Genetic Diversity and Structure for Conservation Genetics of Goldeye Rockfish Sebastes thompsoni (Jordan and Hubbs, 1925) in South Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. Microsatellite Genotyping
2.3. Genetic Diversity Analyses by Microsatellite Markers
2.4. Population Genetic Structure Analysis and Migration Rate
2.5. Historical Effective Population Size Analysis
3. Results
3.1. Microsatellite Genetic Diversity
3.2. Bottleneck Analysis
3.3. Population Structure, Genetic Differentiation Analyses and Gene Flow
3.4. Analysis of Historical Effective Population Size
4. Discussion
4.1. Genetic Diversity and Population Bottleneck
4.2. Population Genetic Structure
4.3. Historical Effective Population Size
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chou, T.-K.; Tang, C.-N. Southward range extension of the goldeye rockfish, Sebastes thompsoni (Actinopterygii: Scorpaeniformes: Scorpaenidae), to northern Taiwan. Acta Ichthyol. Piscat. 2021, 51, 153–158. [Google Scholar] [CrossRef]
- Hauser, L.; Adcock, G.J.; Smith, P.J.; Ramirez, J.H.; Carvalho, G.R. Loss of microsatellite diversity and low effective population size in an overexploited population of New Zealand snapper (Pagrus auratus). Proc. Natl. Acad. Sci. USA 2002, 99, 11742–11747. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; England, P.R.; Luikart, G.; Ritchie, P.A.; Ryman, N. Genetic effects of harvest on wild animal populations. Trends Ecol. Evol. 2008, 23, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Kim, J.K. Upwelling and eddies affect connectivity among local populations of the goldeye rockfish, Sebastes thompsoni (Pisces, Scorpaenoidei). Ecol. Evol. 2018, 8, 4387–4402. [Google Scholar] [CrossRef] [PubMed]
- Andrews, K.; Bartos, B.; Harvey, C.J.; Tonnes, D.; Bhuthimethee, M.; MacCready, P. Testing the potential for larval dispersal to explain connectivity and population structure of threatened rockfish species in Puget Sound. Mar. Ecol. Prog. Ser. 2021, 677, 95–113. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Inference of human population history from individual whole-genome sequences. Nature 2011, 475, 493–496. [Google Scholar] [CrossRef]
- Beichman, A.C.; Huerta-Sanchez, E.; Lohmueller, K.E. Using genomic data to infer historic population dynamics of nonmodel organisms. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 433–456. [Google Scholar] [CrossRef]
- Sekino, M.; Takagi, N.; Hara, M.; Takahashi, H. Microsatellites in rockfish Sebastes thompsoni (Scorpaenidae). Mol. Ecol. 2000, 9, 634–636. [Google Scholar] [CrossRef]
