Short- and Long-Term Effects of Undernutrition During Adolescence on Oxidative Status and Glucose Homeostasis in Male and Female Rats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experimental Design and Diet Treatment
2.3. Assessment of Food Intake, Drinking Water and Body Weight
2.4. Glucose–Insulin Homeostasis Assessment
2.5. Assessment of Body Mass Composition
2.6. Assessment of the Oxidative Status of the Liver and iBAT
2.7. Statistical Analyses
3. Results
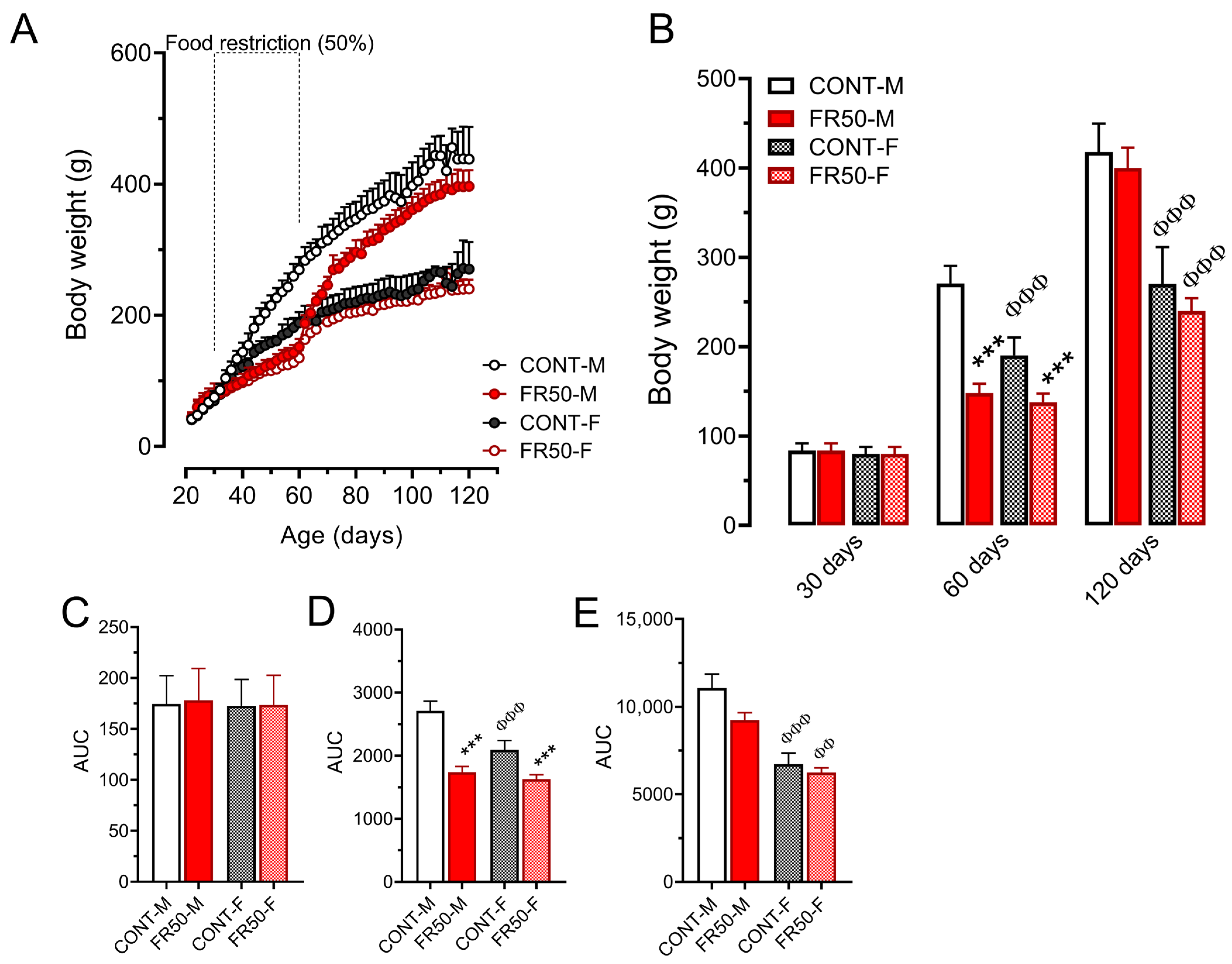
3.1. Food and Drinking Water Intake and Body Weight Gain
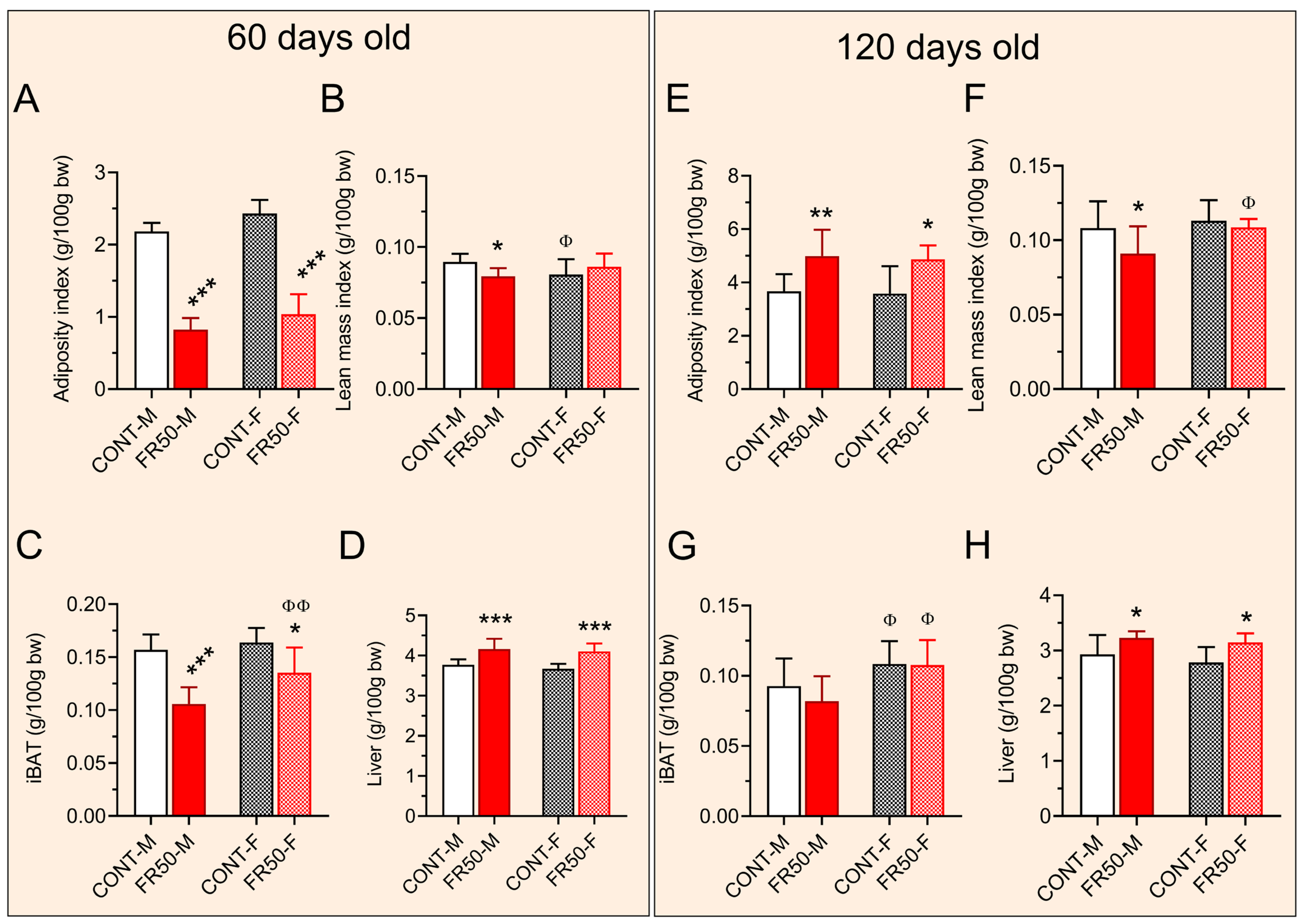
3.2. Murinometric Parameters
3.3. Glucose–Insulin Homeostasis
3.4. Assessment of Liver and iBAT Redox Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Health Organization, Undernutrition. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition/ (accessed on 24 November 2024).
