Characterization of Ingested Microplastics in a Regional Endemic Lizard Apathya cappadocica (Werner, 1902) from Türkiye
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
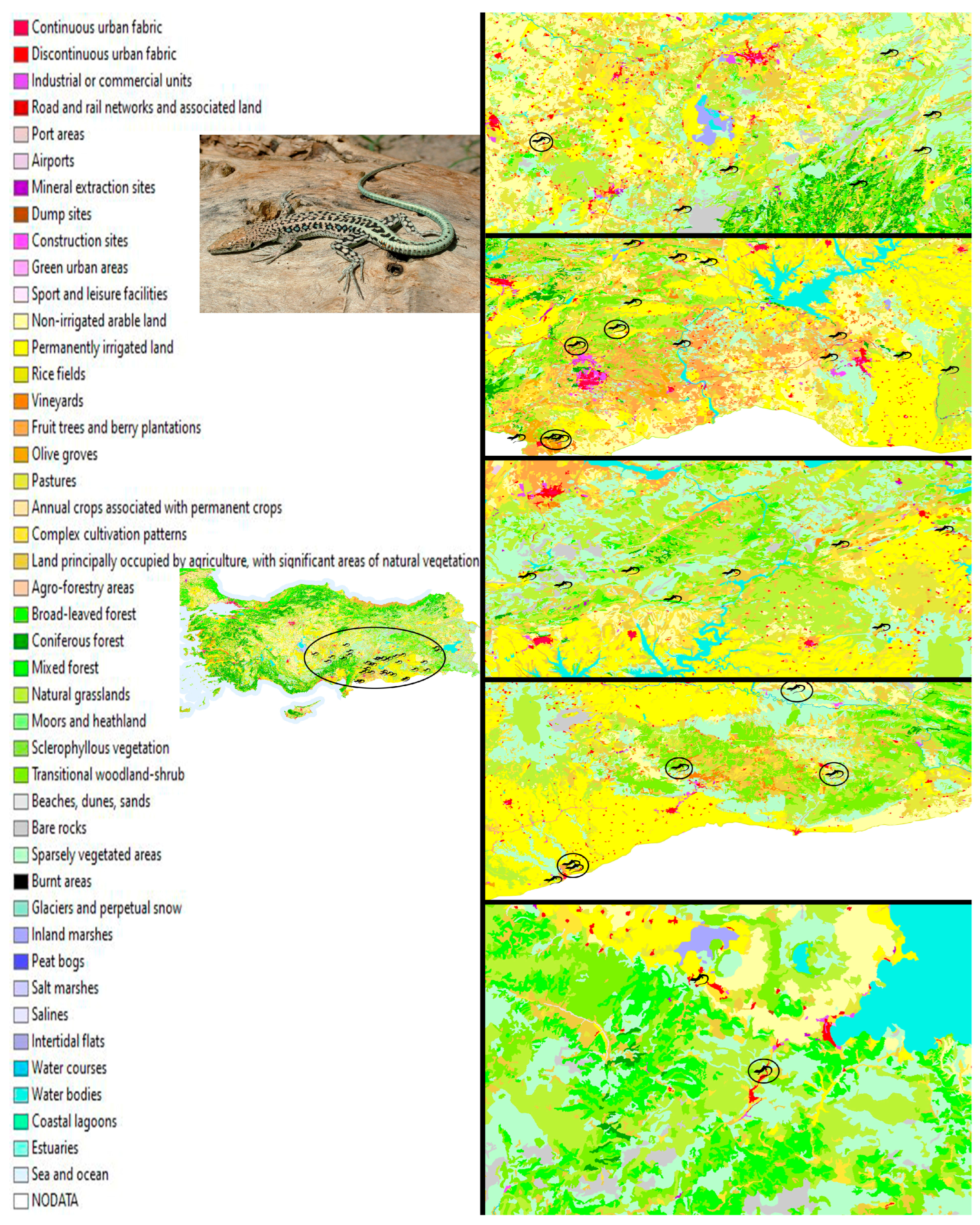
2.1. Sampling
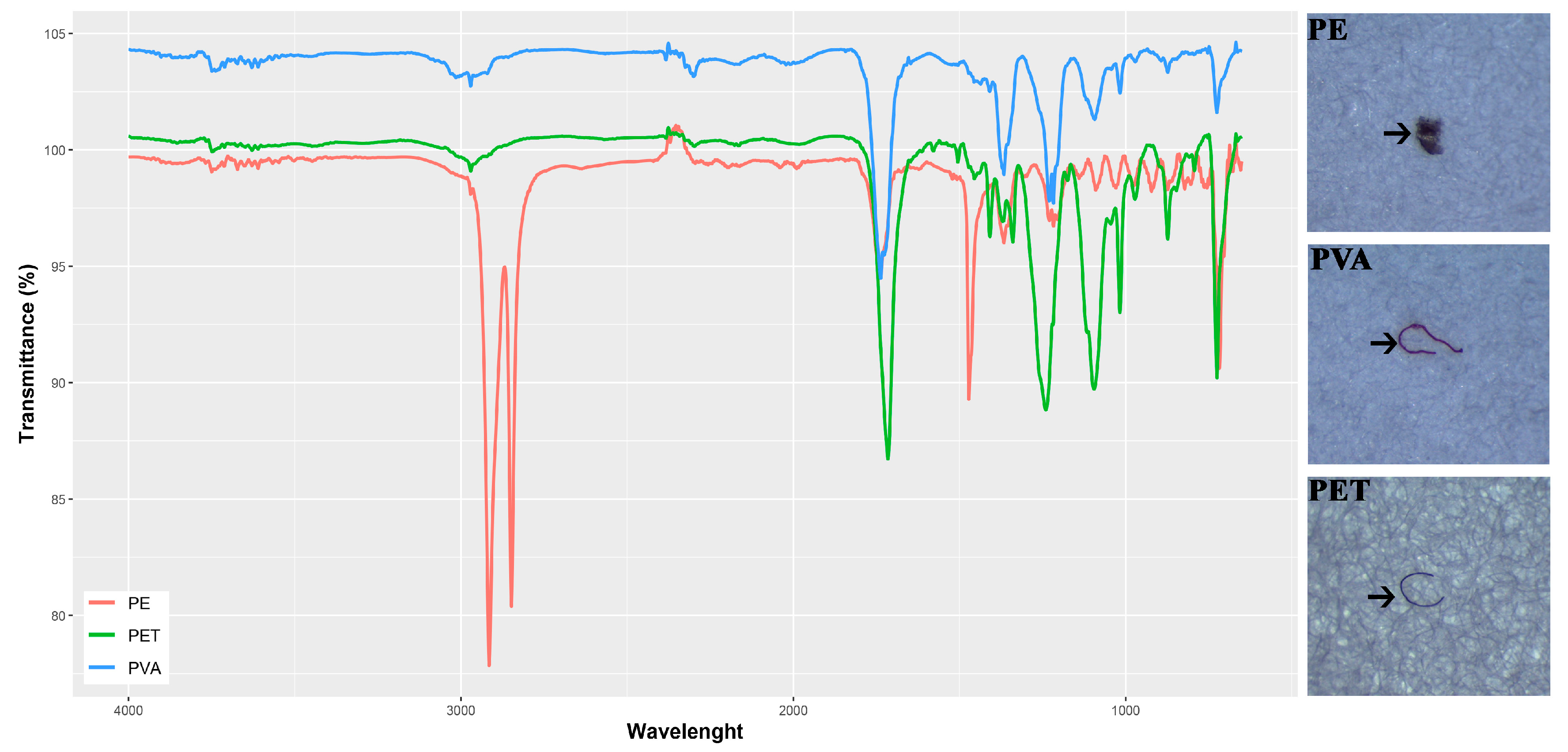
2.2. Microplastic Characterization
2.3. Statistical Process
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Wang, P. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef]
- Caron, A.G.; Thomas, C.R.; Berry, K.L.; Motti, C.A.; Ariel, E.; Brodie, J.E. Ingestion of microplastic debris by green sea turtles (Chelonia mydas) in the Great Barrier Reef: Validation of a sequential extraction protocol. Mar. Pollut. Bull. 2018, 127, 743–751. [Google Scholar] [CrossRef]
- Chemello, G.; Trotta, E.; Notarstefano, V.; Papetti, L.; Di Renzo, L.; Matiddi, M.; Gioacchini, G. Microplastics evidence in yolk and liver of loggerhead sea turtles (Caretta caretta), a pilot study. Environ. Pollut. 2023, 337, 122589. [Google Scholar] [CrossRef]
- Curl, L.F.; Hurst, S.A.; Pomory, C.M.; Lamont, M.M.; Janosik, A.M. Assessing microplastics contamination in unviable loggerhead sea turtle eggs. Sci. Total Environ. 2024, 912, 169434. [Google Scholar] [CrossRef] [PubMed]
- Beigzadeh, K.; Rieland, J.M.; Eastman, C.B.; Duffy, D.J.; Love, B.J. Characterization of ingested plastic microparticles extracted from sea turtle post-hatchlings at necropsy. Microplastics 2022, 1, 254–262. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017; p. 43. [Google Scholar]