- Beaugrand, G.; Brander, K.M.; Alistair Lindley, J.; Souissi, S.; Reid, P.C. Plankton effect on cod recruitment in the North Sea. Nature 2003, 426, 661–664. [Google Scholar] [CrossRef]
- Gallagher, S.J.; Kitamura, A.; Iryu, Y.; Itaki, T.; Koizumi, I.; Hoiles, P.W. The Pliocene to recent history of the Kuroshio and Tsushima Currents: A multi-proxy approach. Prog. Earth Planet. Sci. 2015, 2, 17. [Google Scholar] [CrossRef]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Frankham, R.; Bradshaw, C.J.; Brook, B.W. Genetics in conservation management: Revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biol. Conserv. 2014, 170, 56–63. [Google Scholar] [CrossRef]
- Wang, J.; Santiago, E.; Caballero, A. Prediction and estimation of effective population size. Heredity 2016, 117, 193–206. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Nikolic, N.; Chevalet, C. Detecting past changes of effective population size. Evol. Appl. 2014, 7, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C. Defining ‘Evolutionarily Significant Units’ for conservation. Trends Ecol. Evol. 1994, 9, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Robin, S.W. Evolutionarily Significant Units and the Conservation of Biological Diversity under the Endangered ‘Species Act. In Evolution and the Aquatic Ecosystem: Defining Unique Units in Population Conservation; American Fisheries Society: Bethesda, MD, USA, 1995; Volume 17, pp. 8–27. [Google Scholar]
- Miller, C.V.; Bossu, C.M.; Sarraco, J.F.; Toews, D.P.L.; Rushing, C.S.; Roberto-Charron, A.; Tremblay, J.A.; Chandler, R.B.; DeSaix, M.G.; Fiss, C.J.; et al. Genomics-informed conservation units reveal spatial variation in climate vulnerability in a migratory bird. Mol. Ecol. 2024, 33, e17199. [Google Scholar] [CrossRef]
- Palsboll, P.J.; Berube, M.; Allendorf, F.W. Identification of management units using population genetic data. Trends Ecol. Evol. 2007, 22, 11–16. [Google Scholar] [CrossRef]
- Sakuma, K.; Yoshikawa, A.; Goto, T.; Fujiwara, K.; Ueda, Y. Delineating management units for Pacific cod (Gadus macrocephalus) in the Sea of Japan. Estuar. Coast. Shelf Sci. 2019, 229, 106401. [Google Scholar] [CrossRef]
- Kucinski, M.; Jakubowska-Lehrmann, M.; Gora, A.; Mirny, Z.; Nadolna-Altyn, K.; Szlinder-Richert, J.; Ocalewicz, K. Population Genetic Study on the European Flounder (Platichthys flesus) from the Southern Baltic Sea Using SNPs and Microsatellite Markers. Animals 2023, 13, 1448. [Google Scholar] [CrossRef]
