Climate Change vs. Human Activities: Conflicting Future Impacts on a High-Altitude Endangered Snake (Thermophis baileyi)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Framework
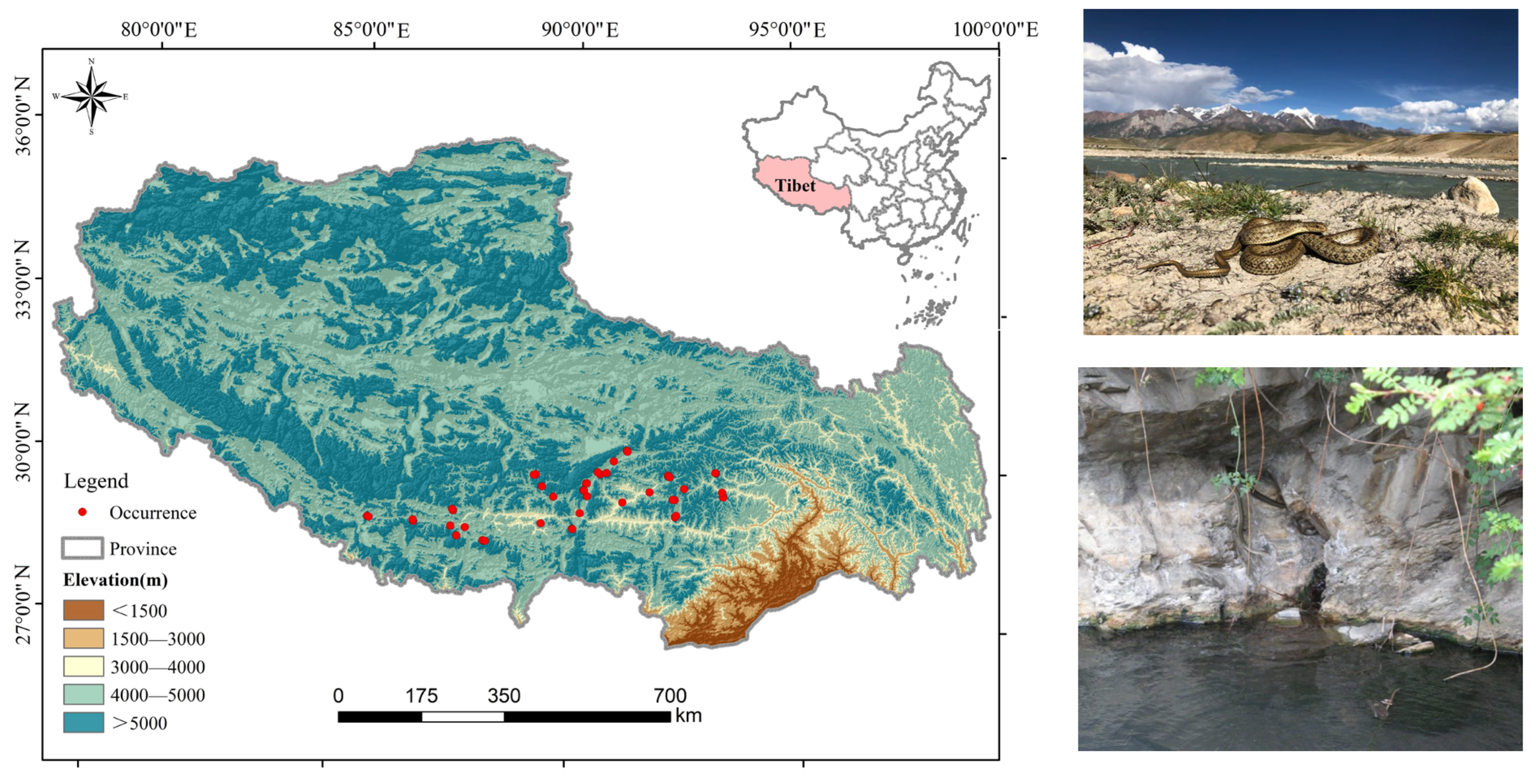
2.2. Species Occurrence Data
2.3. Environmental Variables
2.4. Models
2.4.1. Scenario Settings
2.4.2. Prediction of Future Land Cover
2.4.3. Prediction of Potential Distributions
3. Results
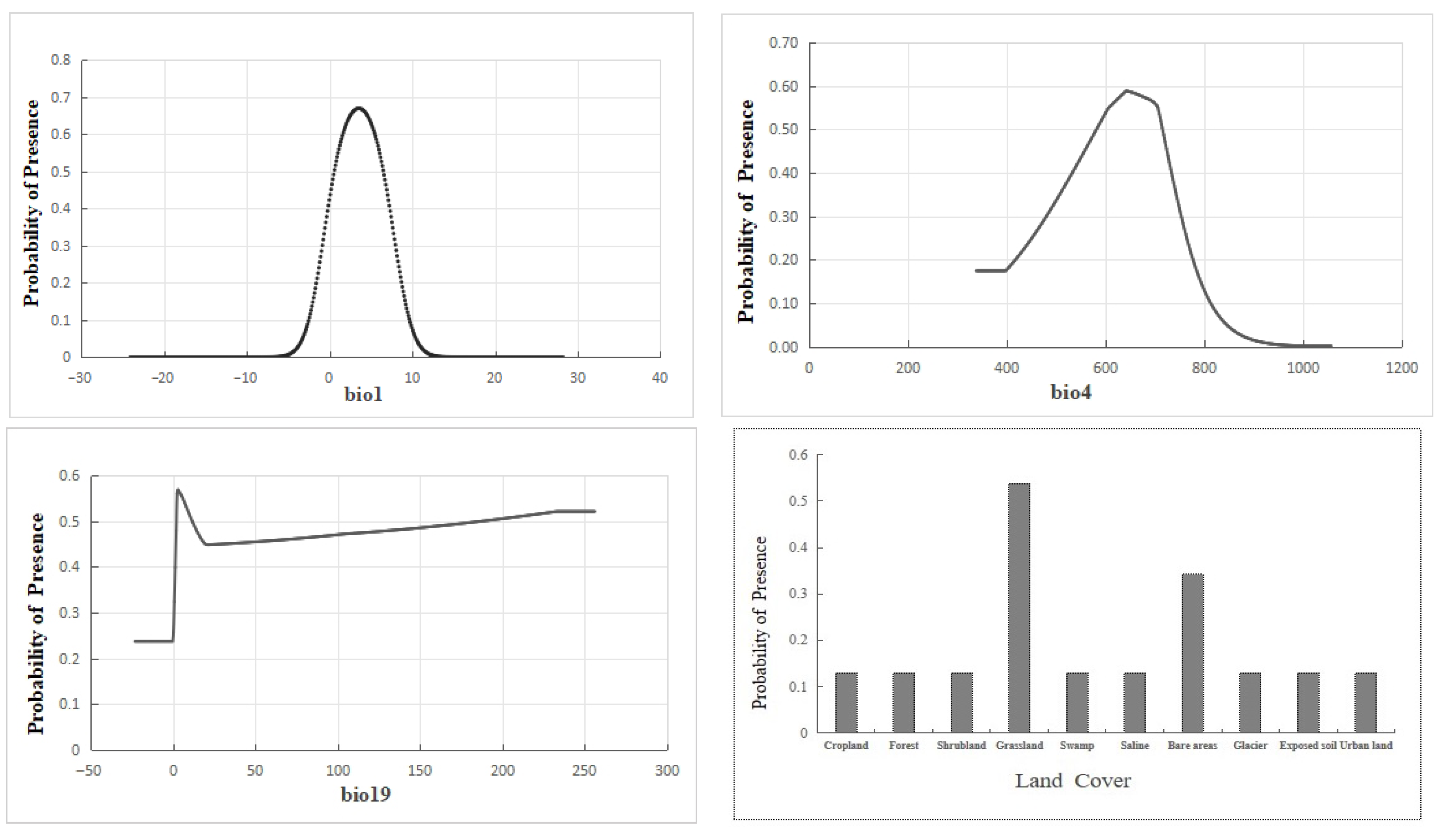
3.1. Dominant Environmental Variables and Response Curves
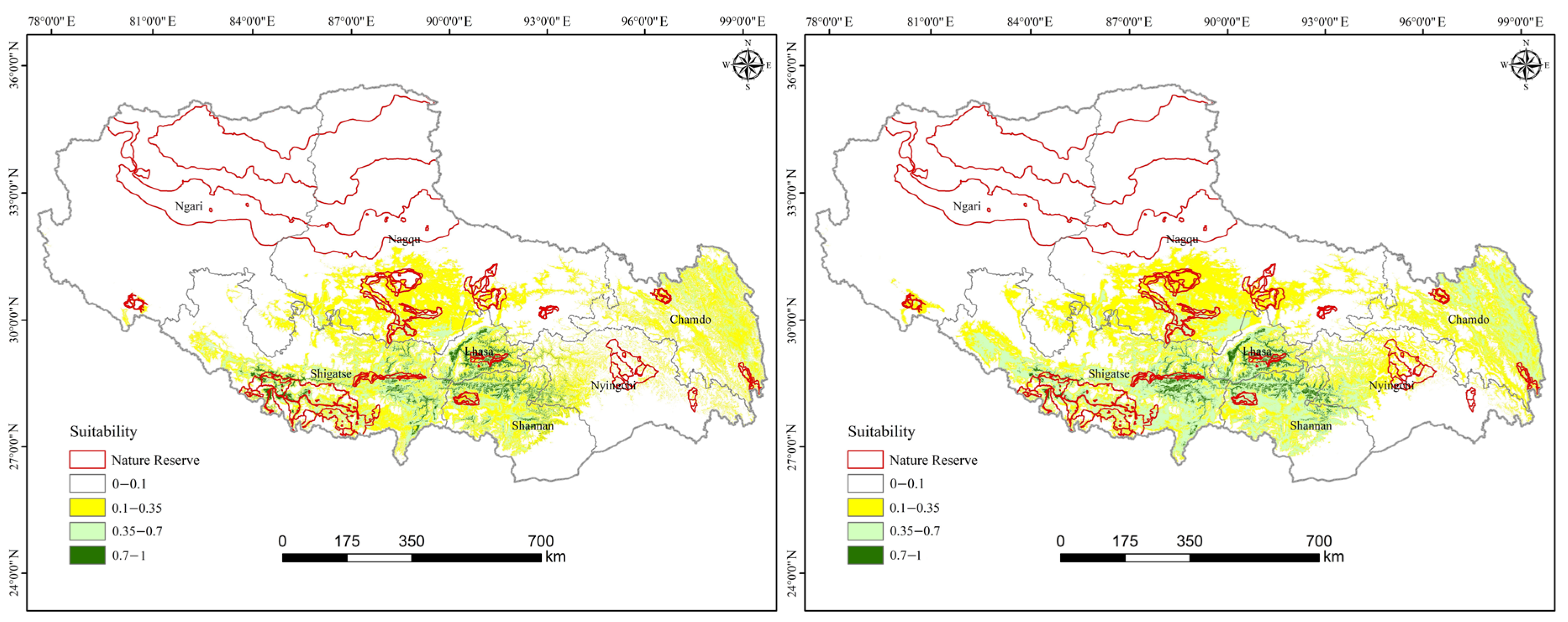
3.2. Comparison of Current and Future Distributions Under Different Scenarios
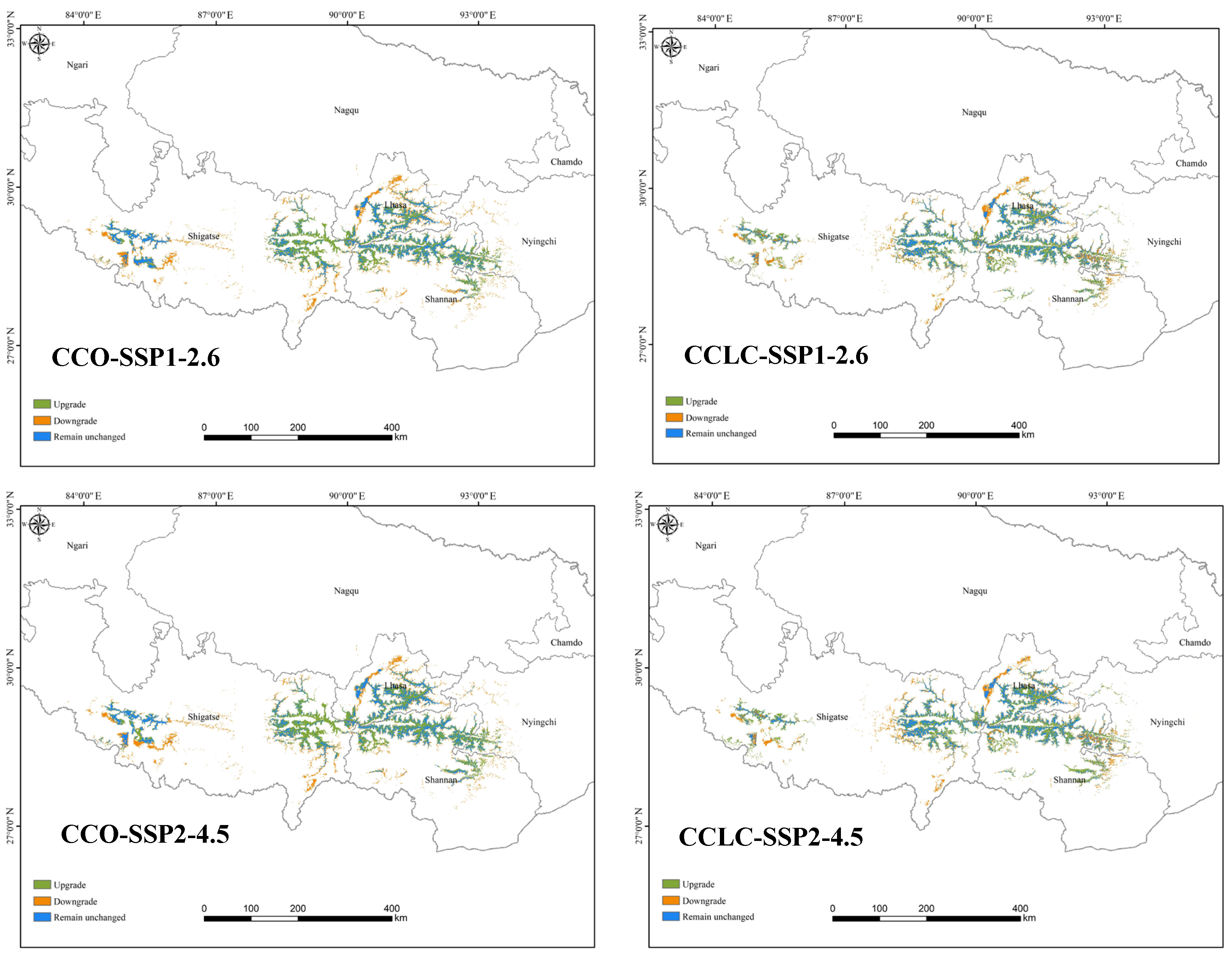
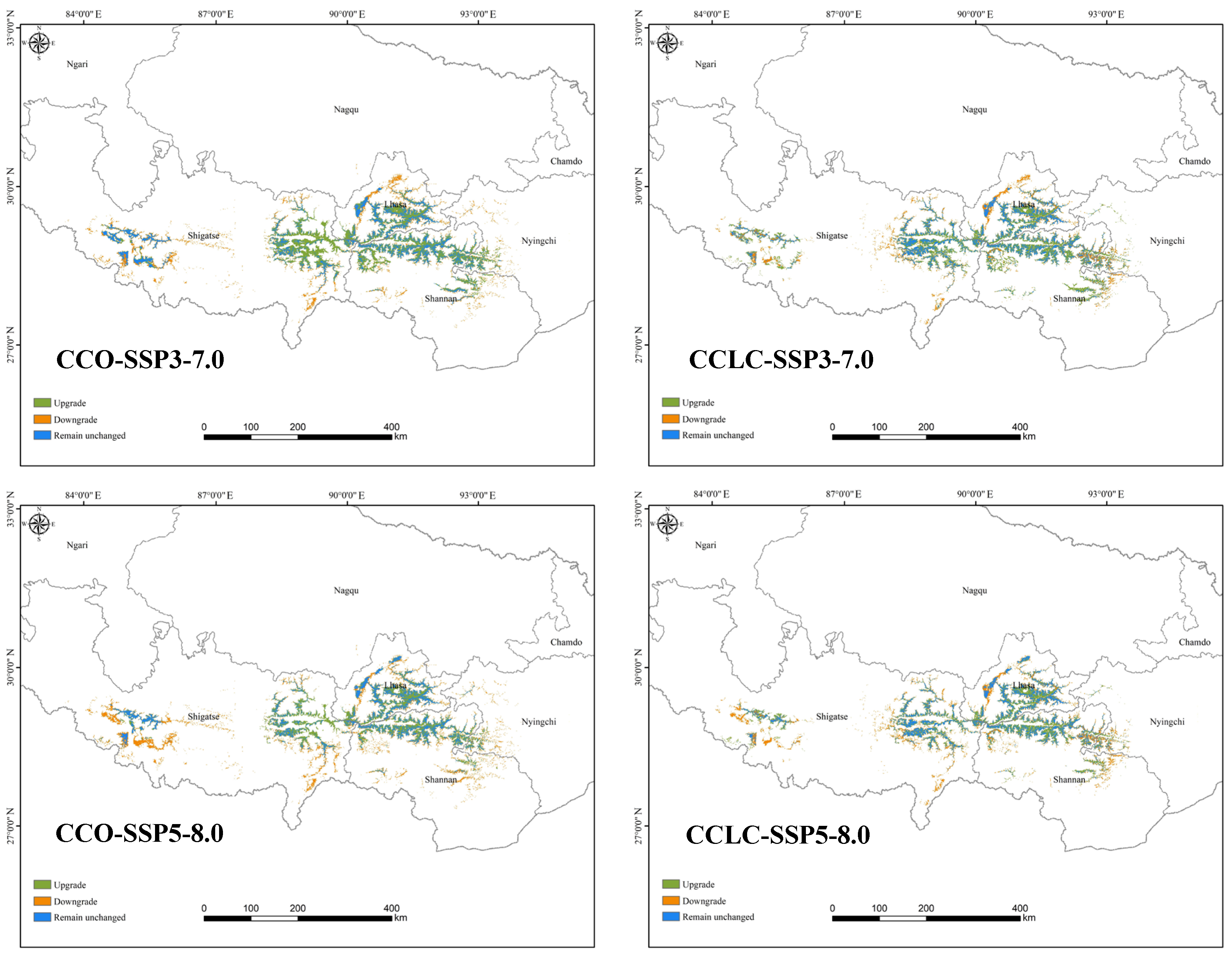
3.2.1. Comparison of Current and Future Distributions Driven by Landscape Change
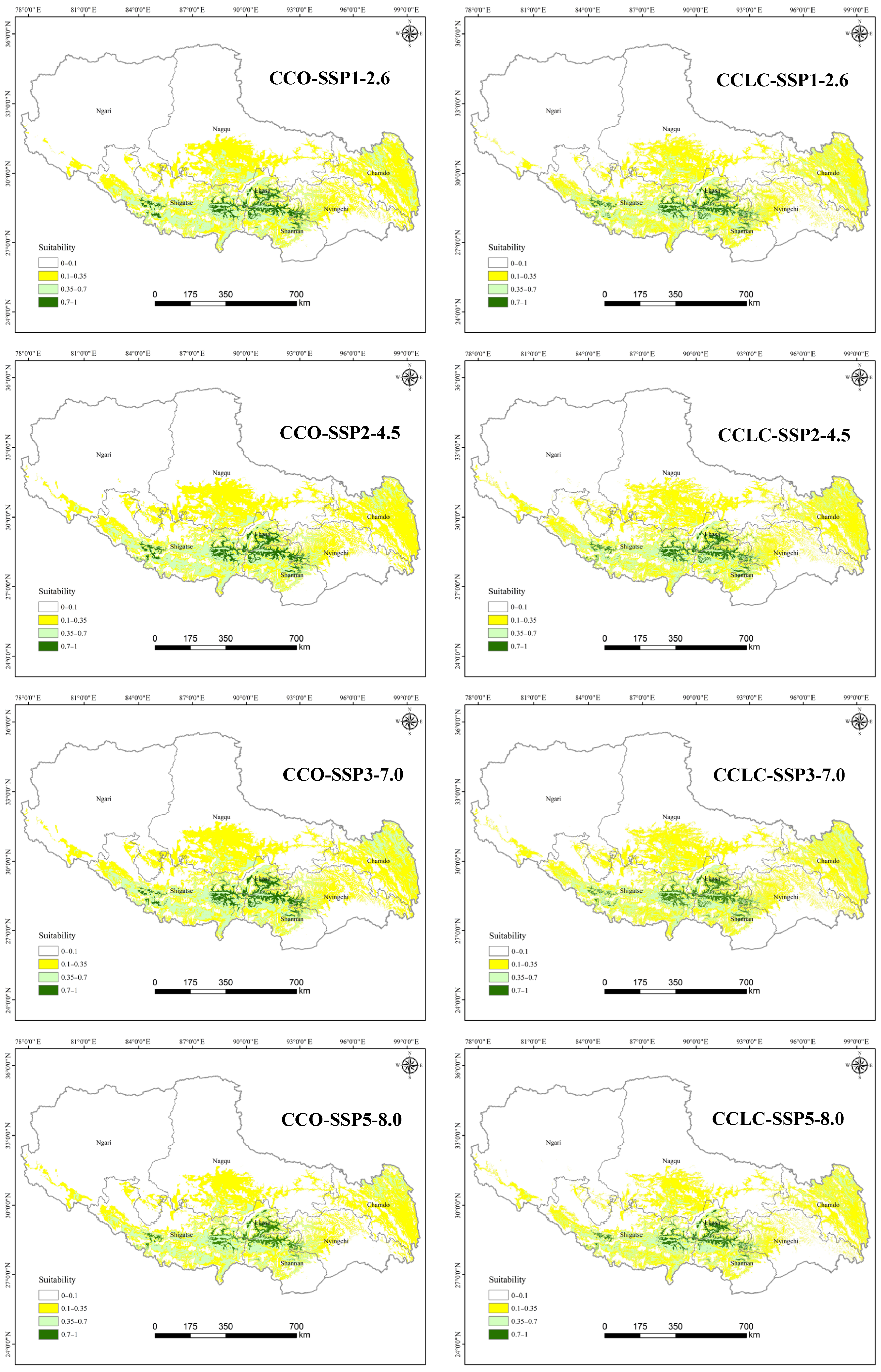
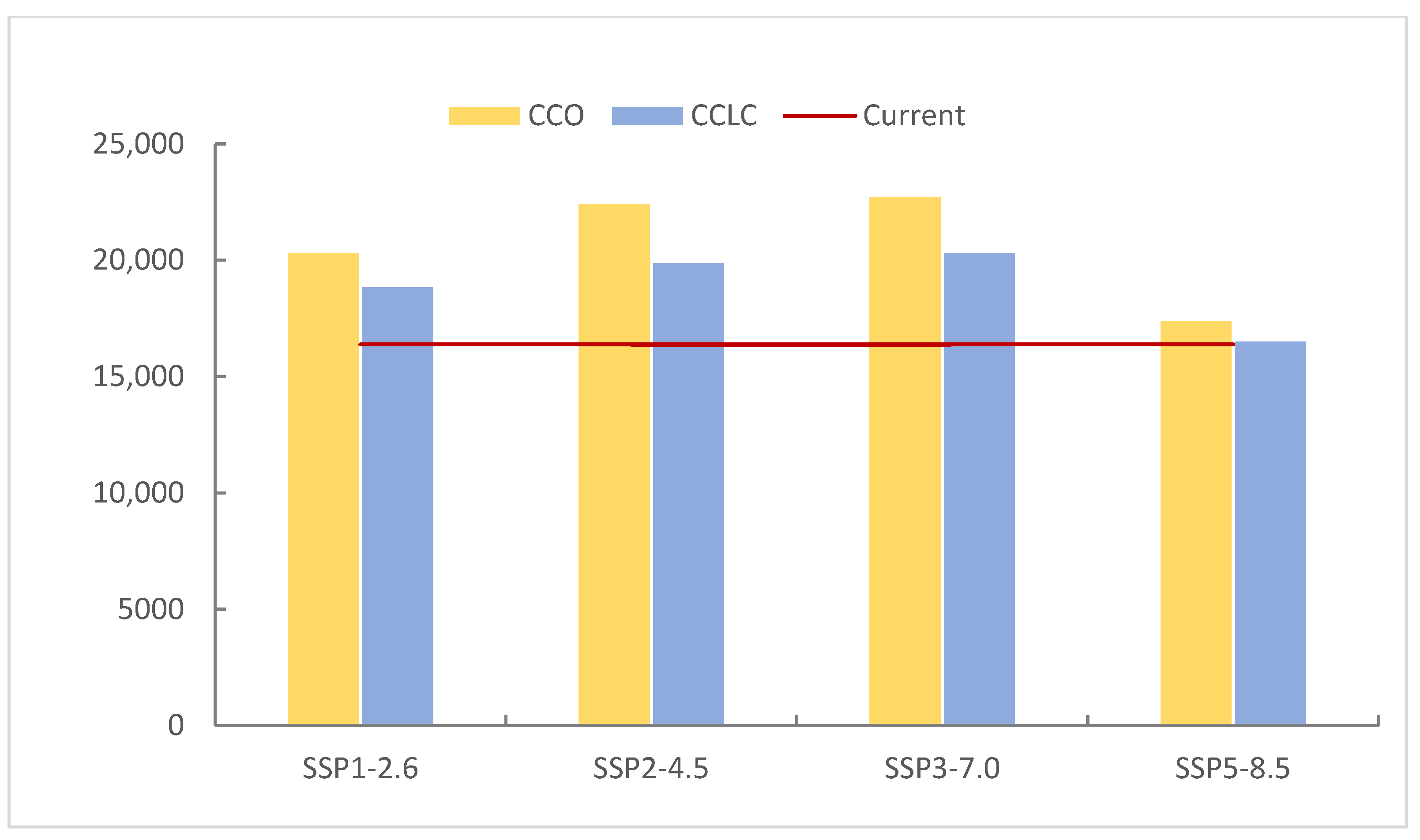
3.2.2. Comparison of Current and Future Distributions Driven by Climate Change
3.2.3. Comparison of Current and Future Distributions Driven by Both Climate Change and Landscape Change
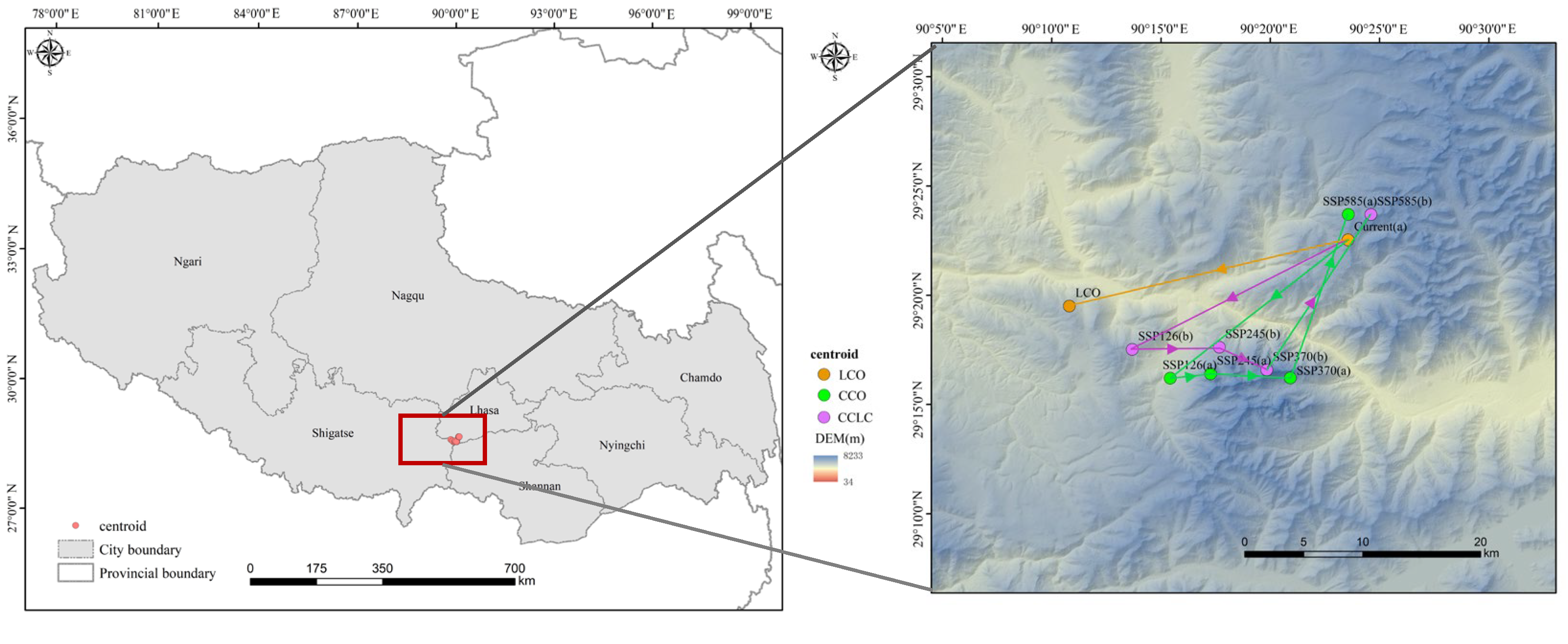
3.3. Centroid Shifts Under Different Scenarios
4. Discussion
4.1. Effects of the Critical Environmental Variables
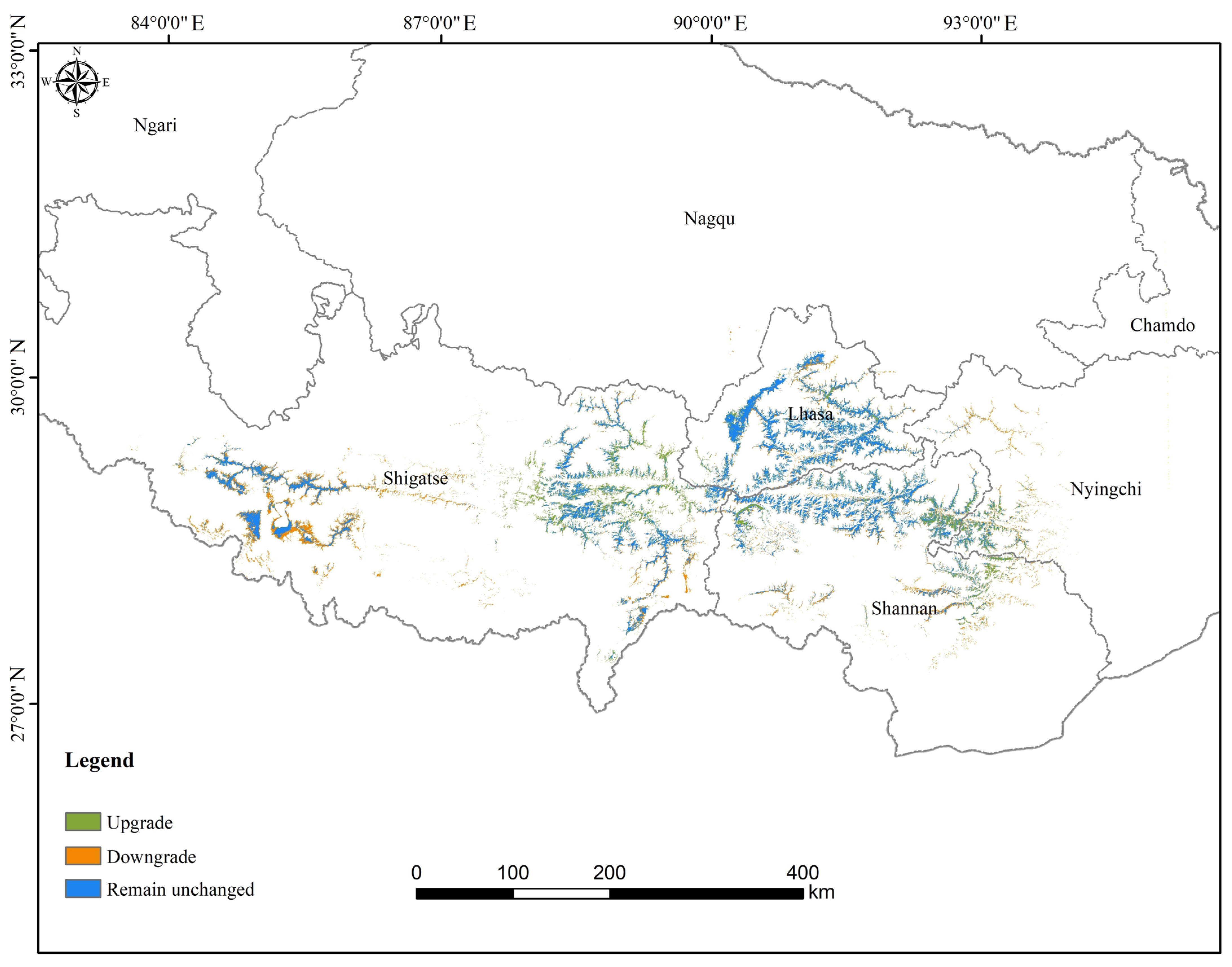
