Infestation Patterns and Climate-Based Projections for European Spongy Moth (Lymantria dispar) in Whirlpool Forest, Ontario, Canada
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
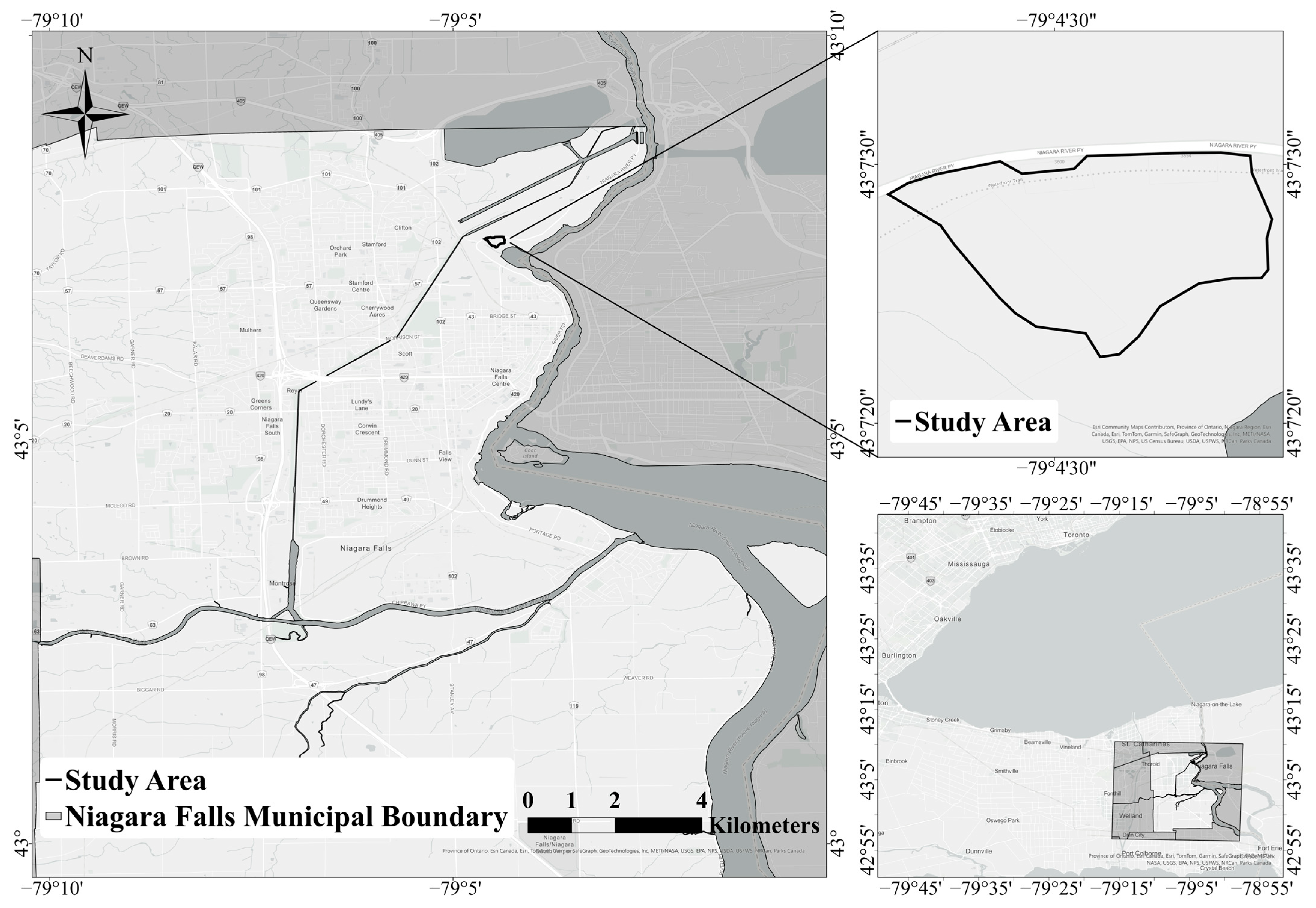
2.1. Study Area
2.2. Field Data Collection
2.2.1. Monitoring of Kaladar Plots (MKPs)
2.2.2. Tree Identification and Calculation of Egg Masses
2.3. Data Analysis and Modeling
2.3.1. Field Data Analysis
2.3.2. Historical Data Analysis and Regression Modeling
2.3.3. Model Validation and Selection
2.3.4. Future Projections and Sensitivity Analysis
3. Results
3.1. Impact of Spongy Moths on Different Tree Species
3.2. Spongy Moth Egg Masses on Trees Above and Below 1 m
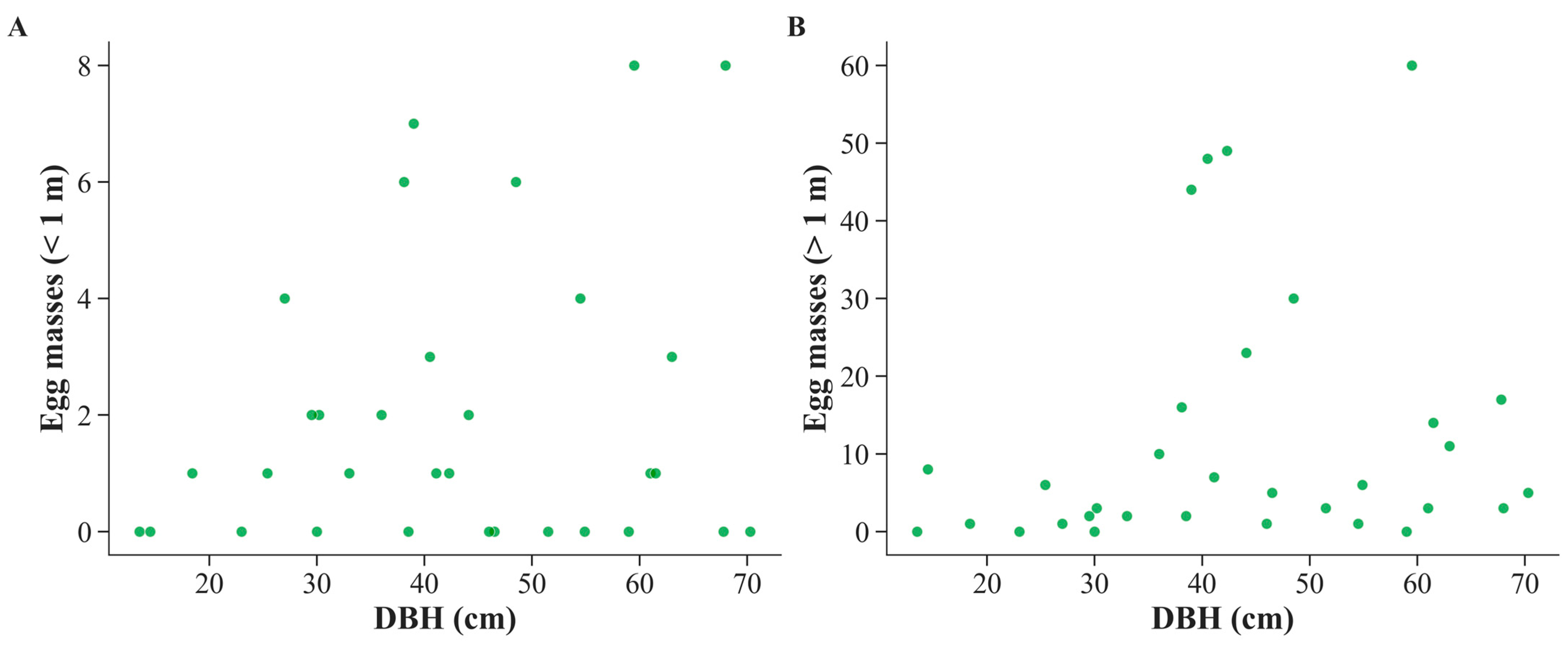
3.3. Relationship Between DBH and Spongy Moth Egg Masses
3.4. Number of Healthy Trees in Relation to Egg Masses
3.5. Historical Climate Analysis and Regression Modeling
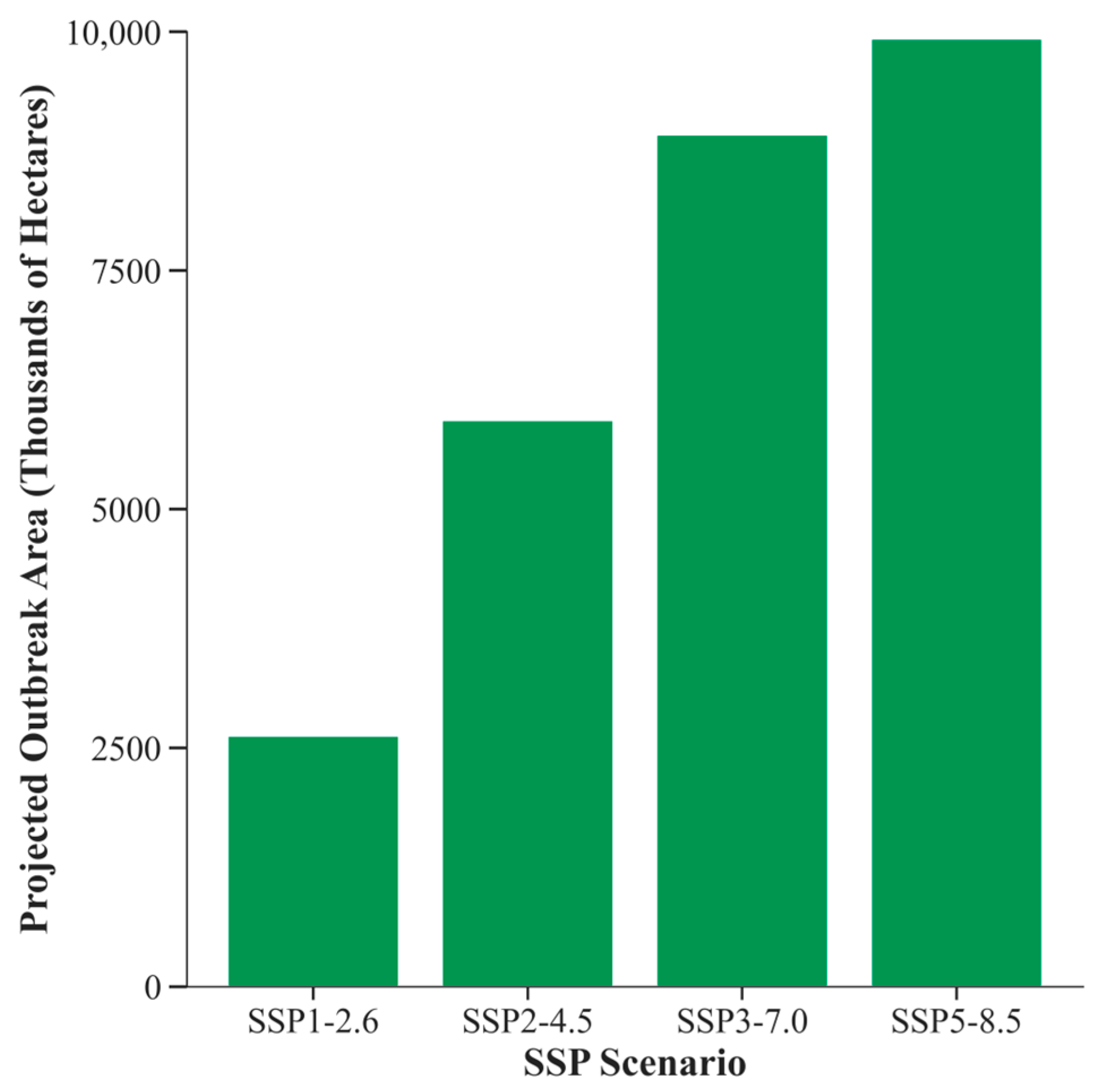
3.6. Future Projections and Sensitivity Analysis
4. Discussion
4.1. Impacts of Spongy Moth on Forest Species and Health
4.2. Egg Mass Density and Defoliation Risk Assessment
4.3. Climate Change and Spongy Moth Outbreaks
4.4. Future Projections and Management Implications
4.5. Integration of Ecological and Climate Modeling Insights
4.6. Research Limitations and Prospections