- Rodríguez-Torres, R.; Rist, S.; Almeda, R.; Nielsen, T.G.; Pedrotti, M.L.; Hartmann, N.B. Research trends in nano- and microplastic ingestion in marine planktonic food webs. Environ. Pollut. 2024, 363, 125136. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Neelam, A.; Hany, O.E.; Ishteyaque, S. Microplastic: A potential threat to marine vertebrates “A mini review”. J. Basic Environ. Sci. 2024, 5, 155–161. [Google Scholar] [CrossRef]
- da Costa, J.R.; de Freitas, D.M. Microplastic occurrence in the diet of green sea turtles (Chelonia mydas) from the coastal region of São Paulo, Brazil. Aquat. Conserv. 2025, 35, e70158. [Google Scholar] [CrossRef]
- Borroto-Paez, R.; León, O.E.S.; Fabres, B.A. Microplastics in invasive geckos (Hemidactylus mabouia and H. angulatus): First evidence in Cuba and the Caribbean, and transfer pathways of concern. Reptiles Amphib. 2024, 31, e22751. [Google Scholar] [CrossRef]
- Teampanpong, J.; Duengkae, P. Terrestrial wildlife as indicators of microplastic pollution in western Thailand. PeerJ 2024, 12, e17384. [Google Scholar] [CrossRef] [PubMed]
- Dursun, C.; Candan, K.; Karaoğlu, K.; Ilgaz, Ç.; Kumlutaş, Y.; Caynak, E.Y.; Gül, S. Microplastic accumulation in snake-eyed lizard (Ophisops elegans Menetries, 1832) after long-term monitoring: Habitats matter, not years. Environ. Sci. Eur. 2025, 37, 8. [Google Scholar] [CrossRef]
- Gonzalez-Jauregui, M.; Borges-Ramirez, M.; Barão-Nóbrega, J.A.L.; Escamilla, A.; Dzul-Caamal, R.; Rendón-von Osten, J. Stomach flushing technique applied to quantify microplastics in Crocodilians. MethodsX 2019, 6, 2677–2685. [Google Scholar] [CrossRef]
- Duncan, E.M.; Broderick, A.C.; Fuller, W.J.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Godley, B.J. Microplastic ingestion ubiquitous in marine turtles. Glob. Change Biol. 2019, 25, 744–752. [Google Scholar] [CrossRef]