4.2. Changes in Suitable Habitats Under Different Scenarios
4.3. Conservation Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asamoah, E.F.; Beaumont, L.J.; Maina, J.M. Climate and land-use changes reduce the benefits of terrestrial protected areas. Nat. Clim. Change 2021, 11, 1105–1110. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.M.; Butchart, S.H.M.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R.; et al. Assessing species vulnerability to climate change. Nat. Clim. Change 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Socolar, J.B.; Mills, S.C.; Gilroy, J.J.; Martínez-Revelo, D.E.; Medina-Uribe, C.A.; Parra-Sanchez, E.; Ramirez-Gutierrez, M.; Sand Sæbø, J.; Meneses, H.S.; Pérez, G.; et al. Tropical biodiversity loss from land-use change is severely underestimated by local-scale assessments. Nat. Ecol. Evol. 2025, 9, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Davison, C.W.; Rahbek, C.; Morueta-Holme, N. Land-use change and biodiversity: Challenges for assembling evidence on the greatest threat to nature. Glob. Change Biol. 2021, 27, 5414–5429. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Cabernard, L.; Pfister, S.; Hellweg, S. Biodiversity impacts of recent land-use change driven by increases in agri-food imports. Nat. Sustain. 2024, 7, 1512–1524. [Google Scholar] [CrossRef]