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tkacz, B.; Moody, B.; Castillo, J.V.; Fenn, M.E. Forest Health Conditions in North America. Environ. Pollut. 2008, 155, 409–425. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest Health and Global Change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Gauthier, S.; Bernier, P.; Kuuluvainen, T.; Shvidenko, A.Z.; Schepaschenko, D.G. Boreal Forest Health and Global Change. Science 2015, 349, 819–822. [Google Scholar] [CrossRef]
- Daniel, S. Invasive Species: What Everyone Needs to Know®; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-992203-1. [Google Scholar]
- Hajek, A.E.; Diss-Torrance, A.L.; Siegert, N.W.; Liebhold, A.M. Inoculative Releases and Natural Spread of the Fungal Pathogen Entomophaga Maimaiga (Entomophthorales: Entomophthoraceae) into U.S. Populations of Gypsy Moth, Lymantria Dispar (Lepidoptera: Erebidae). Environ. Entomol. 2021, 50, 1007–1015. [Google Scholar] [CrossRef]
- Régnière, J.; Nealis, V.; Porter, K. Climate Suitability and Management of the Gypsy Moth Invasion into Canada. In Ecological Impacts of Non-Native Invertebrates and Fungi on Terrestrial Ecosystems; Langor, D.W., Sweeney, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 135–148. ISBN 978-1-4020-9680-8. [Google Scholar]