- Barker, D.J. The developmental origins of chronic adult disease. Acta Paediatr. Suppl. 2004, 93, 26–33. [Google Scholar] [CrossRef]
- Uauy, R.; Kain, J.; Corvalan, C. How can the Developmental Origins of Health and Disease (DOHaD) hypothesis contribute to improving health in developing countries? Am. J. Clin. Nutr. 2011, 94, 1759S–1764S. [Google Scholar] [CrossRef]
- Ravelli, A.C.; van Der Meulen, J.H.; Osmond, C.; Barker, D.J.; Bleker, O.P. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am. J. Clin. Nutr. 1999, 70, 811–816. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; Lisboa, P.C.; de Moura, E.G.; Barella, L.F.; Miranda, R.A.; Malta, A.; Franco, C.C.; Ribeiro, T.A.; Torrezan, R.; Gravena, C.; et al. Poor pubertal protein nutrition disturbs glucose-induced insulin secretion process in pancreatic islets and programs rats in adulthood to increase fat accumulation. J. Endocrinol. 2013, 216, 195–206. [Google Scholar] [CrossRef]
- de Oliveira, J.C.; de Moura, E.G.; Miranda, R.A.; de Moraes, A.M.P.; Barella, L.F.; da Conceicao, E.P.S.; Gomes, R.M.; Ribeiro, T.A.; Malta, A.; Martins, I.P.; et al. Low-protein diet in puberty impairs testosterone output and energy metabolism in male rats. J. Endocrinol. 2018, 237, 243–254. [Google Scholar] [CrossRef]
- Malta, A.; de Oliveira, J.C.; Ribeiro, T.A.; Tofolo, L.P.; Barella, L.F.; Prates, K.V.; Miranda, R.A.; Elmhiri, G.; Franco, C.C.; Agostinho, A.R.; et al. Low-protein diet in adult male rats has long-term effects on metabolism. J. Endocrinol. 2014, 221, 293–303. [Google Scholar] [CrossRef]
- Malta, A.; de Moura, E.G.; Ribeiro, T.A.; Tofolo, L.P.; Abdennebi-Najar, L.; Vieau, D.; Barella, L.F.; de Freitas Mathias, P.C.; Lisboa, P.C.; de Oliveira, J.C. Protein-energy malnutrition at mid-adulthood does not imprint long-term metabolic consequences in male rats. Eur. J. Nutr. 2016, 55, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Thompson, V.; Gildawie, K.; Brenhouse, H.C. Adolescent food restriction in rats alters prefrontal cortex microglia in an experience-dependent manner. Stress 2018, 21, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J. Food restriction in adolescence increases emotional disorder-like behaviors in adult rats. J. Chem. Neuroanat. 2020, 104, 101731. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, C.; Hu, F.; Zhu, W.; Mao, S. Undernutrition-induced lipid metabolism disorder triggers oxidative stress in maternal and fetal livers using a model of pregnant sheep. FASEB J. 2020, 34, 6508–6520. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, N.M.; Chertoff, M.J.; Alberca, C.D.; Berardino, B.G.; Gianatiempo, O.; Brahamian, M.; Levi, V.; Urrutia, L.; Falasco, G.; Canepa, E.T.; et al. Oxidative stress associated with spatial memory impairment and social olfactory deterioration in female mice reveals premature aging aroused by perinatal protein malnutrition. Exp. Neurol. 2023, 368, 114481. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.J. Catch-Up Growth: Basic Mechanisms. Nestle Nutr. Inst. Workshop Ser. 2015, 81, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Sies, H.; Mailloux, R.J.; Jakob, U. Fundamentals of redox regulation in biology. Nat. Rev. Mol. Cell Biol. 2024, 25, 701–719. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Ricken, C.; Dias, G.; Borkenhagen, I.R.; Roecker, A.N.; Bomfim, G.F.; Costermani, H.O.; Dantas Rodrigues, A.M.; Sanches, N.M.; Alves, E.V.; de Oliveira, R.; et al. Okra-supplemented diet prevents hypothalamic inflammation in early overfeeding-programmed obese rats. Brain Res. 2025, 1858, 149641. [Google Scholar] [CrossRef]
