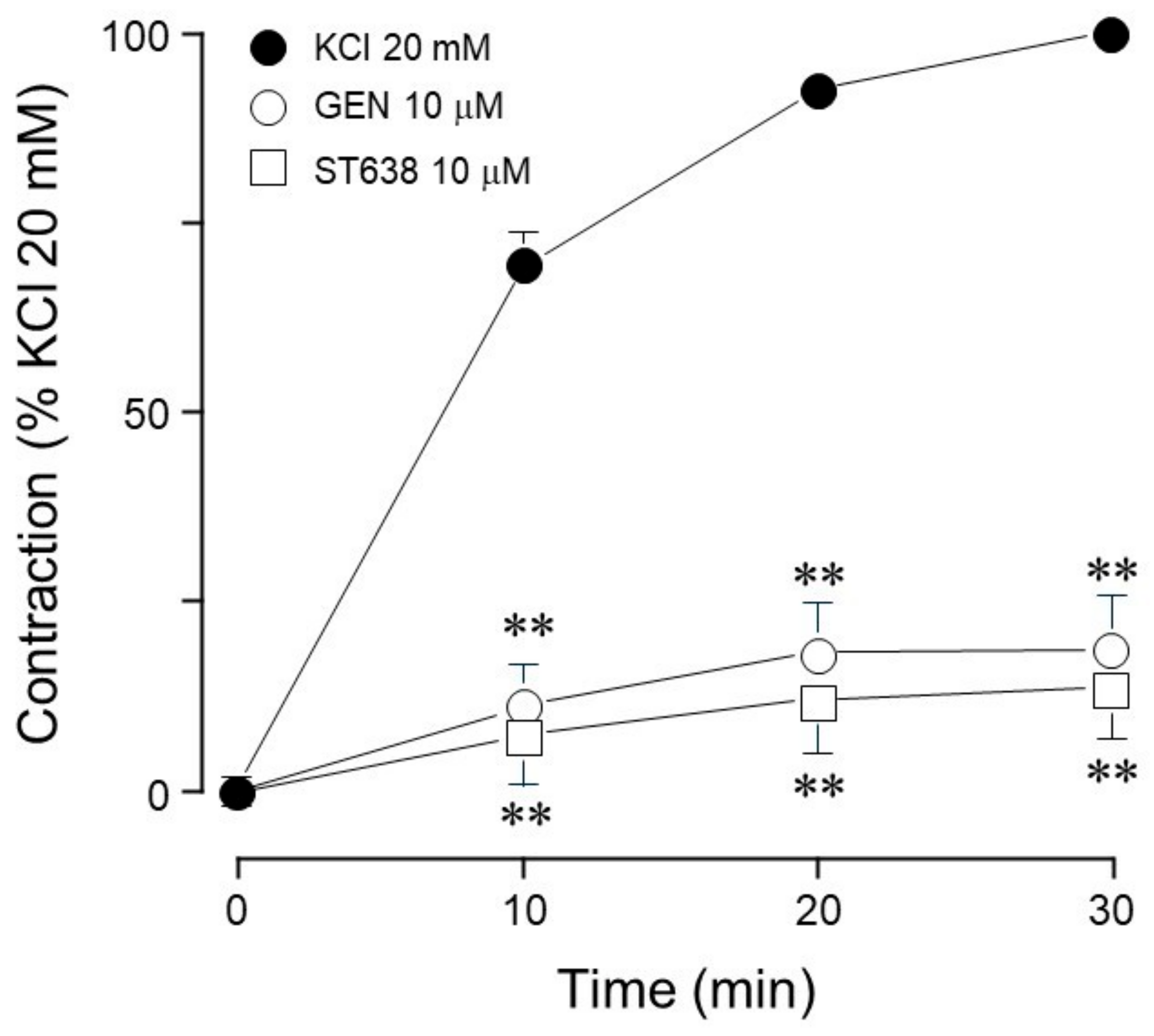

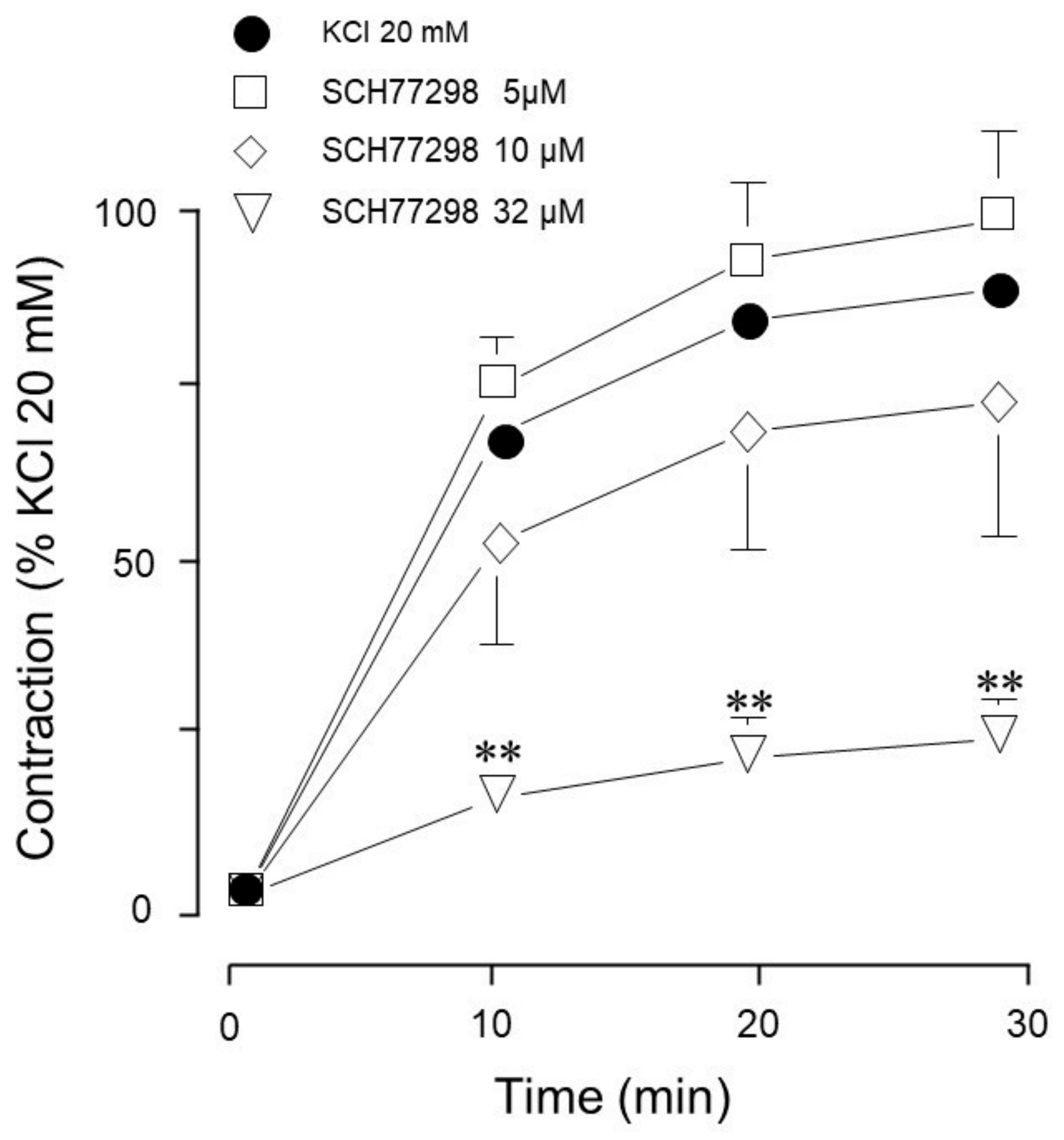
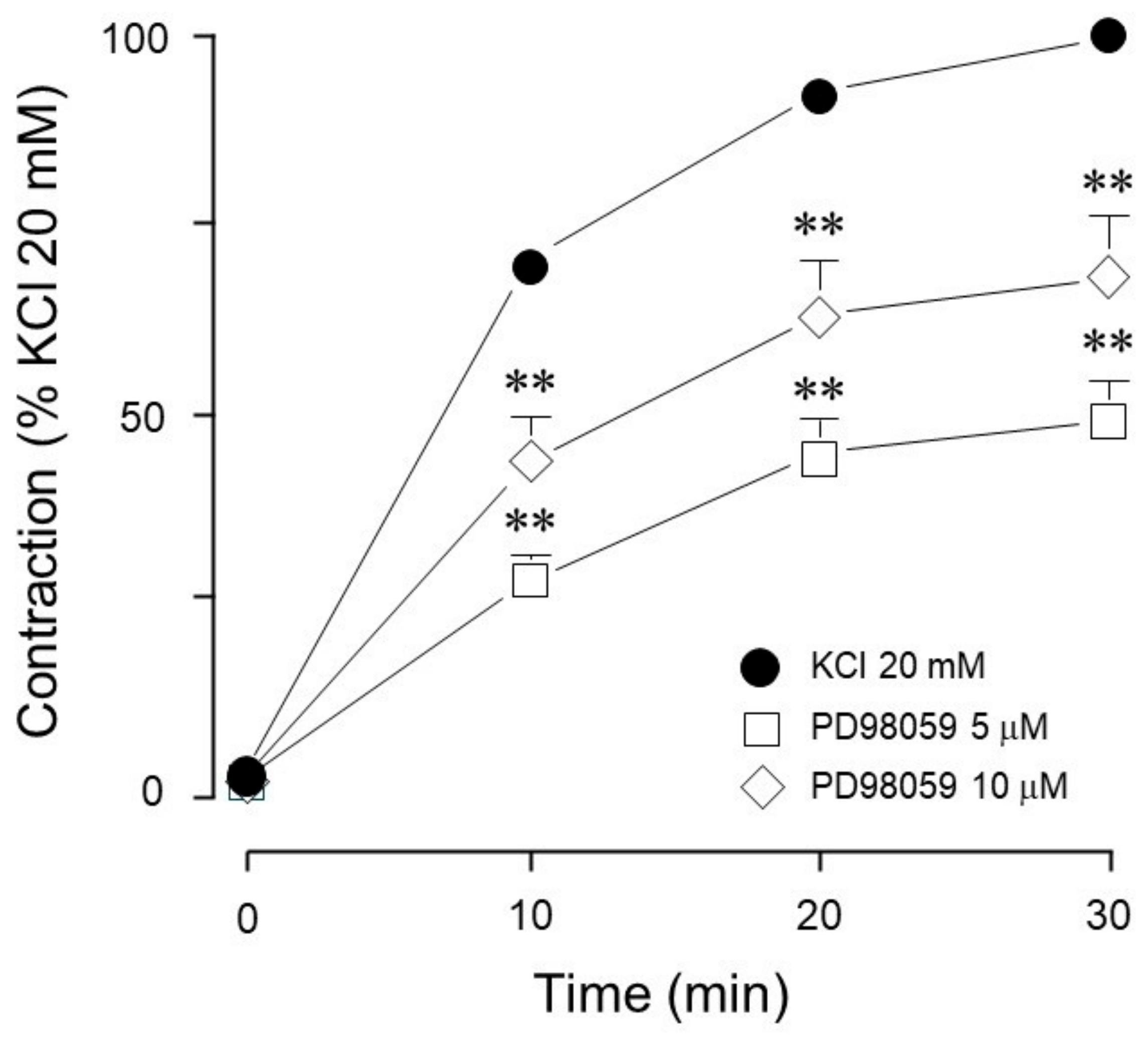
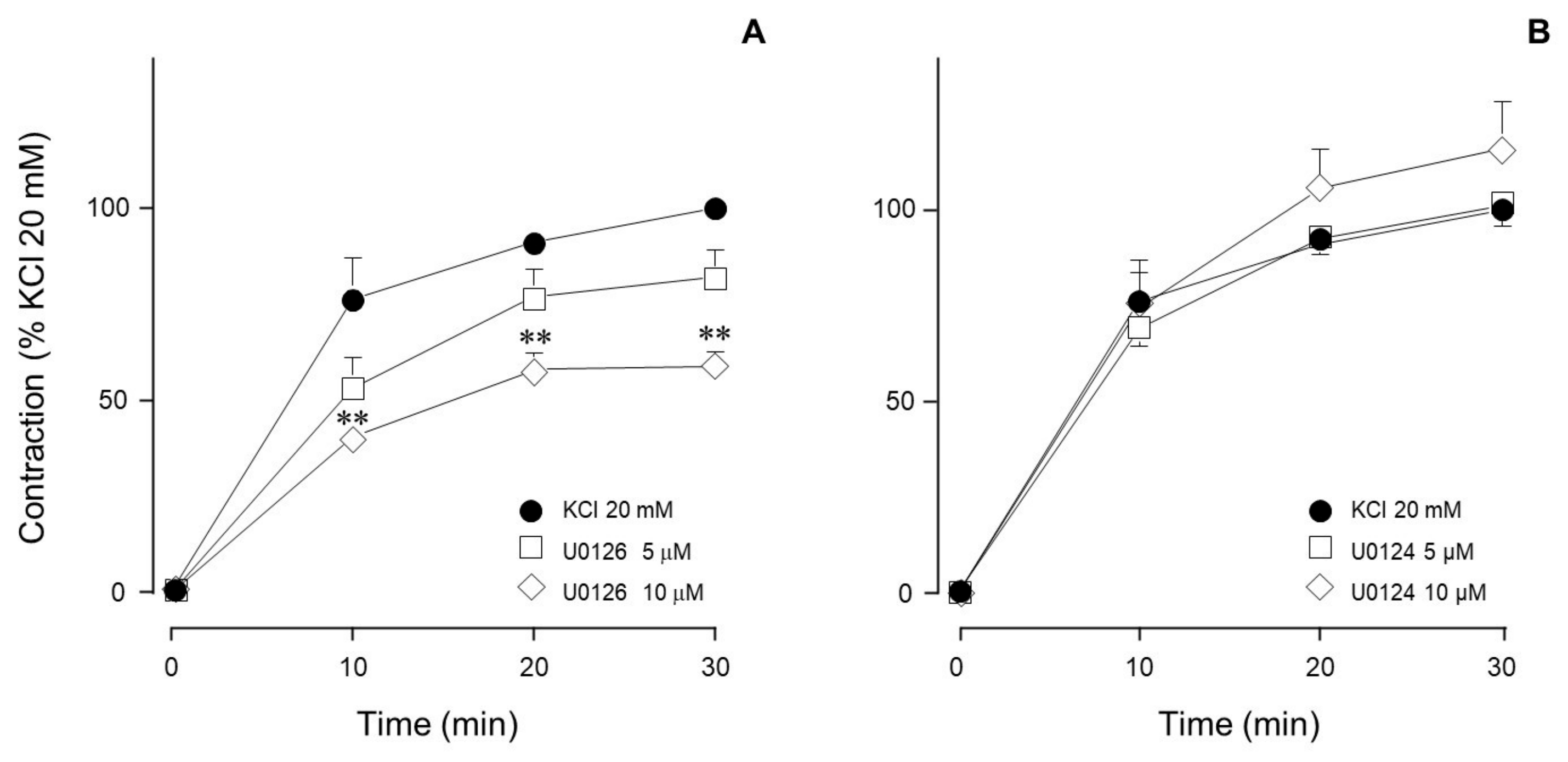
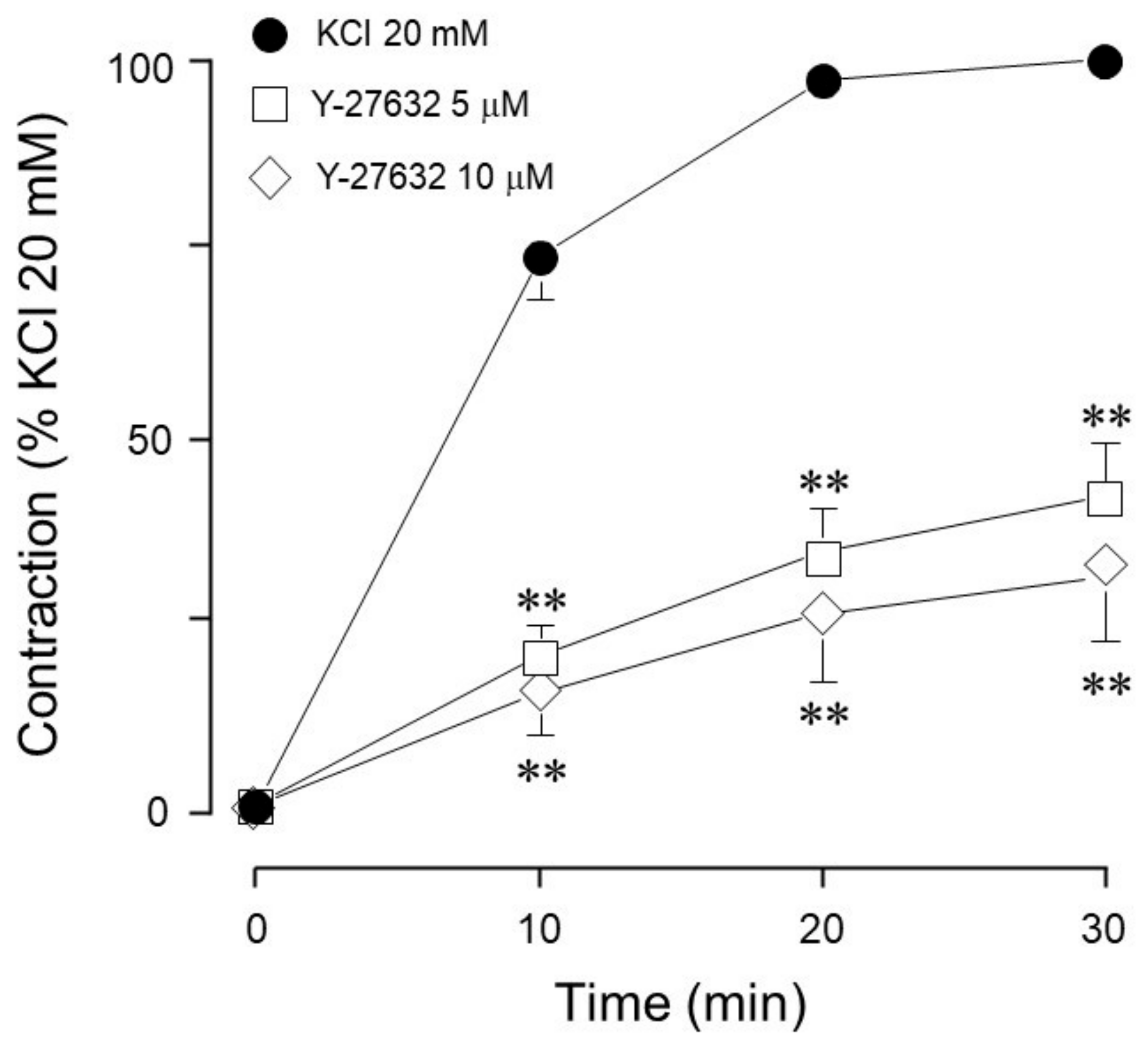

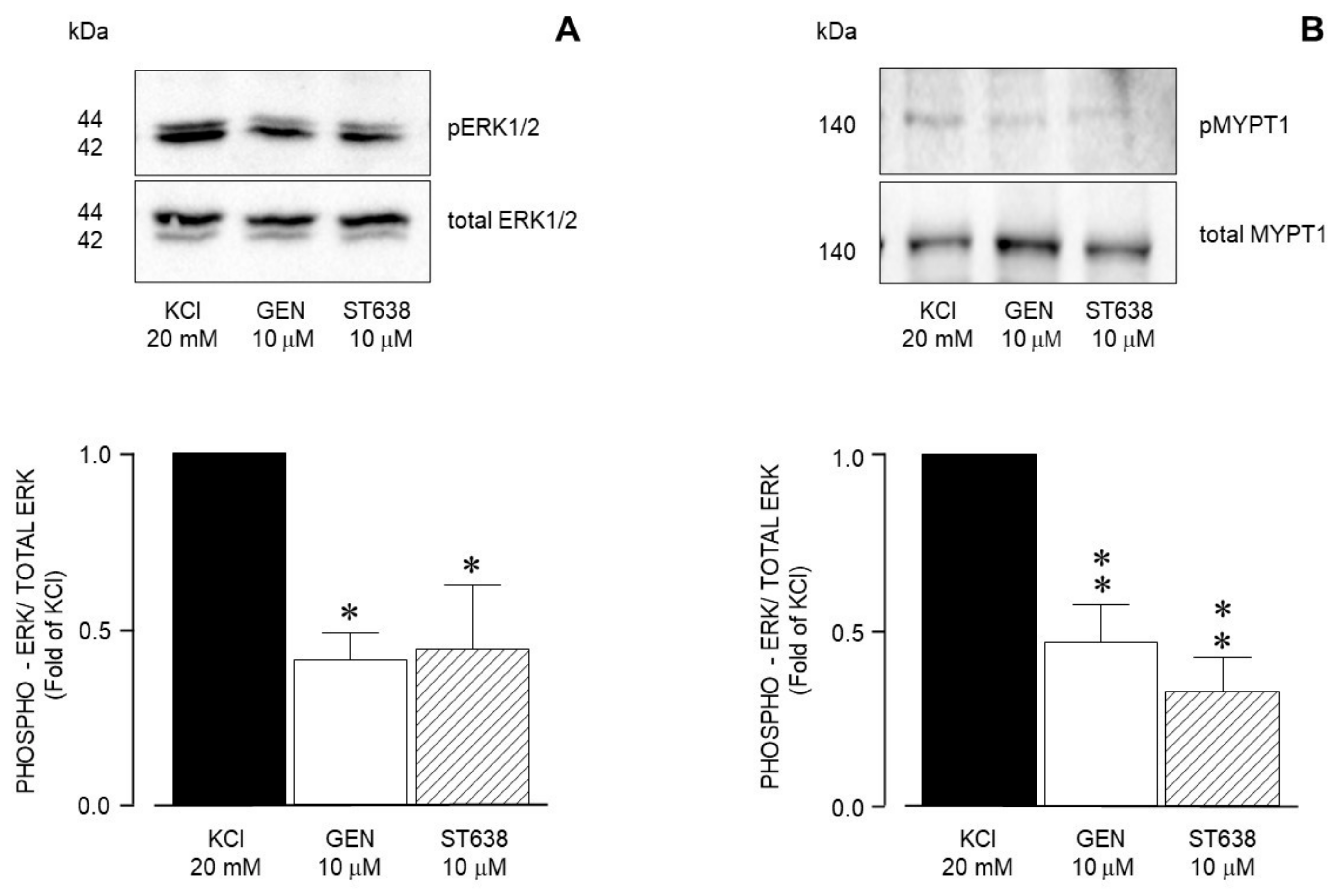
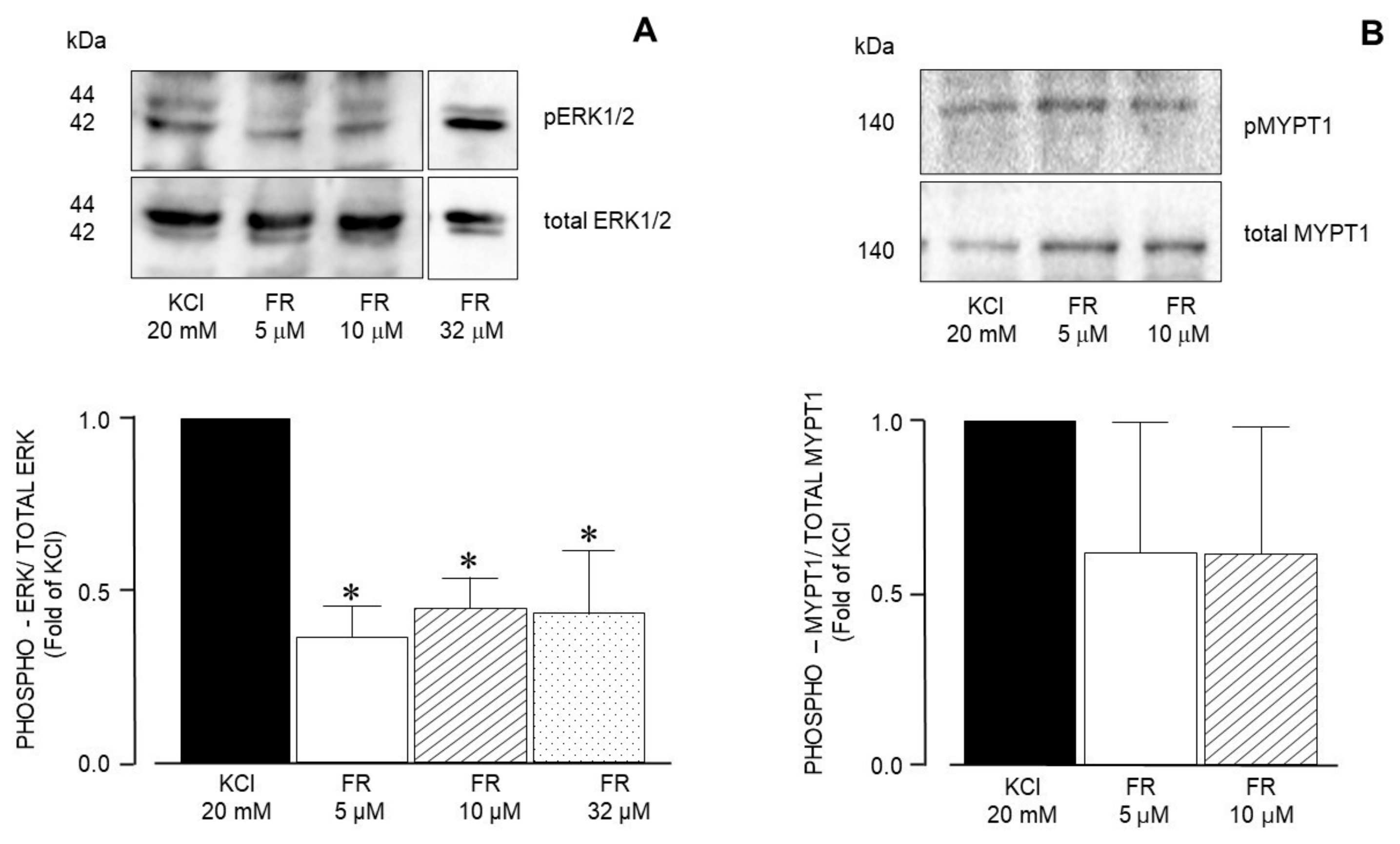