- Digka, N.; Bray, L.; Tsangaris, C.; Andreanidou, K.; Kasimati, E.; Kofidou, E.; Kaberi, H. Evidence of ingested plastics in stranded loggerhead sea turtles along the Greek coastline, East Mediterranean Sea. Environ. Pollut. 2020, 263, 114596. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Stucchi, M.; Ambrosini, R.; Romano, A. A global perspective on microplastic bioaccumulation in marine organisms. Ecol. Indic. 2023, 149, 110179. [Google Scholar] [CrossRef]
- Eastman, C.B.; Farrell, J.A.; Whitmore, L.; Rollinson Ramia, D.R.; Thomas, R.S.; Prine, J.; Eastman, S.F.; Osborne, T.Z.; Martindale, M.Q.; Duffy, D.J. Plastic ingestion in post-hatchling sea turtles: Assessing a major threat in Florida near shore waters. Front. Mar. Sci. 2020, 7, 693. [Google Scholar] [CrossRef]
- Gül, S.; Karaoğlu, K.; Özçifçi, Z.; Candan, K.; Ilgaz, Ç.; Kumlutaş, Y. Occurrence of microplastics in herpetological museum collection: Grass snake (Natrix natrix [Linnaeus, 1758]) and dice snake (Natrix tessellata [Laurenti, 1769]) as model organisms. Water Air Soil Pollut. 2022, 233, 160. [Google Scholar] [CrossRef]
- Dursun, C.; Demirci, N.; Candan, K.; Yıldırım Caynak, E.; Kumlutaş, Y.; Ilgaz, Ç.; Gül, S. Microplastic contamination of the Turkish Worm Lizard (Blanus strauchi Bedriaga, 1884) in Muğla Province (Türkiye). Biology 2025, 14, 441. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; Kim, S.; Sarkar, B.; Oleszczuk, P.; Sang, M.K.; Haque, M.N.; Ahn, J.H.; Bank, M.S.; Ok, Y.S. Effects of microplastics on the terrestrial environment: A critical review. Environ. Res. 2022, 209, 112734. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.J.; Hai, F.I. Microplastics as an emerging contaminant of concern to our environment: A brief overview of the sources and implications. Bioengineered 2023, 14, 2244754. [Google Scholar] [CrossRef] [PubMed]