- Kim, K.-R.; Sung, M.-S.; Kim, K.-S. Population Structure Using Mitochondrial DNA for the Conservation of Liobagrus geumgangensis (Siluriformes: Amblycipitidae), an Endemic Freshwater Fish in Korea. Fishes 2024, 9, 153. [Google Scholar] [CrossRef]
- Wenne, R. Microsatellites as Molecular Markers with Applications in Exploitation and Conservation of Aquatic Animal Populations. Genes 2023, 14, 808. [Google Scholar] [CrossRef] [PubMed]
- Hodel, R.G.; Segovia-Salcedo, M.C.; Landis, J.B.; Crowl, A.A.; Sun, M.; Liu, X.; Gitzendanner, M.A.; Douglas, N.A.; Germain-Aubrey, C.C.; Chen, S.; et al. The report of my death was an exaggeration: A review for researchers using microsatellites in the 21st century. Appl. Plant. Sci. 2016, 4, 1600025. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.R.; Kim, K.Y.; Song, H.Y. Genetic Structure and Diversity of Hatchery and Wild Populations of Yellow Catfish Tachysurus fulvidraco (Siluriformes: Bagridae) from Korea. Int. J. Mol. Sci. 2024, 25, 3923. [Google Scholar] [CrossRef]
- Kim, K.R.; Sung, M.S.; Hwang, Y.; Jeong, J.H.; Yu, J.N. Assessment of the Genetic Diversity and Structure of the Korean Endemic Freshwater Fish Microphysogobio longidorsalis (Gobioninae) Using Microsatellite Markers: A First Glance from Population Genetics. Genes 2024, 15, 69. [Google Scholar] [CrossRef]
- Hou, Y.; Ye, H.; Song, X.; Fan, J.; Li, J.; Shao, J.; Wang, Y.; Lin, D.; Yue, H.; Ruan, R. Genetic diversity and population structure of Chinese longsnout catfish (Leiocassis longirostris) using microsatellite DNA markers. Fishes 2024, 9, 35. [Google Scholar] [CrossRef]
- Asahida, T.; Kobayashi, T.; Saitoh, K.; Nakayama, I. Tissue preservation and total DNA extraction form fish stored at ambient temperature using buffers containing high concentration of urea. Fish. Sci. 1996, 62, 727–730. [Google Scholar] [CrossRef]
- An, H.S.; Park, J.Y.; Kim, M.-J.; Lee, E.Y.; Kim, K.K. Isolation and characterization of microsatellite markers for the heavily exploited rockfish Sebastes schlegeli, and cross-species amplification in four related Sebastes spp. Conserv. Genet. 2009, 10, 1969–1972. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Chapuis, M.-P.; Estoup, A. Microsatellite null alleles and estimation of population differentiation. Mol. Biol. Evol. 2007, 24, 621–631. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Genepop, R.M.R.F. Population genetics software for exact tests and ecumenicism. J. Hered. 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver. 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Piry, S.; Luikart, G.; Cornuet, J.M. Bottleneck: A computer program for detecting recent reductions in the effective population size using allele frequency data. J. Hered. 1999, 90, 502–503. [Google Scholar] [CrossRef]