- Timms, L.L.; Walker, S.C.; Smith, S.M. Establishment and Dominance of an Introduced Herbivore Has Limited Impact on Native Host-Parasitoid Food Webs. Biol. Invasions 2012, 14, 229–244. [Google Scholar] [CrossRef]
- Douce, G.K.; Hamilton, R.D.; Clement, G.L. 1992 Gypsy Moth Programs in the Southeast. J. Entomol. Sci. 1994, 29, 381–397. [Google Scholar] [CrossRef]
- Haq, M.; O’Toole, A.; Beecker, J.; Gooderham, M.J. Return of Lymantria Dispar Dispar (Gypsy Moth): A Case Report. SAGE Open Med. Case Rep. 2021, 9, 2050313X211057926. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, A.M.; MacDonald, W.L.; Bergdahl, D.; Mastro, V.C. Invasion by Exotic Forest Pests: A Threat to Forest Ecosystems. For. Sci. 1995, 41, a0001–z0001. [Google Scholar] [CrossRef]
- Forest Health Conditions in Ontario 2021; Ontario Ministry of Northern Development, Mines, Natural Resources and Forestry, Science and Research Branch: Toronto, ON, Canada, 2022; pp. 65–77.
- Mcmanus, M.; Csóka, G. History and Impact of Gypsy Moth in North America and Comparison to Recent Outbreaks in Europe. Acta Silv. Lignaria Hung. 2007, 3, 47–64. [Google Scholar] [CrossRef]
- Larson, J. Spongy Moth and Kentucky. 2023. Available online: https://entomology.mgcafe.uky.edu/sites/entomology.ca.uky.edu/files/ef425_1.pdf (accessed on 12 November 2024).
- Sadof, C.; Marshall, P. The Spongy Moth in Indiana. 2022. Available online: https://extension.entm.purdue.edu/publications/GM-1.pdf (accessed on 12 November 2024).