- Sinervo, B.; Méndez-de-la-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of Lizard Diversity by Climate Change and Altered Thermal Niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef]
- Elmberg, J.; Palmheden, L.; Edelstam, C.; Hagman, M.; Kärvemo, S. Climate change-induced shifts in survival and size of the worlds’ northernmost oviparous snake: A 68-year study. PLoS ONE 2024, 19, e0300363. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Contu, S.; Hill, S.L.L.; Beck, J.; Liu, Y.; Meyer, C.; Phillips, H.R.P.; Scharlemann, J.P.W.; Purvis, A. Widespread winners and narrow-ranged losers: Land use homogenizes biodiversity in local assemblages worldwide. PLoS Biol. 2018, 16, e2006841. [Google Scholar] [CrossRef]
- Hayden Bofill, S.I.; Blom, M. Climate change from an ectotherm perspective: Evolutionary consequences and demographic change in amphibian and reptilian populations. Biodivers. Conserv. 2024, 33, 905–927. [Google Scholar] [CrossRef]
- He, M.; Feng, J.-C.; Liu, S.; Guo, P.; Zhao, E.-M. The phylogenetic position of Thermophis (Serpentes: Colubridae), an endemic snake from the Qinghai-Xizang Plateau, China. J. Nat. Hist. 2009, 43, 479–488. [Google Scholar] [CrossRef]
- Wall, F. Some new Asian snakes. J. Bombay Nat. Hist. Soc. 1907, 17, 612–618. [Google Scholar]