- Gül, S.; Özdemir, N.; Avcı, A.; Kumlutaş, Y.; Ilgaz, Ç. Altitudinal effects on life history of Anatolian lizard (Apathya cappadocica, Werner 1902) from the southeastern Anatolia, Turkey. Turk. J. Zool. 2015, 39, 507–512. [Google Scholar] [CrossRef]
- Modica, L.; Lanuza, P.; García-Castrillo, G. Surrounded by microplastic, since when? Testing the feasibility of exploring past levels of plastic microfibre pollution using natural history museum collections. Mar. Pollut. Bull. 2020, 151, 110846. [Google Scholar] [CrossRef]
- Nayebi, B.; Khurana, P.; Pulicharla, R.; Karimpour, S.; Brar, S.K. Preservation, storage, and sample preparation methods for freshwater microplastics—A comprehensive review. Environ. Sci. Adv. 2023, 2, 1060–1081. [Google Scholar] [CrossRef]
- CORINE Land Cover 2018 (Vector/Raster 100 m), Europe, 6-Yearly. European Union’s Copernicus Land Monitoring Service Information. Available online: https://land.copernicus.eu/en/products/corine-land-cover/clc2018 (accessed on 25 August 2025).
- Aragón-Sánchez, J.; Quintana-Marrero, Y.; Aragón-Hernández, C.; Hernández-Herero, M.J. ImageJ: A free, easy, and reliable method to measure leg ulcers using digital pictures. Int. J. Low. Extrem. Wounds 2017, 16, 269–273. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2022; Available online: https://CRAN.R-project.org/package=psych (accessed on 1 June 2025).
- Hebbali, A. Olsrr: Tools for Building OLS Regression Models. R Package Version 0.5.3. 2020. Available online: https://CRAN.R-project.org/package=olsrr (accessed on 1 June 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org (accessed on 1 June 2025).