- Lesage, J.; Blondeau, B.; Grino, M.; Breant, B.; Dupouy, J.P. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology 2001, 142, 1692–1702. [Google Scholar] [CrossRef]
- Mathias, P.C.F.; Miranda, G.D.S.; Barella, L.F.; Miranda, R.A.; Pavanello, A.; Martins, I.P.; Facchi, J.C.; Costermani, H.O.; Lima, T.A.L.; de Oliveira, J.C. Cholinergic-pathway-weakness-associated pancreatic islet dysfunction: A low-protein-diet imprint effect on weaned rat offspring. J. Dev. Orig. Health Dis. 2020, 11, 484–491. [Google Scholar] [CrossRef]
- Lundbaek, K. Intravenous glucose tolerance as a tool in definition and diagnosis of diabetes mellitus. Br. Med. J. 1962, 1, 1507–1513. [Google Scholar] [CrossRef]
- Patias, N.S.; Sinhorin, V.D.G.; Ferneda, A.; Ferneda, J.M.A.; Sugui, M.M.; Ferrarini, S.R.; Bomfim, G.F.; Lopes, J.W.; Antoniassi, N.A.B.; Cavalheiro, L.; et al. Study of Liposomes Containing Extract from the Leaves of Protium heptaphyllum (Aubl.) March in Animals Submitted to a Mutagenic Model Induced by Cyclophosphamide. Biology 2024, 13, 706. [Google Scholar] [CrossRef]
- Won, E.T.; Borski, R.J. Endocrine regulation of compensatory growth in fish. Front. Endocrinol. 2013, 4, 74. [Google Scholar] [CrossRef]
- de Wit, C.C.; Sas, T.C.; Wit, J.M.; Cutfield, W.S. Patterns of catch-up growth. J. Pediatr. 2013, 162, 415–420. [Google Scholar] [CrossRef]
- Yamamoto, M.; Iguchi, G.; Fukuoka, H.; Suda, K.; Bando, H.; Takahashi, M.; Nishizawa, H.; Seino, S.; Takahashi, Y. SIRT1 regulates adaptive response of the growth hormone--insulin-like growth factor-I axis under fasting conditions in liver. Proc. Natl. Acad. Sci. USA 2013, 110, 14948–14953. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.V.; Torres, N.; Tovar, A.R. PPAR-alpha as a key nutritional and environmental sensor for metabolic adaptation. Adv. Nutr. 2013, 4, 439–452. [Google Scholar] [CrossRef]
- Breier, B.H. Regulation of protein and energy metabolism by the somatotropic axis. Domest. Anim. Endocrinol. 1999, 17, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat. Rev. Endocrinol. 2012, 8, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef]
- Shin, J.; Zhang, D.; Chen, D. Reversible acetylation of metabolic enzymes celebration: SIRT2 and p300 join the party. Mol. Cell 2011, 43, 3–5. [Google Scholar] [CrossRef]
- Jahoor, F. Effects of decreased availability of sulfur amino acids in severe childhood undernutrition. Nutr. Rev. 2012, 70, 176–187. [Google Scholar] [CrossRef]
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in adolescent growth and development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef]
- Vincent, H.K.; Taylor, A.G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. Int. J. Obes. 2006, 30, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, M.; Sharma, T.K.; Mathur, K.; Rathor, J.S.; Butolia, V.; Gadhok, A.K.; Vardey, S.K.; Sinha, M.; Kaushik, G.G. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin. Lab. 2012, 58, 385–392. [Google Scholar]
- de Onis, M.; Monteiro, C.; Akre, J.; Glugston, G. The worldwide magnitude of protein-energy malnutrition: An overview from the WHO Global Database on Child Growth. Bull. World Health Organ. 1993, 71, 703–712. [Google Scholar]