- Guo, P.; Liu, S.; Feng, J.C.; He, M. The description of a new species of Thermophis (Serpentes: Colubridae). Sichuan J. Zool. 2008, 27, 321. [Google Scholar] [CrossRef]
- Peng, L.; Lu, C.; Huang, S.; Guo, P.; Zhang, Y. A New Species of the Genus Thermophis (Serpentes: Colubridae) from Shangri-La, Northern Yunnan, China, with a Proposal for an Eclectic Rule for Species Delimitation. Asian Herpetol. Res. 2014, 5, 228–239. [Google Scholar] [CrossRef]
- Li, J.-T.; Gao, Y.-D.; Xie, L.; Deng, C.; Shi, P.; Guan, M.-L.; Huang, S.; Ren, J.-L.; Wu, D.-D.; Ding, L.; et al. Comparative genomic investigation of high-elevation adaptation in ectothermic snakes. Proc. Natl. Acad. Sci. USA 2018, 115, 8406–8411. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Fritzsche, P.; Dorge, T.; Miehe, G.; Nothnagel, M. What Makes a Hot-Spring Habitat “Hot” for the Hot-Spring Snake: Distributional Data and Niche Modelling for the Genus Thermophis (Serpentes, Colubridae). Diversity 2021, 13, 325. [Google Scholar] [CrossRef]
- Sills, J.; Huang, S.; Peng, L. Tibetan hot-spring snakes under threat. Science 2019, 366, 193–194. [Google Scholar] [CrossRef]
