Changes in Intestinal Microbial Community of the Black Tiger Shrimp Penaeus monodon in Response to Triclocarban Exposure
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Shrimp and Rearing Conditions
2.3. TCC Exposure Experiment and Sampling
2.4. 16S rDNA High-Throughput Sequencing of Intestinal Microbiota
2.5. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
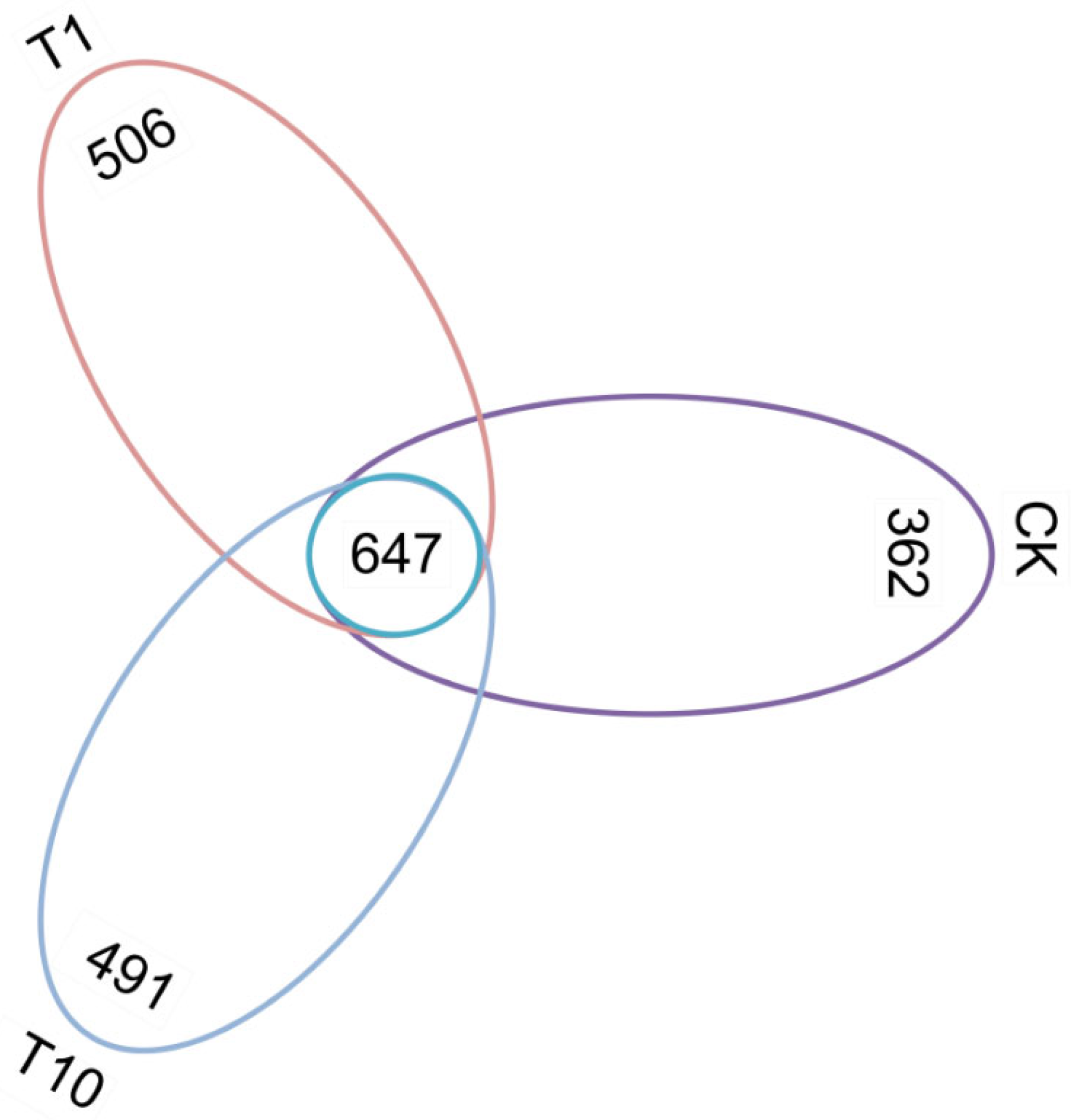
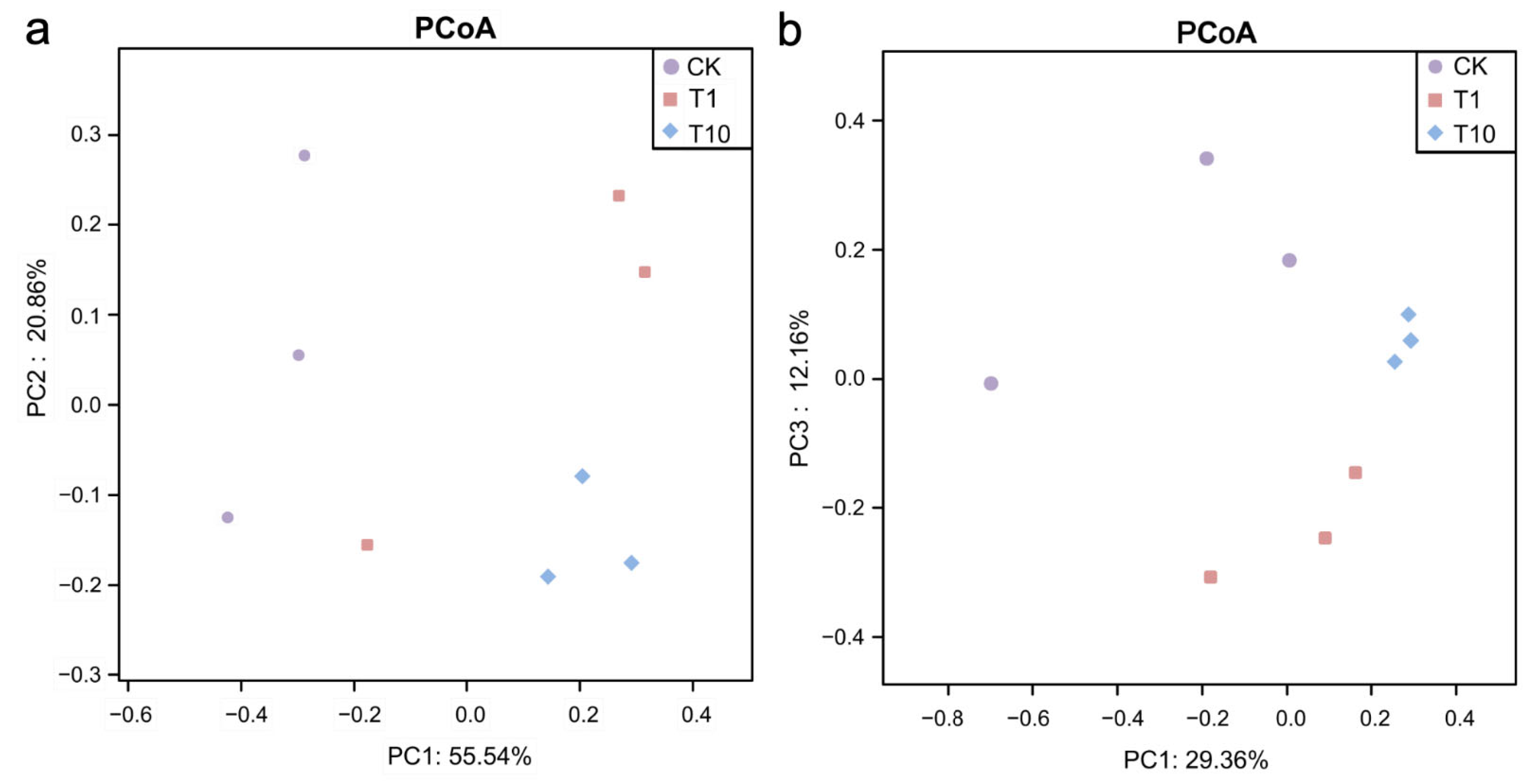
3.1. Changes in Intestinal Microbial Diversity
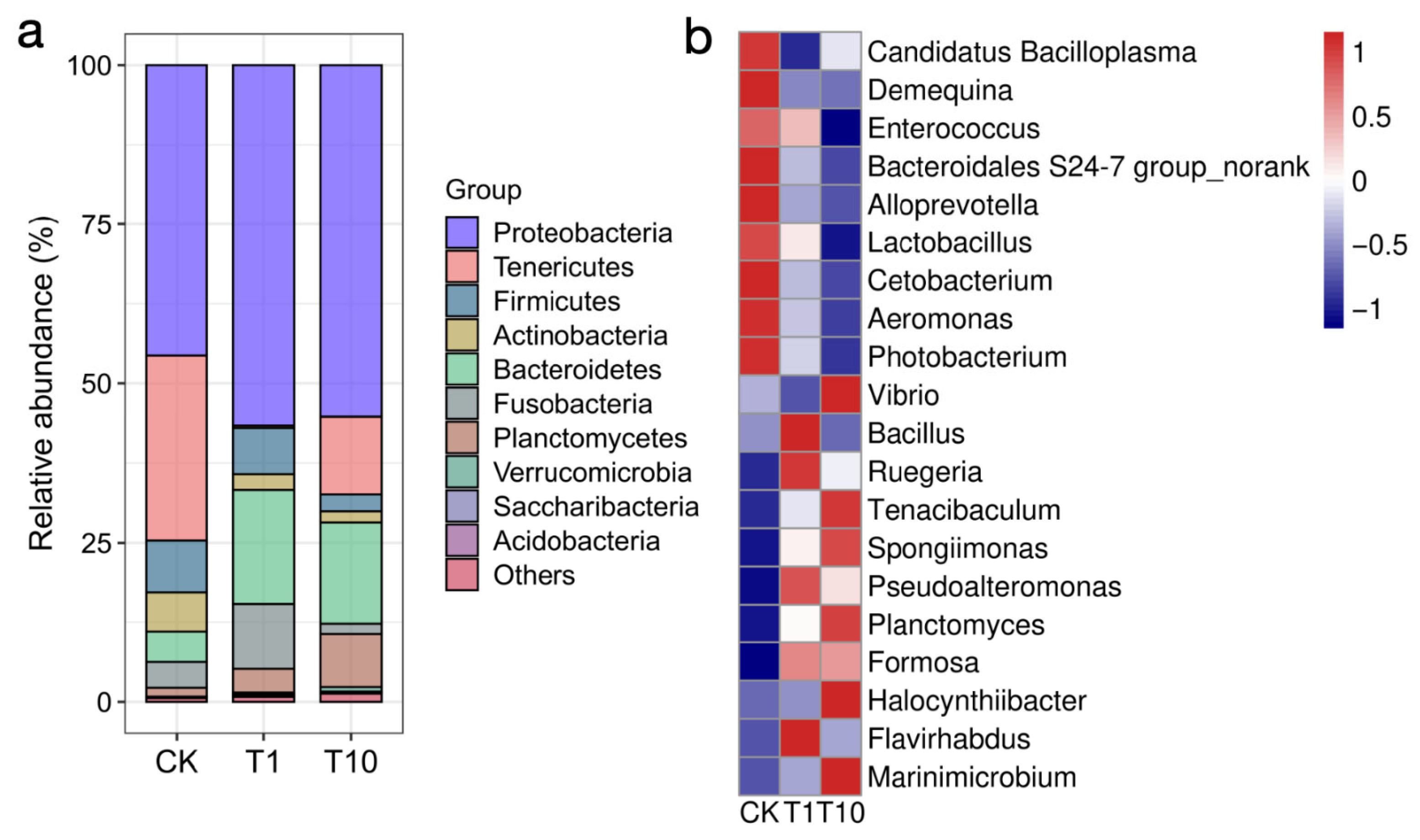
3.2. Changes in the Composition of Intestinal Microbiota
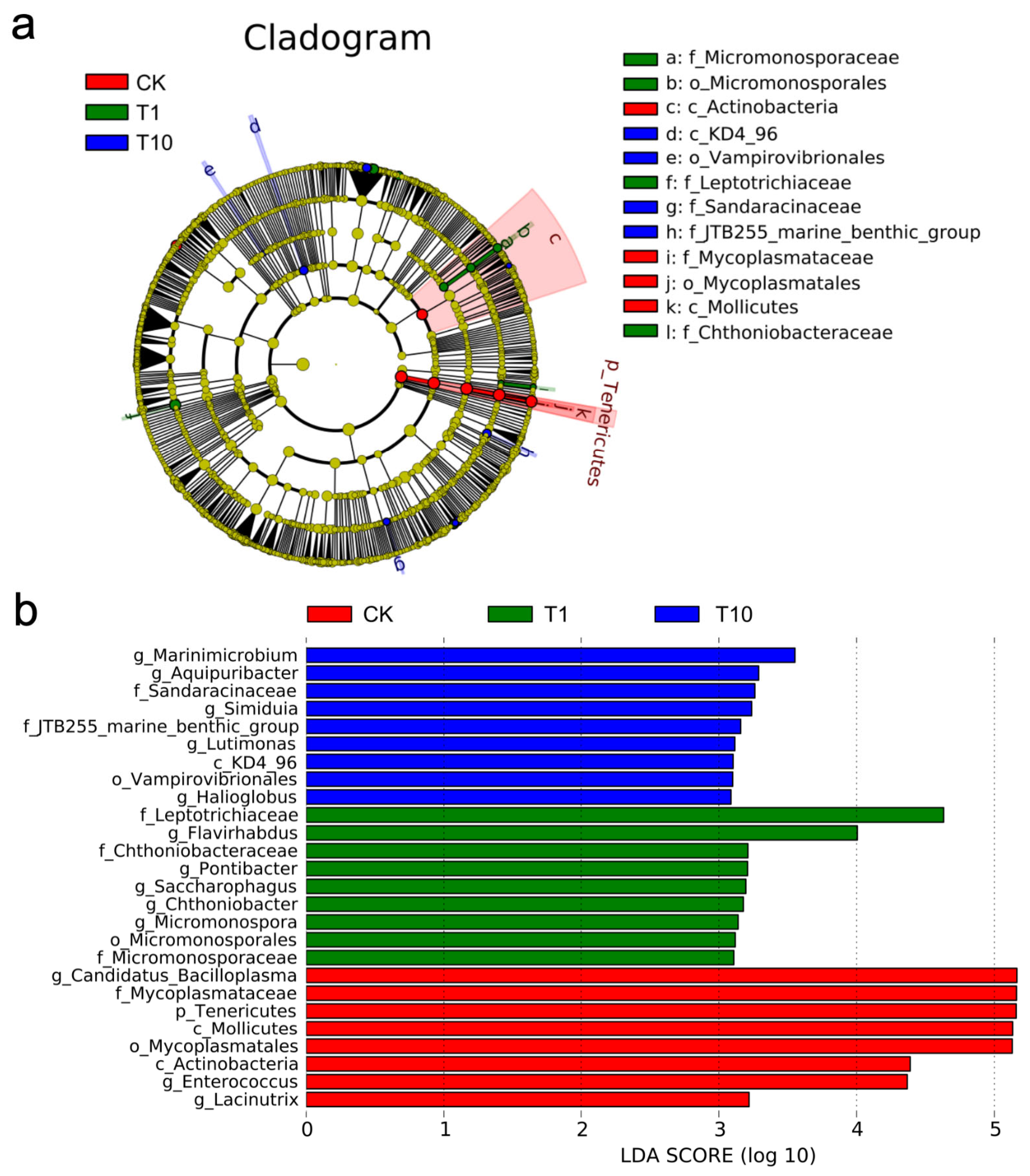
3.3. Identification of Key Biomarkers of Intestinal Microbiota
3.4. Network Relationships Among Intestinal Bacteria
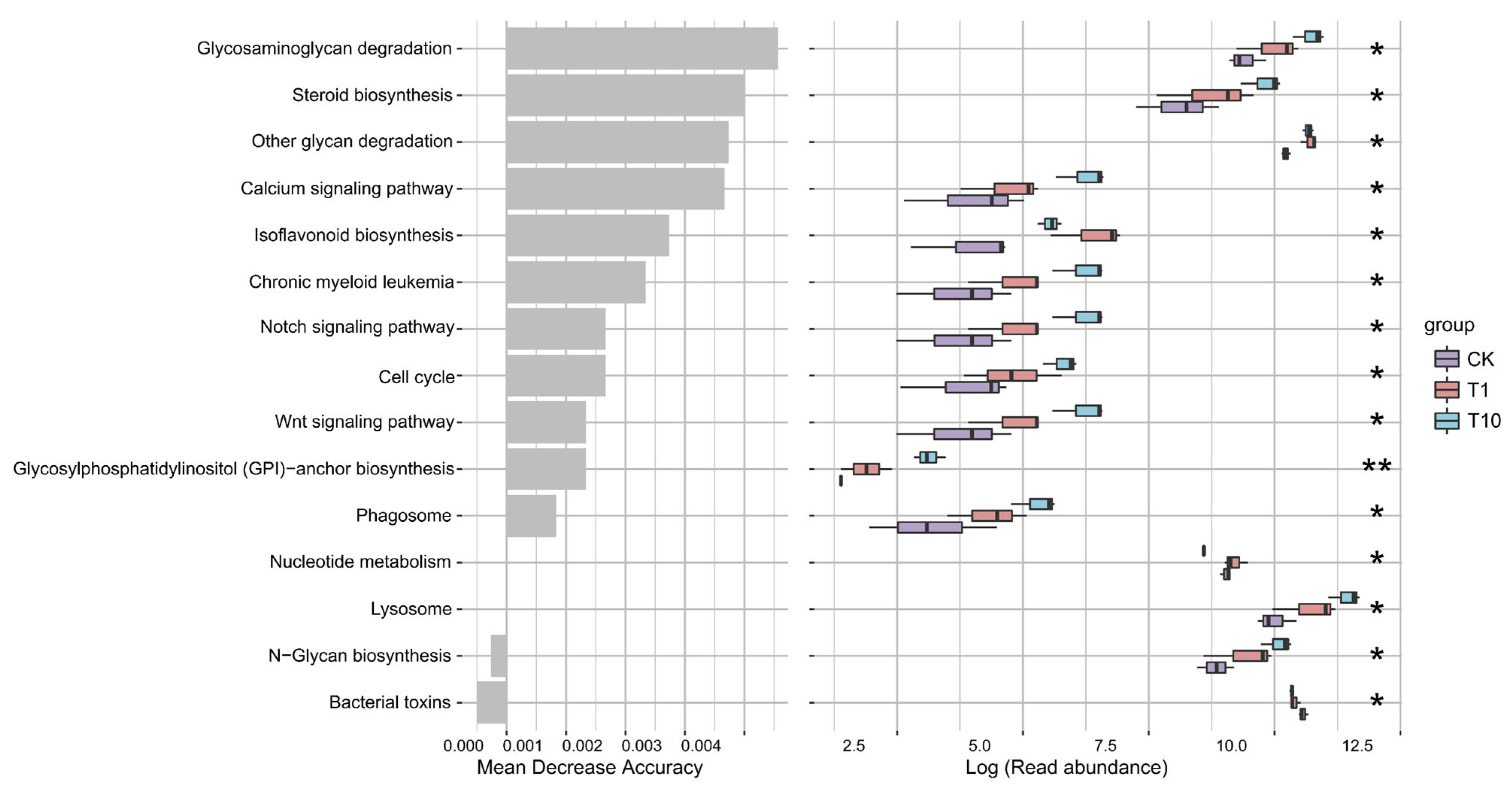
3.5. Alterations in the Intestinal Microbiota Metabolic Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Z.; He, F.; Li, X.; Li, Y.; Huo, C.; Wang, H.; Qi, Y.; Tian, G.; Zong, W.; Liu, R. Response pathways of superoxide dismutase and catalase under the regulation of triclocarban-triggered oxidative stress in Eisenia foetida: Comprehensive mechanism analysis based on cytotoxicity and binding model. Sci. Total Environ. 2022, 854, 158821. [Google Scholar] [CrossRef]
- Clarke, B.O.; Smith, S.R. Review of ’emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environ. Int. 2011, 37, 226–247. [Google Scholar] [CrossRef]