- Benoit, P.; Lachance, D. Gypsy Moth in Canada: Behavior and Control. 1990. Available online: https://cfs.nrcan.gc.ca/pubwarehouse/pdfs/10041_e.pdf (accessed on 2 December 2024).
- Gansner, D.A.; Herrick, O.W.; Herrick, O.W. Host Preferences of Gypsy Moth on a New Frontier of Infestation; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experimental Station: Broomall, PA, USA, 1985; p. NE-RN-330.
- Milanović, S.; Miletić, Z.; Marković, Č.; Šešlija Jovanović, D.; Trailović, Z.; Jankovský, L.; Lazarević, J. Suitability of Turkey Oak, European Beech, and Hornbeam to Gypsy Moth Feeding. Forests 2022, 13, 1006. [Google Scholar] [CrossRef]
- Leroy, B.M.L.; Lemme, H.; Braumiller, P.; Hilmers, T.; Jacobs, M.; Hochrein, S.; Kienlein, S.; Müller, J.; Pretzsch, H.; Stimm, K.; et al. Relative Impacts of Gypsy Moth Outbreaks and Insecticide Treatments on Forest Resources and Ecosystems: An Experimental Approach. Ecol. Solut. Evid. 2021, 2, e12045. [Google Scholar] [CrossRef]
- Philp, R.B. Adverse Health Consequences Following Aerial Spraying with Bacillus Thuringiensis (Var. Kurstakibtk), to Control the Spongy Moth: Flaws in Government Risk Assessments and in Public Health Officials’ Attitudes. 2009. Available online: https://ddata.over-blog.com/xxxyyy/3/19/57/29/Bacillus-thuringiensis--danger.pdf (accessed on 2 December 2024).