- Maruyama, T.; Fuerst, P.A. Population bottlenecks and nonequilibrium models in population genetics. II. Number of alleles in a small population that was formed by a recent bottleneck. Genetics 1985, 111, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Cornuet, J.M.; Luikart, G. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 1996, 144, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Z.; Wang, Y.; Liu, M.; Song, A.; Liu, H.; You, F. Genetic assessment of a black rockfish, Sebastes schlegelii, stock enhancement program in Lidao Bay, China based on mitochondrial and nuclear DNA analysis. Front. Mar. Sci. 2020, 7, 94. [Google Scholar] [CrossRef]
- Priolli, R.H.; Bajay, M.M.; Silvano, R.A.; Begossi, A. Population genetic structure of an estuarine and a reef fish species exploited by Brazilian artisanal fishing. Sci. Mar. 2016, 80, 467–477. [Google Scholar] [CrossRef]
- Luikart, G.; Cornuet, J.-M. Empirical evaluation of a test for identifying recently bottlenecked populations from allele frequency data. Conserv. Biol. 1998, 12, 228–237. [Google Scholar] [CrossRef]
- Do, C.; Waples, R.S.; Peel, D.; Macbeth, G.M.; Tillett, B.J.; Ovenden, J.R. NeEstimator v2: Re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol. Ecol. Resour. 2014, 14, 209–214. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Girod, C.; Vitalis, R.; Leblois, R.; Freville, H. Inferring population decline and expansion from microsatellite data: A simulation-based evaluation of the Msvar method. Genetics 2011, 188, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Beerli, P.; Felsenstein, J. Maximum-likelihood estimation of migration rates and effective population numbers in two populations using a coalescent approach. Genetics 1999, 152, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Peery, M.Z.; Kirby, R.; Reid, B.N.; Stoelting, R.; Doucet-Beer, E.; Robinson, S.; Vasquez-Carrillo, C.; Pauli, J.N.; Palsboll, P.J. Reliability of genetic bottleneck tests for detecting recent population declines. Mol. Ecol. 2012, 21, 3403–3418. [Google Scholar] [CrossRef]
- Suzuki, T.; Ouchi, K.; Ikehara, K. On the determination of the age and growth of Sebastes thompsoni (Jordan et Hubbs). Bull. Jpn. Sea Reg. Fish. Res. Lab. 1978, 111–119. [Google Scholar]