- Caioni, G.; d’Angelo, M.; Panella, G.; Merola, C.; Cimini, A.; Amorena, M.; Benedetti, E.; Perugini, M. Environmentally relevant concentrations of triclocarban affect morphological traits and melanogenesis in zebrafish larvae. Aquat. Toxicol. 2021, 236, 105842. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U.; Paull, D.H. Co-occurrence of triclocarban and triclosan in U.S. water resources. Environ. Sci. Technol. 2005, 39, 6335–6336. [Google Scholar] [CrossRef]
- Vimalkumar, K.; Arun, E.; Krishna-Kumar, S.; Poopal, R.K.; Nikhil, N.P.; Subramanian, A.; Babu-Rajendran, R. Occurrence of triclocarban and benzotriazole ultraviolet stabilizers in water, sediment, and fish from Indian rivers. Sci. Total Environ. 2018, 625, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Ying, G.G.; Liu, Y.S.; Chen, F.; Yang, J.F.; Wang, L. Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: From source to the receiving environment. J. Hazard. Mater. 2010, 179, 215–222. [Google Scholar] [CrossRef]
- Gomes, M.F.; de Carvalho Soares de Paula, V.; Rocha Martins, L.R.; Esquivel Garcia, J.R.; Yamamoto, F.Y.; Martins de Freitas, A. Sublethal effects of triclosan and triclocarban at environmental concentrations in silver catfish (Rhamdia quelen) embryos. Chemosphere 2021, 263, 127985. [Google Scholar] [CrossRef]