- Gerbing, D.W. Enhancement of the command-line environment for use in the introductory statistics course and beyond. J. Stat. Data Sci. Educ. 2021, 29, 251–256. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Jovanović, B. Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef]
- Oza, J.; Rabari, V.; Yadav, V.K.; Sahoo, D.K.; Patel, A.; Trivedi, J. A systematic review on microplastic contamination in fishes of Asia: Polymeric risk assessment and future prospectives. Environ. Toxicol. Chem. 2024, 43, 671–685. [Google Scholar] [CrossRef]
- Kaya, C.; Minaz, M.; Şentürk Koca, Y.; Oral Kaba, M.; Kurtul, I.; Aytan, Ü. Monitoring microplastics in a region with sensitive fish biodiversity: Tigris, Euphrates and Van Lake drainages in Irano-Anatolian hotspot. Environ. Sci. Eur. 2025, 37, 102. [Google Scholar] [CrossRef]
- Dursun, C.; Karaoğlu, K.; Özdemir, N.; Candan, K.; Kumlutaş, Y.; Ilgaz, Ç.; Gül, S. Spatiotemporal distribution of microplastics in true frogs (Ranidae: Pelophylax) populations from Türkiye. Environ. Res. 2023, 236, 116774. [Google Scholar] [CrossRef]
- Gao, C.; Xu, B.; Li, Z.; Wang, Z.; Huang, S.; Jiang, Z.; Yang, H. From plankton to fish: The multifaceted threat of microplastics in freshwater environments. Aquat. Toxicol. 2025, 279, 107242. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Kim, E.S.; Sung, H.C. Microplastics as an emerging threat to amphibians: Current status and future perspectives. Heliyon 2024, 10, e28220. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, G.A.; Ramos, M.C.S.; Carvalho, G.L.D.; Camilo-Cotrim, C.F.; do Amaral, R.B.; Castro, Í.B.; Damacena-Silva, L. Microplastic contamination in wild freshwater fish: Global trends, challenges and perspectives. Environ. Pollut. 2025, 377, 126406. [Google Scholar] [CrossRef]
- Najibzadeh, M.; Kazemi, A.; Esmaeilbeigi, M.; Hassan, H.U. Impact of microplastic contamination derived from human activities on anuran species. Process Saf. Environ. Prot. 2025, 197, 107002. [Google Scholar] [CrossRef]
- Clause, A.G.; Celestian, A.J.; Pauly, G.B. Plastic ingestion by freshwater turtles: A review and call to action. Sci. Rep. 2021, 11, 5672. [Google Scholar] [CrossRef]
- Huo, D.-M.; Rao, D.-Q. Microplastics: Their effects on amphibians and reptiles—A review. Pak. J. Zool. 2022, 54, 503–523. [Google Scholar]