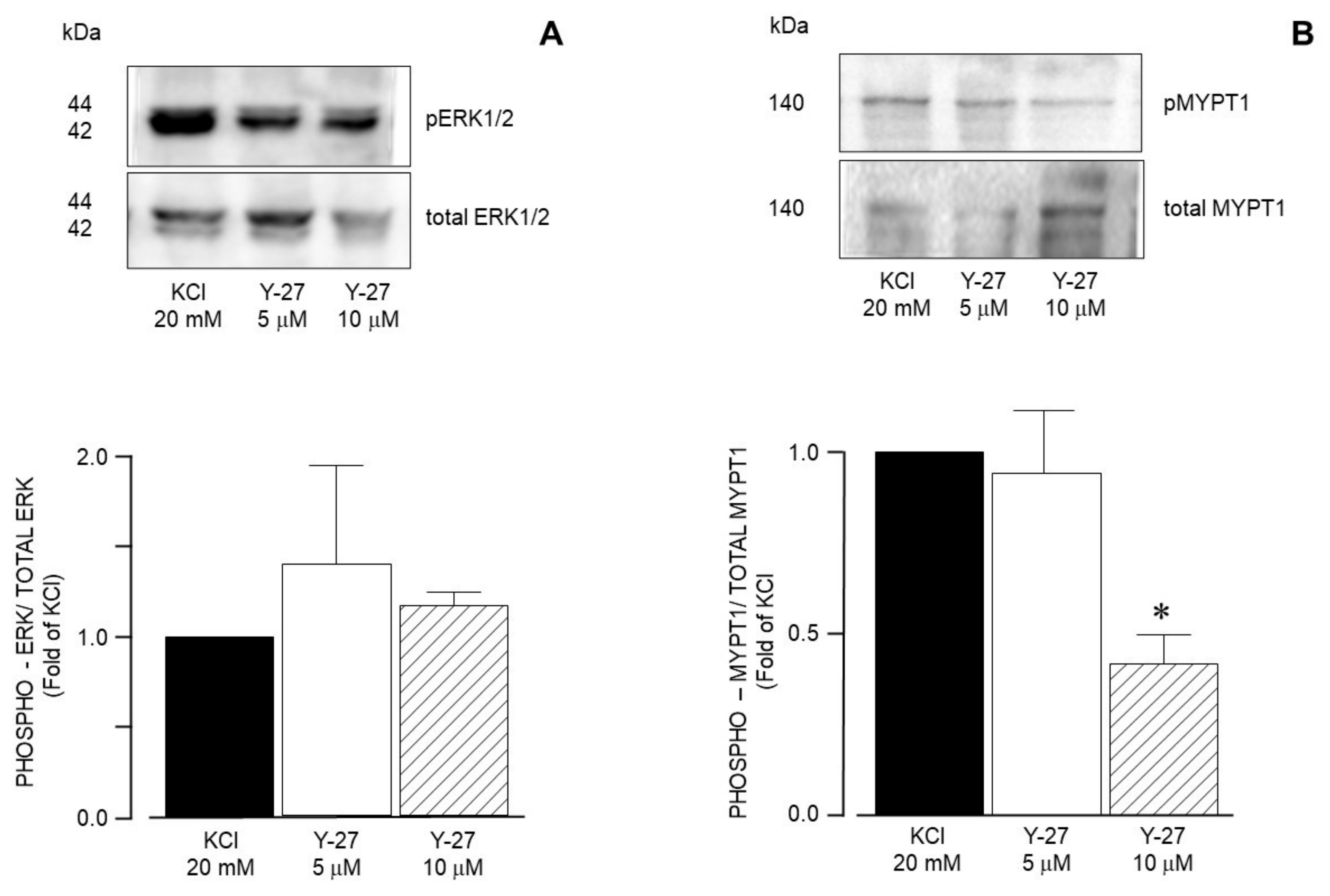
| Inhibitor Name | Target Molecule | Concentration (µM) |
|---|---|---|
| Genistein | RTK | 10 |
| ST638 | RTK | 10 |
| FR180204 | ERK1/ERK2 | 5, 10, 32 |
| SCH772984 | ERK1/ERK2 | 5, 10, 32 |
| PD98059 | MEK | 5, 10 |