- Schultz, M.M.; Bartell, S.E.; Schoenfuss, H.L. Effects of triclosan and triclocarban, two ubiquitous environmental contaminants, on anatomy, physiology, and behavior of the fathead minnow (Pimephales promelas). Arch. Environ. Contam. Toxicol. 2012, 63, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Wang, B.Y.; Jin, Y.T.; Liu, Z.Q.; Zhang, H.J. Triclocarban and triclosan disturb lipometabolism via PPAR in black-spotted frogs: In vivo and molecular dynamics simulation studies. Aquat. Toxicol. 2025, 286, 107436. [Google Scholar] [CrossRef]
- Nan, Y.X.; Zhu, X.Y.; Huang, J.H.; Zhang, Z.; Xing, Y.F.; Yang, Y.K.; Xiao, M.; Duan, Y.F. Toxic effects of triclocarban on the histological morphology, physiological and immune response in the gills of the black tiger shrimp Penaeus monodon. Mar. Environ. Res. 2023, 192, 106245. [Google Scholar] [CrossRef]
- Li, X.M.; Ringø, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618. [Google Scholar] [CrossRef]
- Xiao, F.S.; Zhu, W.G.; Yu, Y.H.; He, Z.L.; Wu, B.; Wang, C. Host development overwhelms environmental dispersal in governing the ecological succession of zebrafish gut microbiota. NPJ Biofilms Microbiol. 2021, 7, 5. [Google Scholar] [CrossRef]
- Chen, L.G.; Ahmad, M.; Li, J.; Li, J.L.; Yang, Z.X.; Hu, C.Y. Gut microbiota manipulation to mitigate the toxicities of environmental pollutants. Aquat. Toxicol. 2025, 285, 107425. [Google Scholar] [CrossRef]
- Gómez, G.D.; Balcázar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar]