- Ribeiro, R.A.; Santos-Silva, J.C.; Vettorazzi, J.F.; Cotrim, B.B.; Mobiolli, D.D.; Boschero, A.C.; Carneiro, E.M. Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic beta-cells. Amino Acids 2012, 43, 1791–1801. [Google Scholar] [CrossRef]
- Marroqui, L.; Batista, T.M.; Gonzalez, A.; Vieira, E.; Rafacho, A.; Colleta, S.J.; Taboga, S.R.; Boschero, A.C.; Nadal, A.; Carneiro, E.M.; et al. Functional and structural adaptations in the pancreatic alpha-cell and changes in glucagon signaling during protein malnutrition. Endocrinology 2012, 153, 1663–1672. [Google Scholar] [CrossRef]
- Miranda, G.D.S.; de Lima, T.A.L.; Costermani, H.O.; Ricken, C.; Parrela, J.; Membrive, B.L.A.; de Almeida, R.E.; Facchi, J.C.; de Oliveira, L.R.; Miranda, R.A.; et al. Breastfeeding undernutrition changes iBAT-involved thermogenesis protein expression and leads to a lean phenotype in adult rat offspring. J. Nutr. Biochem. 2022, 99, 108857. [Google Scholar] [CrossRef]
- de Freitas Mathias, P.C.; Dantas Rodrigues, A.M.; Lisboa, P.C.; Miranda, R.A.; Malta, A.; Ribeiro, T.A.; Barella, L.F.; Dias, G.; Lima, T.A.L.; Gomes, R.M.; et al. Maternal Low-Protein Diet During Nursing Leads to Glucose-Insulin Dyshomeostasis and Pancreatic-Islet Dysfunction by Disrupting Glucocorticoid Responsiveness in Male Rats. Biology 2024, 13, 1036. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31S–38S. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, A.; Madrigal-Santillan, E.; Bautista, M.; Esquivel-Soto, J.; Morales-Gonzalez, A.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sanchez-Rivera, G.; Valadez-Vega, C.; Morales-Gonzalez, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Santanam, N.; Shern-Brewer, R.; McClatchey, R.; Castellano, P.Z.; Murphy, A.A.; Voelkel, S.; Parthasarathy, S. Estradiol as an antioxidant: Incompatible with its physiological concentrations and function. J. Lipid Res. 1998, 39, 2111–2118. [Google Scholar] [CrossRef]
- Tawarayama, H.; Uchida, K.; Hasegawa, H.; Yoshida, M.; Inoue-Yanagimachi, M.; Sato, W.; Himori, N.; Yamamoto, M.; Nakazawa, T. Estrogen, via ESR2 receptor, prevents oxidative stress-induced Muller cell death and stimulates FGF2 production independently of NRF2, attenuating retinal degeneration. Exp. Eye Res. 2024, 248, 110103. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Liu, Y.; Zhou, S.; Zhou, E.; Wang, Y. Protective Effects of Estrogen on Cardiovascular Disease Mediated by Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 5523516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Martinez, M.N.; Emfinger, C.H.; Palmisano, B.T.; Stafford, J.M. Estrogen signaling prevents diet-induced hepatic insulin resistance in male mice with obesity. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1188–E1197. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Zohar, Y.; Shehadeh, N.; Saadi, T. Glycogenic hepatopathy. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 113–118. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.; Scafidi, S.; Wolfgang, M.J. Hepatic Fatty Acid Oxidation Restrains Systemic Catabolism during Starvation. Cell Rep. 2016, 16, 201–212. [Google Scholar] [CrossRef]
- Himms-Hagen, J. Brown adipose tissue thermogenesis: Interdisciplinary studies. FASEB J. 1990, 4, 2890–2898. [Google Scholar] [CrossRef]