- DeFaveri, J.; Merila, J. Temporal stability of genetic variability and differentiation in the three-spined stickleback (Gasterosteus aculeatus). PLoS ONE 2015, 10, e0123891. [Google Scholar] [CrossRef]
- Hall, N.; Mercer, L.; Phillips, D.; Shaw, J.; Anderson, A.D. Maximum likelihood estimation of individual inbreeding coefficients and null allele frequencies. Genet. Res. 2012, 94, 151–161. [Google Scholar] [CrossRef]
- De Meeus, T. Revisiting FIS, FST, Wahlund Effects, and Null Alleles. J. Hered. 2018, 109, 446–456. [Google Scholar] [CrossRef]
- Scheideman, F.F.; Ekernas, L.S.; Swallow, J.G. Genetic viability of small American bison (Bison bison) populations a century after reintroduction. Front. Conserv. Sci. 2025, 6, 1553543. [Google Scholar] [CrossRef]
- Wright, S. Evolution in Mendelian populations. Genetics 1931, 16, 97. [Google Scholar] [CrossRef] [PubMed]
- Shulman, M.J. What can population genetics tell us about dispersal and biogeographic history of coral-reef fishes? Aust. J. Ecol. 1998, 23, 216–225. [Google Scholar] [CrossRef]
- Cowen, R.K.; Sponaugle, S. Larval dispersal and marine population connectivity. Annu. Rev. Mar. Sci. 2009, 1, 443–466. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T. The computer program STRUCTURE does not reliably identify the main genetic clusters within species: Simulations and implications for human population structure. Heredity 2011, 106, 625–632. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Kim, K.-R.; Kim, K.-S.; Bang, I.-C. The impact of weir construction in Korea’s Nakdong River on the population genetic variability of the endangered fish species, rapid small gudgeon (Microphysogobio rapidus). Genes 2023, 14, 1611. [Google Scholar] [CrossRef]
- Kim, K.-R.; Lee, D.; Kim, K.-H.; Kim, H.C.; Kim, S.H.; Park, S.J.; Lee, D.-C. Genetic Diversity and Structure of Korean Pacific Oyster (Crassostrea gigas) for Determining Selective Breeding Groups. Biology 2025, 14, 449. [Google Scholar] [CrossRef]
- Kim, K.-R.; Choi, H.-k.; Lee, T.W.; Lee, H.J.; Yu, J.-N. Population structure and genetic diversity of the spotted sleeper Odontobutis interrupta (Odontobutidae), a fish endemic to Korea. Diversity 2023, 15, 913. [Google Scholar] [CrossRef]
- Wooldridge, B.; Orland, C.; Enbody, E.; Escalona, M.; Mirchandani, C.; Corbett-Detig, R.; Kapp, J.D.; Fletcher, N.; Cox-Ammann, K.; Raimondi, P.; et al. Limited genomic signatures of population collapse in the critically endangered black abalone (Haliotis cracherodii). Mol. Ecol. 2024, e17362. [Google Scholar] [CrossRef]