- Xiao, F.S.; Liao, L.J.; Xu, Q.Q.; He, Z.L.; Xiao, T.Y.; Wang, J.J. Host-microbiota interactions and responses to grass carp reovirus infection in Ctenopharyngodon idellus. Environ. Microbiol. 2021, 23, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lei, H.; Cao, Z.; Zhang, C.; Chen, C.; Wu, M.; Zhang, H.; Du, R.; Li, L.J.; Chen, X.; et al. Long-term triclocarban exposure induces enterotoxicity by triggering intestinal AHR-mediated inflammation and disrupting microbial community in mice. Chem. Res. Toxicol. 2024, 37, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.G.; Du, J.Z.; Zhang, T.X.; Sun, Q.H.; Sun, B.B.; Zhang, Y.; Li, S. Impairment of the gut health in Danio rerio exposed to triclocarban. Sci. Total Environ. 2022, 832, 155025. [Google Scholar] [CrossRef]
- Wang, H.L.; Liu, X.C.; Zhao, C.X.; Yan, J.; Wang, Z.J.; Dahlgren, R.A.; Qian, Q.H.; Wang, X.D. Interference of gut-brain-gonad axis originating from triclocarban exposure to parent zebrafish induces offspring embryonic development abnormality by up-regulation of maternal circSGOL1. Aquat. Toxicol. 2024, 266, 106782. [Google Scholar] [CrossRef]
- Sang, M.Y.; Liu, S.Y.; Yan, H.H.; Zhang, B.; Chen, S.Y.; Wu, B.W.; Ma, T.; Jiang, H.Y.; Zhao, P.C.; Sun, G.J.; et al. Synergistic detoxification efficiency and mechanism of triclocarban degradation by a bacterial consortium in the liver-gut-microbiota axis of zebrafish (Danio rerio). J. Hazard. Mater. 2024, 470, 134178. [Google Scholar] [CrossRef]