| U0126 | MEK | 5, 10 |
| U0124 | Inactive analog of U0126 | 5, 10 |
| ML-7 | MLCK | 5, 10 |
| Y-27632 | ROCK | 5, 10 |
| Antibody | Dilution | Purchased From | Catalog Number |
|---|---|---|---|
| ERK 1/ERK 2 | 1:3000 | Cell Signaling Technology | 9102S |
| p-ERK 1/ERK 2 | 1:3000 | Cell Signaling Technology | 9101S |
| MYPT 1 | 1:1000 | Cell Signaling Technology | 2634S |
| pThr-696-MYPT1 | 1:1000 | Cell Signaling Technology | 5163S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Alcibar, E.; Flores-Soto, E.; López, R.M.; Castillo, E.F.; Campos-Bedolla, P.; Carbajal, V.; Sommer, B. Characterization of the Signaling Pathways Activated by KCl-Induced RTK Stimulation in Guinea Pig Airways. Biology 2025, 14, 1557. https://doi.org/10.3390/biology14111557
Herrera-Alcibar E, Flores-Soto E, López RM, Castillo EF, Campos-Bedolla P, Carbajal V, Sommer B. Characterization of the Signaling Pathways Activated by KCl-Induced RTK Stimulation in Guinea Pig Airways. Biology. 2025; 14(11):1557. https://doi.org/10.3390/biology14111557
Chicago/Turabian StyleHerrera-Alcibar, Eva, Edgar Flores-Soto, Ruth M. López, Enrique F. Castillo, Patricia Campos-Bedolla, Verónica Carbajal, and Bettina Sommer. 2025. "Characterization of the Signaling Pathways Activated by KCl-Induced RTK Stimulation in Guinea Pig Airways" Biology 14, no. 11: 1557. https://doi.org/10.3390/biology14111557
APA StyleHerrera-Alcibar, E., Flores-Soto, E., López, R. M., Castillo, E. F., Campos-Bedolla, P., Carbajal, V., & Sommer, B. (2025). Characterization of the Signaling Pathways Activated by KCl-Induced RTK Stimulation in Guinea Pig Airways. Biology, 14(11), 1557. https://doi.org/10.3390/biology14111557