- Zhan, L.; Yang, J.; Liu, Y. A Neural Networks-Based Evaluation of Ecological Effectiveness and Economic Worth in Forests. Soft Comput. 2023, 27, 19339–19358. [Google Scholar] [CrossRef]
- Addas, A. Impact of Forestry on Environment and Human Health: An Evidence-Based Investigation. Front. Public Health 2023, 11, 1260519. [Google Scholar] [CrossRef]
- Davies, B.W. Forest Ecological Studies in the Northern Conifer Hardwood Region of Central Southern Ontario. Master’s Thesis, McGill University, Montréal, QC, Canada, 1968. [Google Scholar]
- Maycock, P.F. The Phytosociology of the Deciduous Forests of Extreme Southern Ontario. Can. J. Bot. 1963, 41, 379–438. [Google Scholar] [CrossRef]
- Bartlett, R.M.; Matthes-Sears, U.; Larson, D.W. Organization of the Niagara Escarpment Cliff Community. II. Characterization of the Physical Environment. Can. J. Bot. 1990, 68, 1931–1941. [Google Scholar] [CrossRef]
- Lovett-Doust, J.; Kuntz, K. Land Ownership and Other Landscape-Level Effects on Biodiversity in Southern Ontario’s Niagara Escarpment Biosphere Reserve, Canada. Landsc. Ecol. 2001, 16, 743–755. [Google Scholar] [CrossRef]
- McMullin, R.T.; Bennett, L.L.; Bjorgan, O.J.; Bourque, D.A.; Burke, C.J.; Clarke, M.A.; Gutgesell, M.K.; Krawiec, P.L.; Malyon, R.; Mantione, A.; et al. Relationships between Air Pollution, Population Density, and Lichen Biodiversity in the Niagara Escarpment World Biosphere Reserve. Lichenologist 2016, 48, 593–605. [Google Scholar] [CrossRef]
- Calkin, P.E.; Brett, C.E. Ancestral Niagara River Drainage: Stratigraphic and Paleontologic Setting. GSA Bull. 1978, 89, 1140–1154. [Google Scholar] [CrossRef]
- Karrow, P.F.; Terasmae, J. Pollen-Bearing Sediments of the St. Davids Buried Valley Fill at the Whirlpool, Niagara River Gorge, Ontario. Can. J. Earth Sci. 1970, 7, 539–542. [Google Scholar] [CrossRef]
- Sharov, A.A.; Liebhold, A.M. Bioeconomics of Managing the Spread of Exotic Pest Species with Barrier Zones. Ecol. Appl. 1998, 8, 833. [Google Scholar] [CrossRef]
- Tobin, P.C.; Blackburn, L.M. Slow the Spread: A National Program to Manage the Gypsy Moth; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2007; p. NRS-GTR-6.
- Gauthier, S.; May, B.; Vasseur, L. Ecosystem-Based Adaptation to Protect Avian Species in Coastal Communities in the Greater Niagara Region, Canada. Climate 2021, 9, 91. [Google Scholar] [CrossRef]
- Canadian Climate Normals: 1951–1980; Environment Canada: Ottawa, ON, Canada, 1982.
- Gypsy Moth Egg Mass Surveys for the Woodlot Owner. 1999. Available online: https://foca.on.ca/wp-content/uploads/2017/04/Gypsy-moth-surveys-for-woodlot-owners.pdf (accessed on 3 December 2024).
- Thorpe, K.W. Relationship of Gypsy Moth (Lepidoptera: Lymantriidae) Egg Mass Age to Persistence and Color, and an Evaluation of Two Methods to Distinguish New and Old Egg Masses. Environ. Entomol. 1998, 27, 1452–1462. [Google Scholar] [CrossRef]
- Forest Health Conditions in Ontario 2015; Ontario Ministry of Natural Resources and Forestry, Science and Research Branch: Toronto, ON, Canada, 2016; pp. 50–53.
- Forest Health Conditions in Ontario, 2017; Ontario Ministry of Natural Resources and Forestry, Science and Research Branch: Toronto, ON, Canada, 2018; pp. 63–67.
- Forest Health Conditions in Ontario, 2018; Ontario Ministry of Natural Resources and Forestry, Science and Research Branch: Toronto, ON, Canada, 2019; pp. 78–82.
- Forest Health Conditions in Ontario 2019; Ontario Ministry of Natural Resources and Forestry, Science and Research Branch: Toronto, ON, Canada, 2020; pp. 69–77.
- Forest Health Conditions in Ontario 2020; Ontario Ministry of Northern Development, Mines, Natural Resources and Forestry, Science and Research Branch: Toronto, ON, Canada, 2021; pp. 41–55.