- Yan, C.; Wu, W.; Dong, W.; Zhu, B.; Chang, J.; Lv, Y.; Yang, S.; Li, J.-T. Temperature acclimation in hot-spring snakes and the convergence of cold response. Innovation 2022, 3, 100295. [Google Scholar] [CrossRef]
- Hofmann, S. Population genetic structure and geographic differentiation in the hot spring snake Thermophis baileyi (Serpentes, Colubridae): Indications for glacial refuges in southern-central Tibet. Mol. Phylogenetics Evol. 2012, 63, 396–406. [Google Scholar] [CrossRef]
- Deng, T.; Wu, F.; Zhou, Z.; Su, T. Tibetan Plateau: An evolutionary junction for the history of modern biodiversity. Sci. China Earth Sci. 2020, 63, 172–187. [Google Scholar] [CrossRef]
- Dorge, T.; Hofmann, S.; Wangdwei, M.; Duoje, L.; Solhøy, T.; Miehe, G. The ecological specialist, Thermophis baileyi (Wall, 1907): New records, distribution and biogeographic conclusions. Herpetol. Bull. 2007, 31, 8–12. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species, Version 2025-2; IUCN: Gland, Switzerland, 2025.
- IUCN. The IUCN Red List of Threatened Species, Version 2010; IUCN: Gland, Switzerland, 2010.
- Yan, C.; Song, M.H.; Jiang, D.; Ren, J.L.; Lv, Y.; Chang, J.; Huang, S.; Zaher, H.; Li, J.T. Genomic evidence reveals intraspecific divergence of the hot-spring snake (Thermophis baileyi), an endangered reptile endemic to the Qinghai-Tibet plateau. Mol. Ecol. 2023, 32, 1335–1350. [Google Scholar] [CrossRef] [PubMed]