- Koizumi, I. Diatom-derived SSTs (Td′ ratio) indicate warm seas off Japan during the middle Holocene (8.2–3.3 kyr BP). Mar. Micropaleontol. 2008, 69, 263–281. [Google Scholar] [CrossRef]
- Miller, G.H.; Geirsdóttir, Á.; Zhong, Y.; Larsen, D.J.; Otto-Bliesner, B.L.; Holland, M.M.; Bailey, D.A.; Refsnider, K.A.; Lehman, S.J.; Southon, J.R. Abrupt onset of the Little Ice Age triggered by volcanism and sustained by sea-ice/ocean feedbacks. Geophys. Res. Lett. 2012, 39, L02708. [Google Scholar] [CrossRef]
- Bigman, J.S.; Laurel, B.J.; Kearney, K.; Hermann, A.J.; Cheng, W.; Holsman, K.K.; Rogers, L.A. Predicting Pacific cod thermal spawning habitat in a changing climate. ICES J. Mar. Sci. 2025, 82, fsad096. [Google Scholar] [CrossRef]
- Ólafsdóttir, G.Á.; Westfall, K.M.; Edvardsson, R.; Pálsson, S. Historical DNA reveals the demographic history of Atlantic cod (Gadus morhua) in medieval and early modern Iceland. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132976. [Google Scholar]
- Brander, K. The effect of temperature on growth of Atlantic cod (Gadus morhua L.). ICES J. Mar. Sci. 1995, 52, 1–10. [Google Scholar] [CrossRef]
- Chassot, E.; Bonhommeau, S.; Dulvy, N.K.; Melin, F.; Watson, R.; Gascuel, D.; Le Pape, O. Global marine primary production constrains fisheries catches. Ecol. Lett. 2010, 13, 495–505. [Google Scholar] [CrossRef]
- Park, Y.-H.; Khim, B.-K. Development of the East Korea Warm Current in the Hupo Trough of the southwestern East Sea (Japan Sea) since the Last Glacial Maximum based on TEX86 and U37K′ paleothermometers. Org. Geochem. 2022, 170, 104446. [Google Scholar] [CrossRef]
- Pak, G.; Lee, K.-J.; Lee, S.-W.; Jin, H.; Park, J.-H. Quantification of the extremely intensified East Korea Warm Current in the summer of 2021: Offshore and coastal variabilities. Front. Mar. Sci. 2023, 10, 1252302. [Google Scholar] [CrossRef]
- Kim, J.-G.; Kim, J.-G. Changes in climate factors and catches of fisheries in the Republic of Korea over the three decades. Water 2023, 15, 1952. [Google Scholar] [CrossRef]
- Macura, B.; Byström, P.; Airoldi, L.; Eriksson, B.K.; Rudstam, L.; Støttrup, J.G. Impact of structural habitat modifications in coastal temperate systems on fish recruitment: A systematic review. Environ. Evid. 2019, 8, 14. [Google Scholar] [CrossRef]
- Kim, M.-J.; Han, I.-S.; Lee, J.-S.; Kim, D.-H. Determination of the vulnerability of Korean fish stocks using productivity and susceptibility indices. Ocean. Coast. Manag. 2022, 227, 106287. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S. What caused the collapse of walleye pollock population in Korean waters? KMI Int. J. Marit. Aff. Fish. 2015, 7, 43–58. [Google Scholar] [CrossRef]