- Forest Health Conditions in Ontario 2022; Ontario Ministry of Natural Resources and Forestry, Science and Research Branch: Toronto, ON, Canada, 2023; pp. 73–80.
- Forest Health Conditions in Ontario 2023; Ontario Ministry of Natural Resources and Forestry, Science and Research Branch: Toronto, ON, Canada, 2024; pp. 82–85.
- Andow, D.A. Population Responses to Temperature in 12-year Insect Time Series. Ecol. Entomol. 2024, 49, 205–214. [Google Scholar] [CrossRef]
- Ovenden, T.S.; Perks, M.P.; Clarke, T.; Mencuccini, M.; Jump, A.S. Life after Recovery: Increased Resolution of Forest Resilience Assessment Sheds New Light on Post-drought Compensatory Growth and Recovery Dynamics. J. Ecol. 2021, 109, 3157–3170. [Google Scholar] [CrossRef]
- Todman, L.C.; Bush, A.; Hood, A.S.C. ‘Small Data’ for Big Insights in Ecology. Trends Ecol. Evol. 2023, 38, 615–622. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Gasparrini, A.; Hajat, S.; Smeeth, L.; Armstrong, B. Time Series Regression Studies in Environmental Epidemiology. Int. J. Epidemiol. 2013, 42, 1187–1195. [Google Scholar] [CrossRef]
- Davidson, C.B.; Gottschalk, K.W.; Johnson, J.E. Tree Mortality Following Defoliation by the European Gypsy Moth (Lymantria dispar L.) In the United States: A Review. For. Sci. 1999, 45, 74–84. [Google Scholar] [CrossRef]
- Hough, J.A.; Pimentel, D. Influence of Host Foliage on Development, Survival, and Fecundity of the Gypsy Moth 2. Environ. Entomol. 1978, 7, 97–102. [Google Scholar] [CrossRef]
- Inoue, M.N.; Suzuki-Ohno, Y.; Haga, Y.; Aarai, H.; Sano, T.; Martemyanov, V.V.; Kunimi, Y. Population Dynamics and Geographical Distribution of the Gypsy Moth, Lymantria Dispar, in Japan. For. Ecol. Manag. 2019, 434, 154–164. [Google Scholar] [CrossRef]
- Srivastava, V.; Keena, M.A.; Maennicke, G.E.; Hamelin, R.C.; Griess, V.C. Potential Differences and Methods of Determining Gypsy Moth Female Flight Capabilities: Implications for the Establishment and Spread in Novel Habitats. Forests 2021, 12, 103. [Google Scholar] [CrossRef]
- Thorpe, K.W.; Ridgway, R.L. Gypsy Moth (Lepidoptera: Lymantriidae) Egg Mass Distribution and Sampling in a Residential Setting. Environ. Entomol. 1992, 21, 722–730. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Halverson, J.A.; Elmes, G.A. Gypsy Moth Invasion in North America: A Quantitative Analysis. J. Biogeogr. 1992, 19, 513. [Google Scholar] [CrossRef]
- Lyons, D.B.; Liebhold, A.M. Spatial Distribution and Hatch Times of Egg Masses of Gypsy Moth (Lepidoptera: Lymantriidae). Environ. Entomol. 1992, 21, 354–358. [Google Scholar] [CrossRef]
- Skaller, P.M. Patterns in the Distribution of Gypsy Moth (Lymantria Dispar) (Lepidoptera: Lymantriidae) Egg Masses over an 11-Year Population Cycle. Environ. Entomol. 1985, 14, 106–117. [Google Scholar] [CrossRef]
- Cook, S.P.; Hain, F.P.; Smith, H.R. Oviposition and Pupal Survival of Gypsy Moth (Lepidoptera: Lymantriidae) in Virginia and North Carolina Pine-Hardwood Forests. Environ. Entomol. 1994, 23, 360–366. [Google Scholar] [CrossRef]
- Kärvemo, S.; Johansson, V.; Schroeder, M.; Ranius, T. Local Colonization-Extinction Dynamics of a Tree-Killing Bark Beetle during a Large-Scale Outbreak. Ecosphere 2016, 7, e01257. [Google Scholar] [CrossRef]