- Oitaven, L.P.C.; Calado, S.S.; de Moura, M.S.; Monrós, J.S.; de Moura, G.J.B. Influence of Seasonality and Habitat Variability on the Thermal Ecology of Gymnodactylus geckoides Spix, 1825 (Squamata, Phyllodactylidae) in Semi-Arid Caatinga Biome in Northeastern Brazil. Austral Ecol. 2025, 50, e70055. [Google Scholar] [CrossRef]
- Caruccio, R.; Vieira, R.C.; Verrastro, L.; Machado, D.M. Thermal biology, activity, and population parameters of Cnemidophorus vacariensis (Squamata, Teiidae), a lizard endemic to southern Brazil. Iheringia. Série Zool. 2011, 101, 283–295. [Google Scholar] [CrossRef]
- Giacometti, D.; Yagi, K.T.; Abney, C.R.; Jung, M.P.; Tattersall, G.J. Staying warm is not always the norm: Behavioural differences in thermoregulation of two snake species. Can. J. Zool. 2021, 99, 974–983. [Google Scholar] [CrossRef]
- Fey, S.B.; Vasseur, D.A.; Alujević, K.; Kroeker, K.J.; Logan, M.L.; O’Connor, M.I.; Rudolf, V.H.W.; DeLong, J.P.; Peacor, S.; Selden, R.L.; et al. Opportunities for behavioral rescue under rapid environmental change. Glob. Change Biol. 2019, 25, 3110–3120. [Google Scholar] [CrossRef]
- GBIF. Occurrence Download. Available online: https://www.gbif.org/species/2454079 (accessed on 16 July 2024).