- Wang, B.Y.; Sun, W.H.; Ye, X.D.; Hoskins, T.D.; Han, Y.; Yuan, X.; Chen, Q.; Liu, Z.Q.; Zhang, H.J. Sustained exposure to triclocarban and triclosan at environmental relevant concentration disrupts gut-liver axis in the black-spotted frog. Aquat. Toxicol. 2025, 286, 107463. [Google Scholar] [CrossRef]
- Lin, Z.; He, Z.; Liao, W.; Liao, M.; Zhou, J.; Xue, Y.; Sun, C. Dietary supplementation with chondroitin sulfate improved the growth, immunity, and intestinal microbiota structure of Penaeus monodon. Aquaculture 2023, 563, 738952. [Google Scholar] [CrossRef]
- Fan, B.; Li, J.; Wang, X.; Gao, X.; Chen, J.; Ai, S.; Li, W.; Huang, Y.; Liu, Z. Study of aquatic life criteria and ecological risk assessment for triclocarban (TCC). Environ. Pollut. 2019, 254, 112956. [Google Scholar] [CrossRef]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, K.; Wu, J.; Qiuqian, L.; Yang, K.; Qian, Y. Changes in intestinal bacterial communities are closely associated with shrimp disease severity. Appl. Microbiol. Biotechnol. 2015, 99, 6911–6919. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- Graça, A.P.; Calisto, R.; Lage, O.M. Planctomycetes as novel source of bioactive molecules. Front. Microbiol. 2016, 7, 1241. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E. A core gut microbiome in obese and lean twins. Nature 2008, 457, 480. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Duan, Y.F.; Nan, Y.X.; Zhu, X.Y.; Yang, Y.K.; Xing, Y.F. The adverse impacts of ammonia stress on the homeostasis of intestinal health in Pacific white shrimp (Litopenaeus vannamei). Environ. Pollut. 