- Buonanduci, M.S.; Morris, J.E.; Agne, M.C.; Harvey, B.J. Neighborhood Context Mediates Probability of Host Tree Mortality in a Severe Bark Beetle Outbreak. Ecosphere 2020, 11, e03236. [Google Scholar] [CrossRef]
- Scriber, J.M. Non-Target Impacts of Forest Defoliator Management Options: Decision for No Spraying May Have Worse Impacts on Non-Target Lepidoptera than Bacillus Thuringiensis Insecticides. J. Insect Conserv. 2004, 8, 241–261. [Google Scholar] [CrossRef]
- Wagner, D.L.; Van Driesche, R.G. Threats Posed to Rare or Endangered Insects by Invasions of Nonnative Species. Annu. Rev. Entomol. 2010, 55, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.A.; Lugo, A.E.; Cintron, G. Tree Mortality in Mangrove Forests. Biotropica 1985, 17, 177. [Google Scholar] [CrossRef]
- Lazarević, J.; Milanović, S.; Šešlija Jovanović, D.; Janković-Tomanić, M. Temperature- and Diet-Induced Plasticity of Growth and Digestive Enzymes Activity in Spongy Moth Larvae. Biomolecules 2023, 13, 821. [Google Scholar] [CrossRef]
- Lance, D.R.; Elkinton, J.S.; Schwalbe, C.P. Microhabitat and Temperature Effects Explain Accelerated Development during Outbreaks of the Gypsy Moth (Lepidoptera: Lymantriidae)1. Environ. Entomol. 1987, 16, 202–205. [Google Scholar] [CrossRef]
- Ponomarev, V.I.; Klobukov, G.I.; Napalkova, V.V.; Akhanaev, Y.B.; Pavlushin, S.V.; Yakimova, M.E.; Subbotina, A.O.; Picq, S.; Cusson, M.; Martemyanov, V.V. Phenological Features of the Spongy Moth, Lymantria Dispar (L.) (Lepidoptera: Erebidae), in the Northernmost Portions of Its Eurasian Range. Insects 2023, 14, 276. [Google Scholar] [CrossRef]
- Onufrieva, K.S.; Thorpe, K.W.; Hickman, A.D.; Tobin, P.C.; Leonard, D.S.; Anderson Roberts, E. Effects of SPLAT® GM Sprayable Pheromone Formulation on Gypsy Moth Mating Success. Entomol. Exp Appl. 2010, 136, 109–115. [Google Scholar] [CrossRef]
- Mannu, R.; Cocco, A.; Luciano, P.; Lentini, A. Influence of Bacillus Thuringiensis Application Timing on Population Dynamics of Gypsy Moth in Mediterranean Cork Oak Forests. Pest Manag. Sci. 2020, 76, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Podgwaite, J.D.; Martemyanov, V.V.; Slavicek, J.M.; Bakhvalov, S.A.; Pavlushin, S.V.; Hayes-Plazolles, N.; Zerillo, R.T. Potency of Nucleopolyhedrovirus Genotypes for European and Asian Gypsy Moth (Lepidoptera: Lymantriidae). J. Entomol. Sci. 2013, 48, 332–344. [Google Scholar] [CrossRef]
- Webb, R.E.; White, G.B.; Sukontarak, T.; Podgwaite, J.D.; Schumacher, D.; Diss, A.; Reardon, R.C. Biological Efficacy of Gypchek Against a Low-Density Leading-Edge Gypsy Moth Population. North. J. Appl. For. 2004, 21, 144–149. [Google Scholar] [CrossRef]
- Leroy, B.M.L. Global Insights on Insecticide Use in Forest Systems: Current Use, Impacts and Perspectives in a Changing World. Curr. For. Rep. 2024, 11, 6. [Google Scholar] [CrossRef]

| Tree Species (Scientific Name) | Not Infected | Infected | Total |
|---|---|---|---|
| Quercus rubra (Red oak) | 1 | 31 | 32 |
| Acer saccharum (Sugar maple) | 12 | 27 | 39 |
| Quercus macrocarpa (Bur oak) | 2 | 17 | 19 |
| Quercus spp. (Oak) | 1 | 7 | 8 |
| Tilia americana (Basswood) | 2 | 5 | 7 |
| Acer rubrum (Red maple) | 2 | 4 | 6 |
| Fraxinus americana (White ash) | 3 | 4 | 7 |
| Quercus alba (White oak) | 2 | 4 | 6 |
| Unknown | 0 | 3 | 3 |