- MEE. Technical Regulations for the Survey and Assessment of Amphibian and Reptile Diversity in County Areas; The Ministry of Ecology and Environment: Beijing, China, 2017.
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.F.; Lowe, J.; et al. The Scenario Model Intercomparison Project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Ermida, S.L.; Soares, P.; Mantas, V.; Göttsche, F.-M.; Trigo, I.F. Google Earth Engine Open-Source Code for Land Surface Temperature Estimation from the Landsat Series. Remote Sens. 2020, 12, 1471. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chen, X.; Gao, Y.; Xie, S.; Mi, J. GLC_FCS30: Global land-cover product with fine classification system at 30 m using time-series Landsat imagery. Earth Syst. Sci. Data 2021, 13, 2753–2776. [Google Scholar] [CrossRef]
- Tobler, W. Cellular Geography. Philos. Geogr. 1979, 20, 379–386. [Google Scholar] [CrossRef]
- Bell, E.J. Markov analysis of land use change—An application of stochastic processes to remotely sensed data. Socio-Econ. Plan. Sci. 1974, 8, 311–316. [Google Scholar] [CrossRef]
- de Oliveira Barros, K.; Alvares Soares Ribeiro, C.A.; Marcatti, G.E.; Lorenzon, A.S.; Martins de Castro, N.L.; Domingues, G.F.; Romário de Carvalho, J.; Rosa dos Santos, A. Markov chains and cellular automata to predict environments subject to desertification. J. Environ. Manag. 2018, 225, 160–167. [Google Scholar] [CrossRef]
- Myint, S.W.; Wang, L. Multicriteria decision approach for land use land cover change using Markov chain analysis and a cellular automata approach. Can. J. Remote Sens. 2006, 32, 390–404. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, X. Dominant transition probability: Combining CA-Markov model to simulate land use change. Environ. Dev. Sustain. 2023, 25, 6829–6847. [Google Scholar] [CrossRef]
- Pontius, G.R.; Malanson, J. Comparison of the structure and accuracy of two land change models. Int. J. Geogr. Inf. Sci. 2005, 19, 243–265. [Google Scholar] [CrossRef]
- Mu, H.; Li, X.; Wen, Y.; Huang, J.; Du, P.; Su, W.; Miao, S.; Geng, M. A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci. Data 2022, 9, 176. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.; Liu, Z.; Li, Q.; Zhang, H.; Wei, W. Conflict or coordination? The spatiotemporal relationship between humans and nature on the Qinghai-Tibet Plateau. Earth’s Future 2023, 11, e2022EF003452. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, Z.; Chen, B.; Gong, P.; Xu, B.; Fu, H. A Prolonged Artificial Nighttime-light Dataset of China (1984–2020). Sci. Data 2024, 11, 414. [Google Scholar] [CrossRef]
- Zhan, N.; Liu, W.; Ye, T.; Li, H.; Chen, S.; Ma, H. High-resolution livestock seasonal distribution data on the Qinghai-Tibet Plateau in 2020. Sci. Data 2023, 10, 142. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Jaynes, E.T. Information Theory and Statistical Mechanics. Phys. Rev. 1957, 106, 620–630. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, S.; Chen, S. Predicting the potential habitat suitability of Saussurea species in China under future climate scenarios using the optimized Maximum Entropy (MaxEnt) model. J. Clean. Prod. 2024, 474, 143552. [Google Scholar] [CrossRef]
- Raffini, F.; Bertorelle, G.; Biello, R.; D’Urso, G.; Russo, D.; Bosso, L. From Nucleotides to Satellite Imagery: Approaches to Identify and Manage the Invasive Pathogen Xylella fastidiosa and Its Insect Vectors in Europe. Sustainability 2020, 12, 4508. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef]
- De Marco, P.J.; Nóbrega, C.C. Evaluating collinearity effects on species distribution models: An approach based on virtual species simulation. PLoS ONE 2018, 13, e0202403. [Google Scholar] [CrossRef]
- Gong, S.; Gao, Y.; Duan, H.; Ge, Y.; Wei, Y. Incorporating physiological data into species distribution models to predict the potential distribution range of the red-eared slider in China. Ecol. Indic. 2023, 154, 110749. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Li, R.; Wang, X.; Cheng, J.; Yang, Q.; Kong, H. AHP-GIS and MaxEnt for delineation of potential distribution of Arabica coffee plantation under future climate in Yunnan, China. Ecol. Indic. 2021, 132, 108339. [Google Scholar] [CrossRef]
- Oitaven, L.P.C.; Silva, B.B.M.R.; Gavilan, S.A.; Dias, E.J.R.; Chaves, M.F.; Lobo, L.M.; Gonzalez, J.S.M.; Mesquita, D.O.; de Moura, G.J.B. Relationships between body growth indices and environmental factors on the reproductive cycle of the Gymnodactylus geckoides Spix, 1825 (Squamata, Gymnophthalmidae) in Northeast Brazil. Cuad. De Herpetol. 2023, 37, 37–56. [Google Scholar]
- Li, J. Diet and Feeding Behavior of Thermophis baileyi, a Chinese Endemic Species (in Chinese). J. Tibet Univ. 2007, 22, 84–90. [Google Scholar]
- Clark, M.I.; Hileman, E.T.; Moore, J.A.; Faust, L.J.; Junge, R.E.; Reid, B.N.; Bradke, D.R.; Bradburd, G.S.; Fitzpatrick, S.W. Inbreeding reduces fitness in spatially structured populations of a threatened rattlesnake. Proc. Natl. Acad. Sci. USA 2025, 122, e2501745122. [Google Scholar] [CrossRef]
- Liu, W.-l.; Liu, P.; Cui, L.-x.; Meng, Y.; Tao, S.-a.; Han, X.-z.; Sun, B.-j. Moderate climate warming scenarios during embryonic and post-embryonic stages benefit a cold-climate lizard. Funct. Ecol. 2022, 36, 1137–1150. [Google Scholar] [CrossRef]
- Mizsei, E.; Sos, T.; Móré, A.; Wenner, B.; Rák, G.; Mebert, K. Restriction times on the rise: Mechanistic modelling of activity time of grassland vipers (Vipera spp.) in the face of climate change. Front. Zool. 2025, 22, 10. [Google Scholar] [CrossRef]