2024, 340, 122762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Q.; Zeng, S.; Deng, Z.; Liu, Z.; Li, X. Succeed to culture a novel lineage symbiotic bacterium of Mollicutes which widely found in arthropods intestine uncovers the potential double-edged sword ecological function. Front. Microbiol. 2024, 15, 1458382. [Google Scholar] [CrossRef]
- Downes, J.; Dewhirst, F.E.; Tanner, A.C.R.; Wade, W.G. Description of Alloprevotella rava gen. nov., sp. nov., isolated from the human oral cavity, and reclassification of Prevotella tannerae Moore et al. 1994 as Alloprevotella tannerae gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2013, 63, 1214–1218. [Google Scholar] [CrossRef]
- Frolova, M.S.; Suvorova, I.A.; Iablokov, S.N.; Petrov, S.N.; Rodionov, D.A. Genomic reconstruction of short-chain fatty acid production by the human gut microbiota. Front. Mol. Biosci. 2022, 9, 949563. [Google Scholar] [CrossRef]
- Wang, A.R.; Zhang, Z.; Ding, Q.W.; Yang, Y.L.; Bindelle, J.; Ran, C.; Zhou, Z.G. Intestinal cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Mayo, B.; BelénFlórez, A.B. Lactic acid bacteria: Lactobacillus spp.: Lactobacillus plantarum. In Encyclopedia of Dairy Sciences, 3rd ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 206–217. [Google Scholar]