| Carya cordiformis (Bitternut hickory) | 0 | 2 | 2 |
| Ostrya virginiana (Ironwood) | 1 | 2 | 3 |
| Acer spp. (Maple) | 3 | 2 | 5 |
| Carya ovata (Shagbark hickory) | 10 | 2 | 12 |
| Fraxinus spp. (Ash) | 0 | 1 | 1 |
| Fraxinus pennsylvanica (Green ash) | 6 | 0 | 6 |
| Acer saccharinum (Silver maple) | 1 | 0 | 1 |
| Tree Species (Scientific Name) | Above 1 m | Below 1 m | Total |
|---|---|---|---|
| Quercus rubra (Red oak) | 381 | 64 | 445 |
| Quercus macrocarpa (Bur oak) | 138 | 21 | 159 |
| Quercus spp. (Oak) | 78 | 12 | 90 |
| Unknown | 37 | 3 | 40 |
| Acer saccharum (Sugar maple) | 27 | 19 | 46 |
| Fraxinus americana (White ash) | 25 | 9 | 34 |
| Ostrya virginiana (Ironwood) | 11 | 1 | 12 |
| Acer rubrum (Red maple) | 8 | 3 | 11 |
| Quercus alba (White oak) | 6 | 3 | 9 |
| Carya ovata (Shagbark hickory) | 16 | 3 | 19 |
| Carya cordiformis (Bitternut hickory) | 1 | 2 | 3 |
| Acer spp. (Maple) | 4 | 1 | 5 |
| Tilia americana (Basswood) | 1 | 1 | 2 |
| Fraxinus spp. (Ash) | 2 | 0 | 2 |
| Fraxinus pennsylvanica (Green ash) | 0 | 0 | 0 |
| Acer saccharinum (Silver maple) | 0 | 0 | 0 |
| Source of Variation | % of Total Variation | DF | MS | F(DFn, DFd) | p Value | Significance |
|---|---|---|---|---|---|---|
| Interaction | 7.76 | 15 | 96.06 | F(15, 282) = 1.96 | 0.018 | * |
| Tree species (Row) | 12.37 | 15 | 153.10 | F(15, 282) = 3.12 | <0.001 | *** |
| Height (Column) | 0.90 | 1 | 167.60 | F(1, 282) = 3.42 | 0.066 | ns |
| Residual | 79.00 | 282 | 49.08 | — | — | — |
| Tree Species | Mean Diff. (Below − Above) | 95% CI of Diff. | Adjusted p Value | Significance |
|---|---|---|---|---|
| Quercus rubra (Red oak) | –9.91 | –15.11 to –4.70 | <0.001 | *** |
| Quercus macrocarpa (Bur oak) | –6.16 | –12.92 to 0.60 | 0.109 | ns |
| Quercus spp. (Unidentified) | –11.33 | –28.34 to 5.68 | 0.549 | ns |
| Quercus alba (White oak) | +1.67 | –10.36 to 13.69 | >0.999 | ns |
| Acer saccharum (Sugar maple) | –0.21 | –4.92 to 4.51 | >0.999 | ns |
| Fraxinus americana (White ash) | –2.00 | –13.13 to 9.14 | >0.999 | ns |
| Carya ovata (Shagbark hickory) | –0.17 | –8.67 to 8.34 | >0.999 | ns |
| All other species | ns | — | — | ns |
| Egg Masses (Presence/Absence) | Unhealthy (0) | Healthy (1) | Total |
|---|---|---|---|
| Absence (0) | 23 | 23 | 46 |
| Presence (1) | 80 | 31 | 111 |
| Total | 103 | 54 | 157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Wang, Q. Infestation Patterns and Climate-Based Projections for European Spongy Moth (Lymantria dispar) in Whirlpool Forest, Ontario, Canada. Biology 2025, 14, 1506. https://doi.org/10.3390/biology14111506
Guo X, Wang Q. Infestation Patterns and Climate-Based Projections for European Spongy Moth (Lymantria dispar) in Whirlpool Forest, Ontario, Canada. Biology. 2025; 14(11):1506. https://doi.org/10.3390/biology14111506
Chicago/Turabian StyleGuo, Xiaolong, and Qianqian Wang. 2025. "Infestation Patterns and Climate-Based Projections for European Spongy Moth (Lymantria dispar) in Whirlpool Forest, Ontario, Canada" Biology 14, no. 11: 1506. https://doi.org/10.3390/biology14111506
APA StyleGuo, X., & Wang, Q. (2025). Infestation Patterns and Climate-Based Projections for European Spongy Moth (Lymantria dispar) in Whirlpool Forest, Ontario, Canada. Biology, 14(11), 1506. https://doi.org/10.3390/biology14111506