- Siple, M.C.; Shelton, A.O.; Francis, T.B.; Lowry, D.; Lindquist, A.P.; Essington, T.E. Contributions of adult mortality to declines of Puget Sound Pacific herring. ICES J. Mar. Sci. 2018, 75, 319–329. [Google Scholar] [CrossRef]
- Licandeo, R.; de la Puente, S.; Christensen, V.; Hilborn, R.; Walters, C. A delay-differential model for representing small pelagic fish stock dynamics and its application for assessing alternative management strategies under environmental uncertainty. Fish Fish. 2023, 24, 544–566. [Google Scholar] [CrossRef]
- Uriarte, A.; Ibaibarriaga, L.; Sánchez-Maroño, S.; Abaunza, P.; Andrés, M.; Duhamel, E.; Jardim, E.; Pawlowski, L.; Prellezo, R.; Roel, B.A. Lessons learnt on the management of short-lived fish from the Bay of Biscay anchovy case study: Satisfying fishery needs and sustainability under recruitment uncertainty. Mar. Policy 2023, 150, 105512. [Google Scholar] [CrossRef]
- Lee, K.N.; Gates, J.; Lee, J. Recent developments in Korean fisheries management. Ocean. Coast. Manag. 2006, 49, 355–366. [Google Scholar]
- Pinsky, M.L.; Palumbi, S.R. Meta-analysis reveals lower genetic diversity in overfished populations. Mol. Ecol. 2014, 23, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Bang, M.; Joh, Y.; Ham, Y.-G.; Kang, N.; Jang, C.J. Characteristics and mechanisms of marine heatwaves in the East Asian marginal seas: Regional and seasonal differences. Remote Sens. 2022, 14, 3522. [Google Scholar] [CrossRef]
- Free, C.M.; Thorson, J.T.; Pinsky, M.L.; Oken, K.L.; Wiedenmann, J.; Jensen, O.P. Impacts of historical warming on marine fisheries production. Science 2019, 363, 979–983. [Google Scholar] [CrossRef]
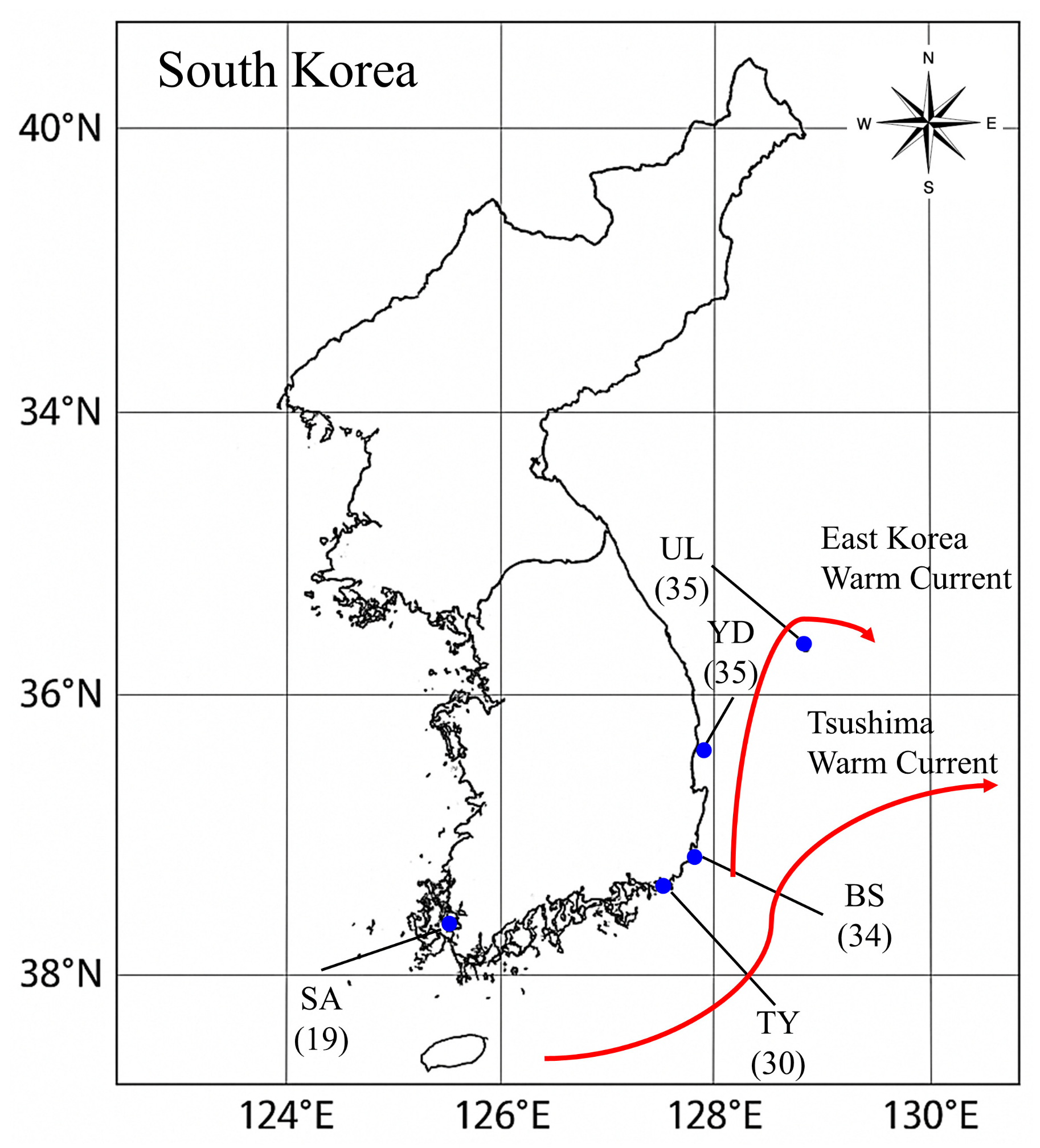
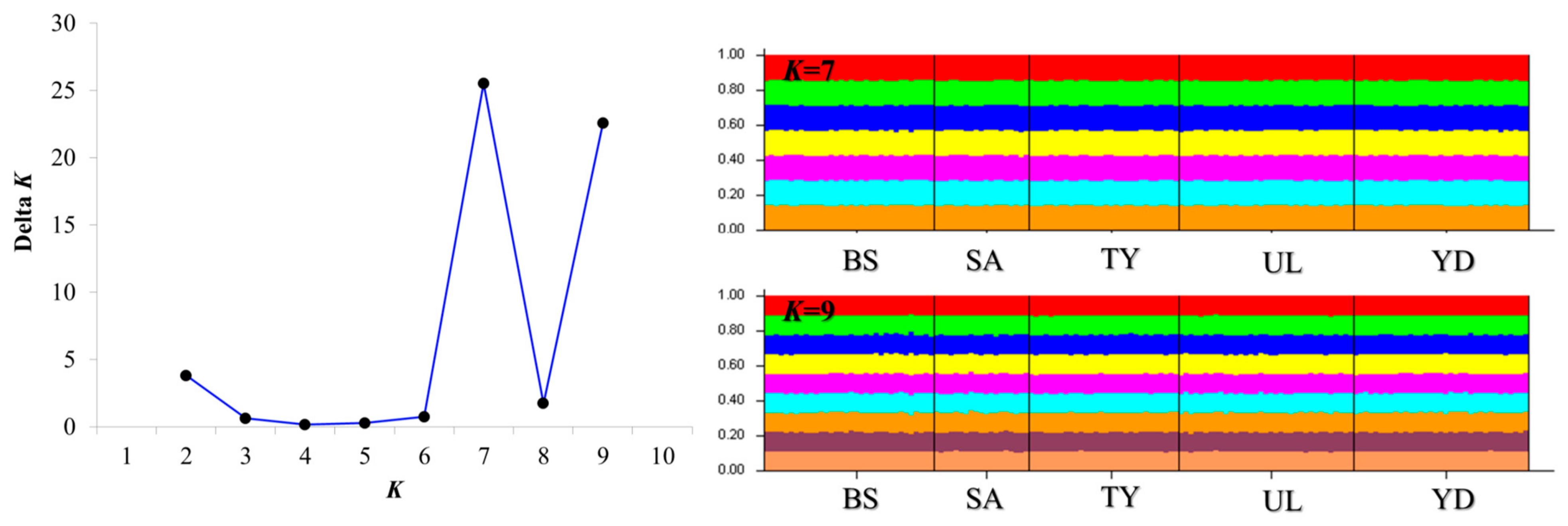
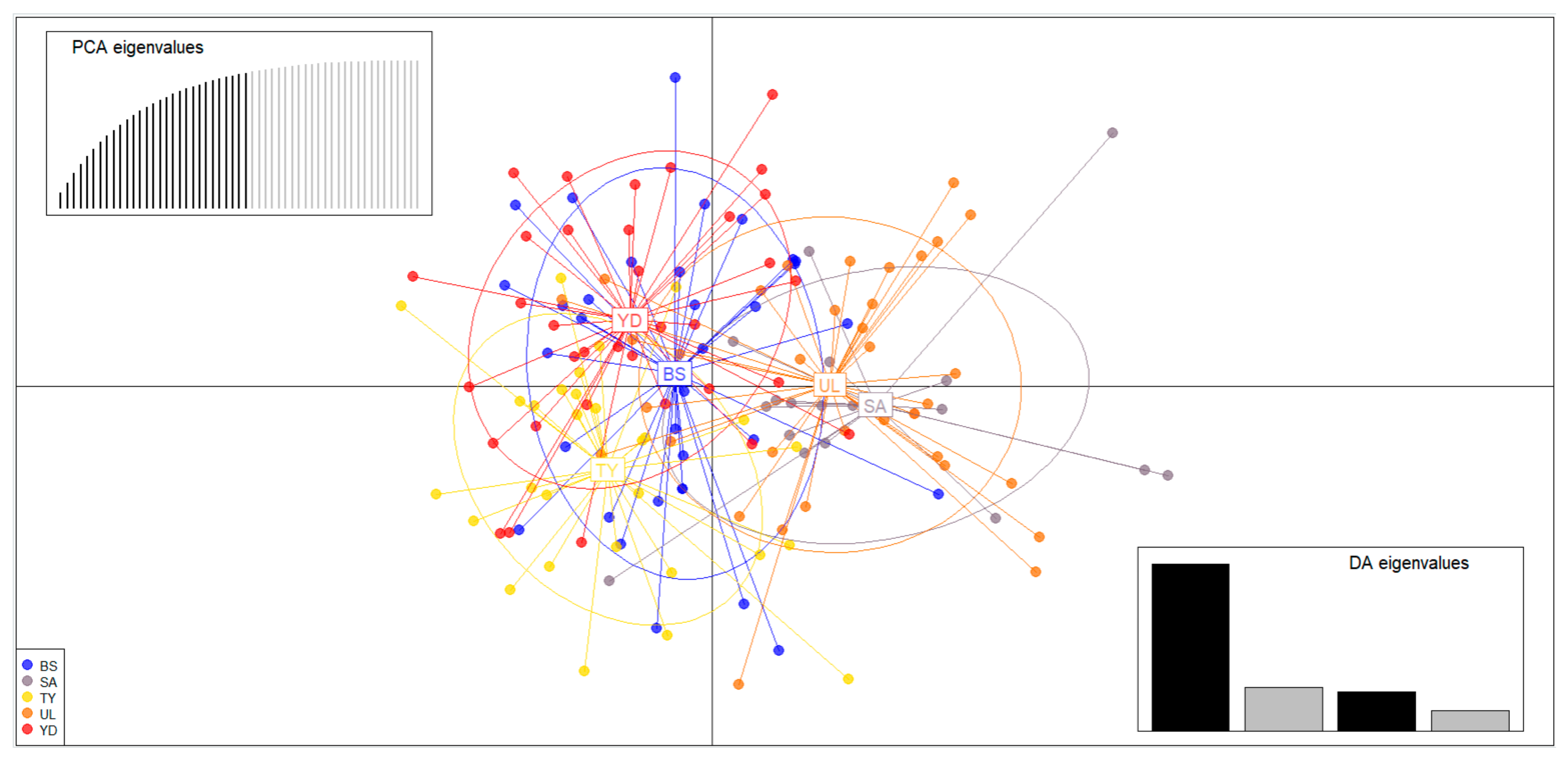
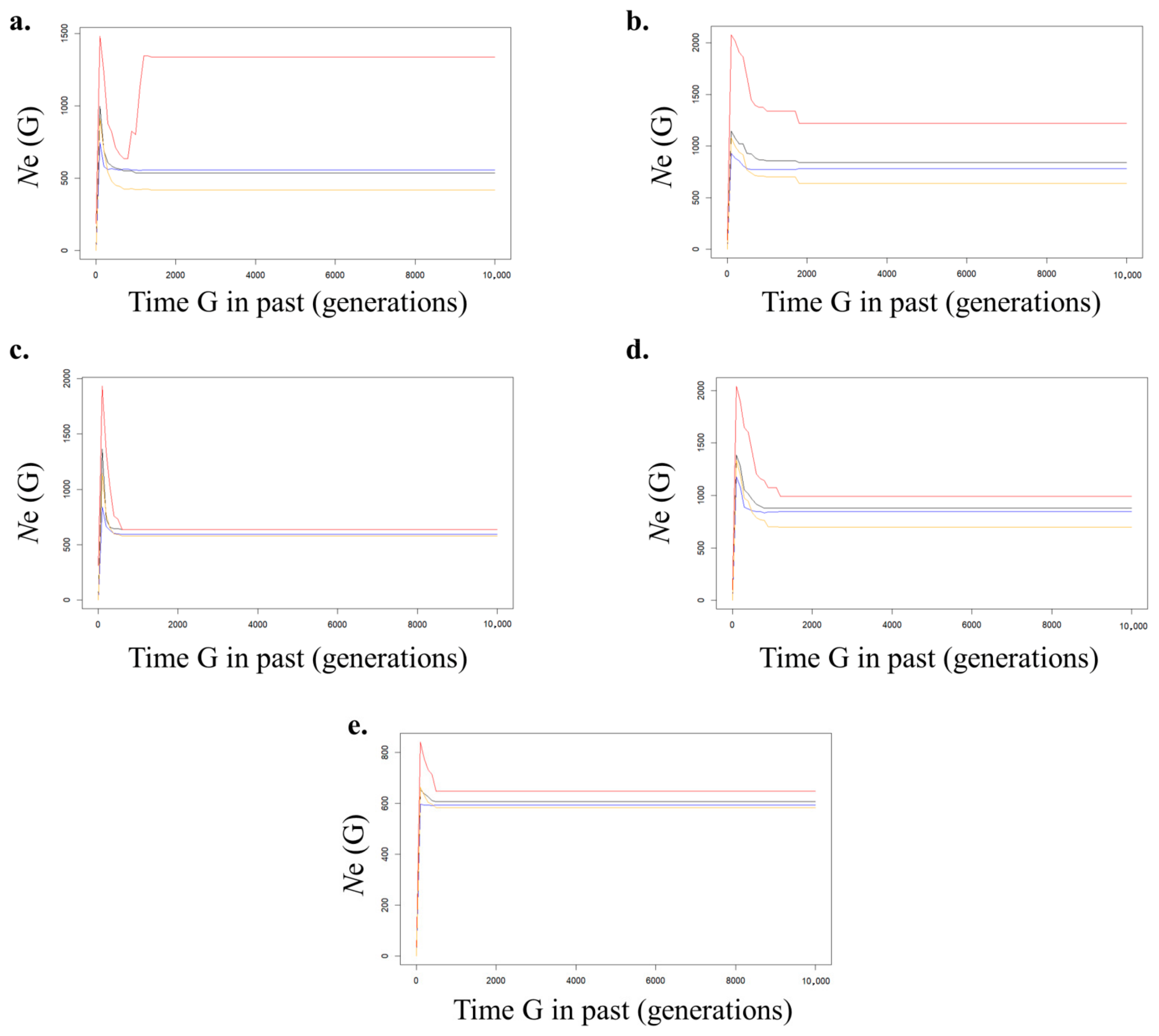
| ID | Region Name | N | NA | AR | HO | HE | PHWE | FIS |
|---|---|---|---|---|---|---|---|---|
| BS | Busan | 34 | 7.3 | 6.54 | 0.790 | 0.680 | 0.000 *** | −0.160 |
| SA | Sinan | 19 | 6.3 | 6.29 | 0.759 | 0.686 | 0.000 *** | −0.111 |
| TY | Tongyeong | 30 | 7.0 | 6.38 | 0.814 | 0.699 | 0.000 *** | −0.168 |
| UL | Ulleungdo | 35 | 7.3 | 6.55 | 0.767 | 0.696 | 0.000 *** | −0.105 |
| YD | Yeongduk | 35 | 7.3 | 6.30 | 0.816 | 0.659 | 0.000 *** | −0.243 *** |
| Population ID | N | Wilcoxon Signed-Rank Test | Ne | (95% CI) | |||
|---|---|---|---|---|---|---|---|