- Sun, X.; Fang, Z.; Yu, H.; Zhao, H.; Wang, Y.; Zhou, F.; Zhao, L.; Sun, J.; Tian, Y. Effects of Enterococcus faecium (R8a) on nonspecific immune gene expression, immunity and intestinal flora of giant tiger shrimp (Penaeus monodon). Sci. Rep. 2024, 14, 1823. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.W.; Wang, L.H.; Hassana, M.; Xie, B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresour. Technol. 2018, 256, 333–341. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, C.D.; Tang, Y.; Sun, B.C.; Huang, J.; Song, X.L. Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture 2018, 494, 30–36. [Google Scholar] [CrossRef]
- Mann, A.J.; Hahnke, R.L.; Huang, S.; Werner, J.; Xing, P.; Barbeyron, T. The genome of the alga-associated marine Flavobacterium Formosa agariphila KMM 3901™ reveals a broad potential for degradation of algal polysaccharides. Appl. Environ. Microbiol. 2013, 79, 6813–6822. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, S.; Xiong, J.; Hou, D.; Zhou, R.; Xing, C. Microecological Koch’s postulates reveal that intestinal microbiota dysbiosis contributes to shrimp white feces syndrome. Microbiome 2020, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, G.; Li, F. Characterization of two pathogenic Photobacterium strains isolated from Exopalaemon carinicauda causing mortality of shrimp. Aquaculture 2016, 464, 129–135. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; MoralesCovarrubias, M.S. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in northwestern Mexico. Appl. Environ. Microbiol. 