- Cinti, S. The role of brown adipose tissue in human obesity. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 569–574. [Google Scholar] [CrossRef]
- Chua, S.C., Jr.; Chung, W.K.; Wu-Peng, X.S.; Zhang, Y.; Liu, S.M.; Tartaglia, L.; Leibel, R.L. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science 1996, 271, 994–996. [Google Scholar] [CrossRef]
- Phillips, M.S.; Liu, Q.; Hammond, H.A.; Dugan, V.; Hey, P.J.; Caskey, C.J.; Hess, J.F. Leptin receptor missense mutation in the fatty Zucker rat. Nat. Genet. 1996, 13, 18–19. [Google Scholar] [CrossRef]
- Romsos, D.R. Efficiency of energy retention in genetically obese animals and in dietary-induced thermogenesis. Fed. Proc. 1981, 40, 2524–2529. [Google Scholar]
- Tschop, M.; Heiman, M.L. Overview of rodent models for obesity research. Curr. Protoc. Neurosci. 2002, 17, 9–10. [Google Scholar] [CrossRef]
- Morris, M.J.; Tortelli, C.F.; Filippis, A.; Proietto, J. Reduced BAT function as a mechanism for obesity in the hypophagic, neuropeptide Y deficient monosodium glutamate-treated rat. Regul. Pept. 1998, 75–76, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Suzuki, W.; Iizuka, S.; Tabuchi, M.; Maruyama, H.; Takeda, S.; Aburada, M.; Miyamoto, K. Type 2 diabetes mellitus in obese mouse model induced by monosodium glutamate. Exp. Anim. 2006, 55, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ihemelandu, E.C. Fibre number and sizes of mouse soleus muscle in early postnatal protein malnutrition. Cells Tissues Organs 1985, 121, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.W. Effect of low nutrition on size of striated muscle fibres in the mouse. J. Exp. Zool. 1968, 167, 353–358. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saint Paul, J.; Ribeiro, A.J.R.; Kipper, A.C.S.; de Oliveira Souza, M.; Santos, T.C.d.; da Silva, K.P.; Rodrigues, A.M.D.; Antunes, M.F.; Fabiane, I.Z.; Ferneda, A.J.L.B.; et al. Short- and Long-Term Effects of Undernutrition During Adolescence on Oxidative Status and Glucose Homeostasis in Male and Female Rats. Biology 2025, 14, 1352. https://doi.org/10.3390/biology14101352
Saint Paul J, Ribeiro AJR, Kipper ACS, de Oliveira Souza M, Santos TCd, da Silva KP, Rodrigues AMD, Antunes MF, Fabiane IZ, Ferneda AJLB, et al. Short- and Long-Term Effects of Undernutrition During Adolescence on Oxidative Status and Glucose Homeostasis in Male and Female Rats. Biology. 2025; 14(10):1352. https://doi.org/10.3390/biology14101352
Chicago/Turabian StyleSaint Paul, Joskame, Antônio José Rocha Ribeiro, Ana Caroline Schoenberger Kipper, Mariele de Oliveira Souza, Thiara Chaves dos Santos, Karoline Paiva da Silva, Aline Milena Dantas Rodrigues, Manoela Fontenele Antunes, Isabelle Zanata Fabiane, Ana Júlia Lopes Braga Ferneda, and et al. 2025. "Short- and Long-Term Effects of Undernutrition During Adolescence on Oxidative Status and Glucose Homeostasis in Male and Female Rats" Biology 14, no. 10: 1352. https://doi.org/10.3390/biology14101352
APA StyleSaint Paul, J., Ribeiro, A. J. R., Kipper, A. C. S., de Oliveira Souza, M., Santos, T. C. d., da Silva, K. P., Rodrigues, A. M. D., Antunes, M. F., Fabiane, I. Z., Ferneda, A. J. L. B., Sinhorin, V. D. G., Luvizotto, R. d. A. M., & de Oliveira, J. C. (2025). Short- and Long-Term Effects of Undernutrition During Adolescence on Oxidative Status and Glucose Homeostasis in Male and Female Rats. Biology, 14(10), 1352. https://doi.org/10.3390/biology14101352