- Mu, H.; Guo, S.; Zhang, X.; Yuan, B.; Xia, Z.; Tang, P.; Zhang, W.; Zhang, P.; Li, X.; Du, P. Moving in the landscape: Omnidirectional connectivity dynamics in China from 1985 to 2020. Environ. Impact Assess. Rev. 2025, 110, 107721. [Google Scholar] [CrossRef]
- Lee-Yaw, J.A.; McCune, J.L.; Pironon, S.; Sheth, S.N. Species distribution models rarely predict the biology of real populations. Ecography 2022, 2022, e05877. [Google Scholar] [CrossRef]
| Category | Variables | Description | Unit | Source | Resolution |
|---|---|---|---|---|---|
| Bioclimatic variables | bio1 | Annual mean temperature | °C | WorldClim 2 database for the current and future periods [32] | 30 s |
| bio2 | Mean diurnal range | °C | |||
| bio3 | Isothermality (bio2/bio7) (×100) | – | |||
| bio4 | Temperature seasonality (standard deviation ×100) | – | |||
| bio5 | Max temperature of the warmest month | °C | |||
| bio6 | Min temperature of the coldest month | °C | |||
| bio7 | Temperature annual range (Bio5-Bio6) | °C | |||
| bio8 | The mean temperature of the wettest quarter | °C | |||
| bio9 | The mean temperature of the driest quarter | °C | |||
| bio10 | The mean temperature of the warmest quarter | °C | |||
| bio11 | The mean temperature of the coldest quarter | °C | |||
| bio12 | Annual precipitation | mm | |||
| bio13 | Precipitation of the wettest month | mm | |||
| bio14 | Precipitation of the driest month | mm | |||
| bio15 | Precipitation seasonality | mm | |||
| bio16 | Precipitation of the wettest quarter | mm | |||
| bio17 | Precipitation of the driest quarter | mm | |||
| bio18 | Precipitation of the warmest quarter | mm | |||
| bio19 | Precipitation of the coldest quarter | mm | |||
| Terrain factors | DEM | Digital Elevation Model | m | Geospatial Data Cloud | 90 m |
| Slope | Slope | m | Extracted from DEM | 90 m | |
| Habitat factors | Land cover | 35 land-cover categories for 1990, 2000, 2010 and 2020 | _ | [36] | 30 m |
| LST | Land Surface Temperature | °C | Computed from Landsat 4, 5, 7, and 8 within Google Earth Engine (GEE) [35] | 30 m |
| Scenarios | Scenario Description | |
|---|---|---|
| Landscape Change-Only Scenario | LCO | The predicted 2050 land-cover layer was imported into the simulation model to replace the 2020 land-cover layer, while the other variables remained unchanged. |
| Climate Change-Only (CCO) Scenario | CCO-SSP1-2.6 | The future 19 bioclimate variables (averages for 2041–2060) under SSP1-2.6, SSP2-4.5, SSP3-7.0, and SSP5-8.5 scenarios were separately imported into the simulation models to replace the current 19 bioclimate variables, while the other variables remained unchanged. |
| CCO-SSP2-4.5 | ||
| CCO-SSP3-7.0 | ||
| CCO-SSP5-8.5 | ||
| Combined Climate–Landscape Change (CCLC) Scenario | CCLC-SSP1-2.6 | Both the future land-cover layer and future 19 bioclimate variables were imported to replace the corresponding current data. |
| CCLC-SSP2-4.5 | ||
| CCLC-SSP3-7.0 | ||
| CCLC-SSP5-8.5 | ||
| Variable | Percent Contribution (%) | Permutation Importance |
|---|---|---|
| bio4 | 41.8 | 7.3 |
| bio19 | 15.1 | 2.9 |
| Land cover | 13.8 | 5.4 |
| bio1 | 10.2 | 3.2 |
| bio12 | 6.2 | 6.3 |
| bio11 | 3.8 | 47.1 |
| LST | 3.7 | 0.5 |
| bio9 | 2.4 | 16.4 |
| bio14 | 2.1 | 1.8 |
| Current and Future Scenarios | Simulated Areas/km2 | Increase/Decrease Rates (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unsuitable | Low Suitability | Medium Suitability | High Suitability | Unsuitable | Low Suitability | Medium Suitability | High Suitability | ||
| Current | 903,712.01 | 191,890.35 | 90,217.44 | 16,380.21 | / | / | / | / | |
| LCO | 838,683.21 | 197,494.50 | 150,620.97 | 15,401.32 | −7.20 | 2.92 | 66.95 | −5.98 | |
| CCO | SSP1-2.6 | 855,617.77 | 220,188.49 | 106,077.03 | 20,316.71 | −5.32 | 14.75 | 17.58 | 24.03 |
| SSP2-4.5 | 835,625.62 | 244,272.78 | 99,887.64 | 22,413.96 | −7.53 | 27.30 | 10.72 | 36.84 | |
| SSP3-7.0 | 835,072.98 | 235,748.58 | 108,683.78 | 22,694.66 | −7.60 | 22.86 | 20.47 | 38.55 | |
| SSP580 | 890,052.23 | 202,048.28 | 92,731.71 | 17,367.78 | −1.51 | 5.29 | 2.79 | 6.03 | |
| CCLC | SSP126 | 876,995.89 | 212,011.51 | 94,371.51 | 18,821.08 | −2.96 | 10.49 | 4.60 | 14.90 |
| SSP245 | 858,734.02 | 234,776.35 | 88,810.78 | 19,878.84 | −4.98 | 22.35 | −1.56 | 21.36 | |
| SSP370 | 855,752.27 | 228,920.30 | 97,215.83 | 20,311.60 | −5.31 | 19.30 | 7.76 | 24.00 | |
| SSP580 | 908,668.61 | 194,841.80 | 82,188.82 | 16,500.78 | 0.55 | 1.54 | −8.90 | 0.74 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Han, R.; Dai, F.; Liu, Y.; Song, T.; Ren, Y.; Huang, S.; Chang, J. Climate Change vs. Human Activities: Conflicting Future Impacts on a High-Altitude Endangered Snake (Thermophis baileyi). Biology 2025, 14, 1531. https://doi.org/10.3390/biology14111531
Pan Y, Han R, Dai F, Liu Y, Song T, Ren Y, Huang S, Chang J. Climate Change vs. Human Activities: Conflicting Future Impacts on a High-Altitude Endangered Snake (Thermophis baileyi). Biology. 2025; 14(11):1531. https://doi.org/10.3390/biology14111531
Chicago/Turabian StylePan, Yuxue, Ruiying Han, Fengbin Dai, Yu Liu, Tianjian Song, Yueheng Ren, Song Huang, and Jiang Chang. 2025. "Climate Change vs. Human Activities: Conflicting Future Impacts on a High-Altitude Endangered Snake (Thermophis baileyi)" Biology 14, no. 11: 1531. https://doi.org/10.3390/biology14111531
APA StylePan, Y., Han, R., Dai, F., Liu, Y., Song, T., Ren, Y., Huang, S., & Chang, J. (2025). Climate Change vs. Human Activities: Conflicting Future Impacts on a High-Altitude Endangered Snake (Thermophis baileyi). Biology, 14(11), 1531. https://doi.org/10.3390/biology14111531