| PIAM | PTPM | PSMM | Mode-Shift | ||||
| BS | 34 | 0.008 ** | 0.039 * | 0.078 | Shifted | 127 | (56–∞) |
| SA | 19 | 0.008 ** | 0.016 * | 0.015 * | Shifted | 254 | (31–∞) |
| TY | 30 | 0.016 * | 0.016 * | 0.023 * | Shifted | - | (69–∞) |
| UL | 35 | 0.008 ** | 0.008 ** | 0.008 * | Shifted | 166 | (55–∞) |
| YD | 35 | 0.008 ** | 0.008 * | 0.008 * | Shifted | 108 | (44–∞) |
| BS | SA | TY | UL | YD | |
|---|---|---|---|---|---|
| BS | - | 0.139 | 0.218 | 0.322 | 0.177 |
| SA | 0.000 | - | 0.032 | 0.995 | 0.008 |
| TY | 0.002 | 0.008 | - | 0.163 | 0.070 |
| UL | 0.000 | 0.000 | 0.001 | - | 0.019 |
| YD | 0.000 | 0.008 | 0.002 | 0.005 | - |
| K | Estimated Ln Prob of Data (L(K)) | Mean Value of Ln Likelihood | Variance of Ln Likelihood | Mean Value of Alpha (α) |
|---|---|---|---|---|
| 1 | −3331.3 | −3316.7 | 29.1 | - |
| 2 | −3406.4 | −3272.0 | 268.9 | 0.9695 |
| 3 | −3487.6 | −3293.1 | 389.1 | 1.5947 |
| 4 | −3383.8 | −3302.1 | 163.4 | 2.7589 |
| 5 | −3348.2 | −3307.7 | 80.9 | 1.697 |
| 6 | −3352.4 | −3307.1 | 90.6 | 1.8328 |
| 7 | −3337.5 | −3307.5 | 60.2 | 3.844 |
| 8 | −3333.1 | −3310.2 | 45.8 | 4.5462 |
| 9 | −3370.5 | −3303.9 | 133.3 | 1.5993 |
| 10 | −3348.7 | −3305.1 | 87.2 | 2.758 |
| Source of Variation | Sum of Squares | Variance Components | Percentage of Variance | FST |
|---|---|---|---|---|
| Among groups | 10.311 | 0.00304 | 0.13 | |
| Within populations | 720.444 | 2.39350 | 99.87 | 0.000 |
| Total | 730.755 | 2.39654 | 100.00 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-R.; Kim, K.-S.; Yoon, S.J. Genetic Diversity and Structure for Conservation Genetics of Goldeye Rockfish Sebastes thompsoni (Jordan and Hubbs, 1925) in South Korea. Biology 2025, 14, 1559. https://doi.org/10.3390/biology14111559
Kim K-R, Kim K-S, Yoon SJ. Genetic Diversity and Structure for Conservation Genetics of Goldeye Rockfish Sebastes thompsoni (Jordan and Hubbs, 1925) in South Korea. Biology. 2025; 14(11):1559. https://doi.org/10.3390/biology14111559
Chicago/Turabian StyleKim, Kang-Rae, Keun-Sik Kim, and Sung Jin Yoon. 2025. "Genetic Diversity and Structure for Conservation Genetics of Goldeye Rockfish Sebastes thompsoni (Jordan and Hubbs, 1925) in South Korea" Biology 14, no. 11: 1559. https://doi.org/10.3390/biology14111559
APA StyleKim, K.-R., Kim, K.-S., & Yoon, S. J. (2025). Genetic Diversity and Structure for Conservation Genetics of Goldeye Rockfish Sebastes thompsoni (Jordan and Hubbs, 1925) in South Korea. Biology, 14(11), 1559. https://doi.org/10.3390/biology14111559