2015, 81, 1689–1699. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kawato, S.; Imaizumi, K.; Furukawa, M.; Konishi, K.; Nozaki, R.; Koiwai, K.; Kondo, H.; Hirono, I. Studies on pathogenic mechanisms in Tenacibaculum mesophilum isolated from the gills of kuruma shrimp (Penaeus japonicus) with fusarium disease. Fish Shellfish Immunol. 2022, 131, 1321. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Nan, Y.; Huang, J.; Zhang, Z.; Sui, Y.; Dan, X. Changes in Intestinal Microbial Community of the Black Tiger Shrimp Penaeus monodon in Response to Triclocarban Exposure. Biology 2025, 14, 1542. https://doi.org/10.3390/biology14111542
Duan Y, Nan Y, Huang J, Zhang Z, Sui Y, Dan X. Changes in Intestinal Microbial Community of the Black Tiger Shrimp Penaeus monodon in Response to Triclocarban Exposure. Biology. 2025; 14(11):1542. https://doi.org/10.3390/biology14111542
Chicago/Turabian StyleDuan, Yafei, Yuxiu Nan, Jianhua Huang, Zhe Zhang, Yanming Sui, and Xueming Dan. 2025. "Changes in Intestinal Microbial Community of the Black Tiger Shrimp Penaeus monodon in Response to Triclocarban Exposure" Biology 14, no. 11: 1542. https://doi.org/10.3390/biology14111542
APA StyleDuan, Y., Nan, Y., Huang, J., Zhang, Z., Sui, Y., & Dan, X. (2025). Changes in Intestinal Microbial Community of the Black Tiger Shrimp Penaeus monodon in Response to Triclocarban Exposure. Biology, 14(11), 1542. https://doi.org/10.3390/biology14111542