- Botterell, Z.L.; Ardren, J.; Dove, E.; McArthur, E.; Addison, D.S.; Adegbile, O.M.; Tucker, A.D. A global assessment of microplastic abundance and characteristics on marine turtle nesting beaches. Mar. Pollut. Bull. 2025, 215, 117768. [Google Scholar] [CrossRef]
- Mackenzie, C.M.; Vladimirova, V. Preliminary study and first evidence of presence of microplastics in terrestrial herpetofauna from Southwestern Paraguay. Stud. Neotrop. Fauna Environ. 2023, 58, 16–24. [Google Scholar] [CrossRef]
- Teampanpong, J.; Duengkae, P. Using feces to indicate plastic pollution in terrestrial vertebrate species in western Thailand. PeerJ 2024, 12, e17596. [Google Scholar] [CrossRef]
- Lu, S.; Qiu, R.; Hu, J.; Li, X.; Chen, Y.; Zhang, X.; Cao, C.; Shi, H.; Xie, B.; Wu, W.M.; et al. Prevalence of microplastics in animal-based traditional medicinal materials: Widespread pollution in terrestrial environments. Sci. Total Environ. 2020, 709, 136214. [Google Scholar] [CrossRef]
- Mishra, S.; Charan Rath, C.; Das, A.P. Marine microfiber pollution: A review on present status and future challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef]
- Liu, J.; Liang, J.; Ding, J.; Zhang, G.; Zeng, X.; Yang, Q.; Gao, W. Microfiber pollution: An ongoing major environmental issue related to the sustainable development of textile and clothing industry. Environ. Dev. Sustain. 2021, 23, 11240–11256. [Google Scholar] [CrossRef]
- Athey, S.N.; Erdle, L.M. Are we underestimating anthropogenic microfiber pollution? A critical review of occurrence, methods, and reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Haave, M.; Gomiero, A.; Schönheit, J.; Nilsen, H.; Olsen, A.B. Documentation of microplastics in tissues of wild coastal animals. Front. Environ. Sci. 2021, 9, 575058. [Google Scholar] [CrossRef]
- Rebelein, A.; Int-Veen, I.; Kammann, U.; Scharsack, J.P. Microplastic fibers—Underestimated threat to aquatic organisms? Sci. Total Environ. 2021, 777, 146045. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Dargahi, A.; Almasi, A.; Mousavi, S.A.; Mohammadi, P. Occurrence of microplastic, antibiotics, hormones, and heavy metals in livestock and poultry manure in West Iran. Sci. Rep. 2025, 15, 27228. [Google Scholar] [CrossRef] [PubMed]
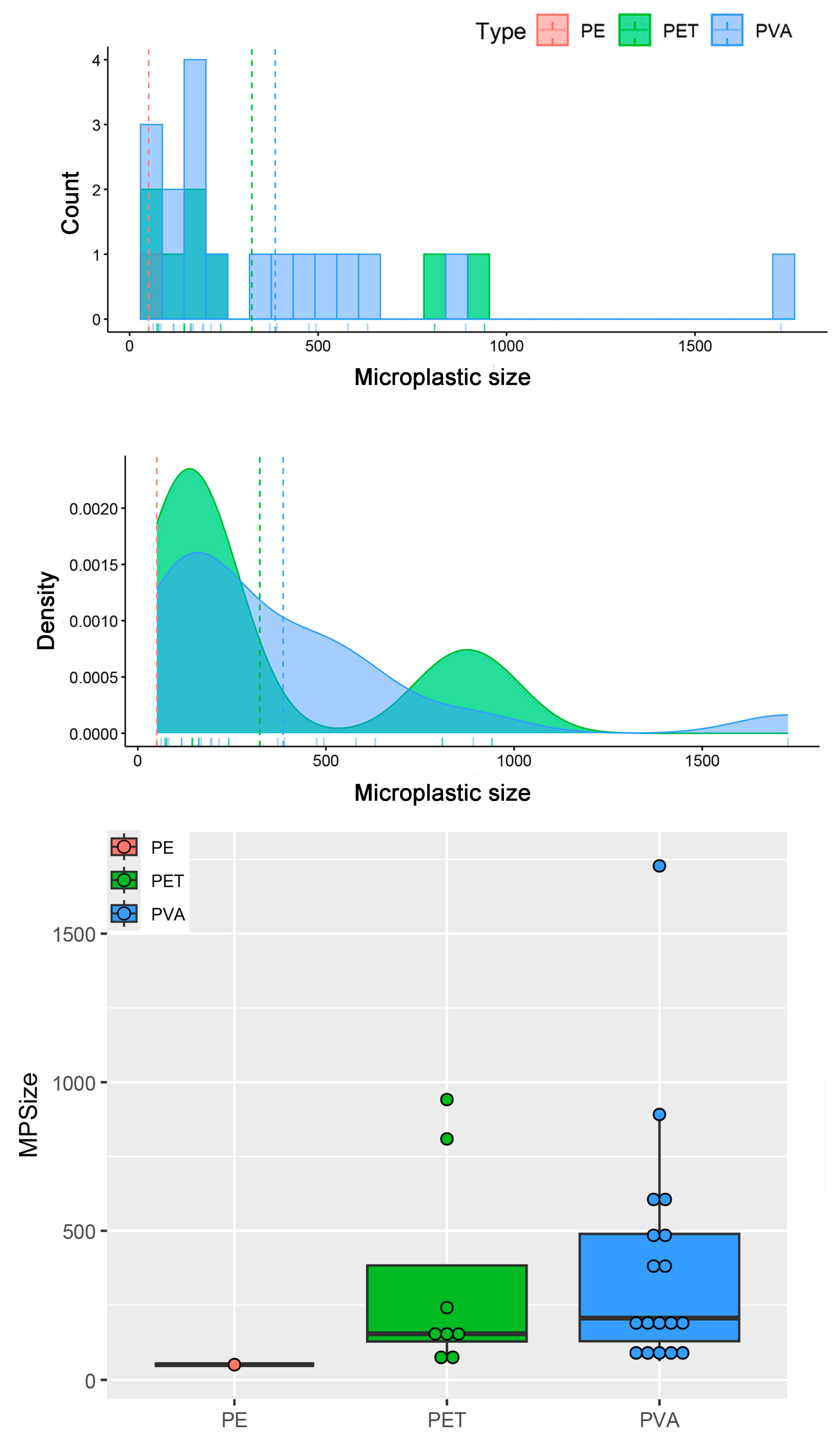
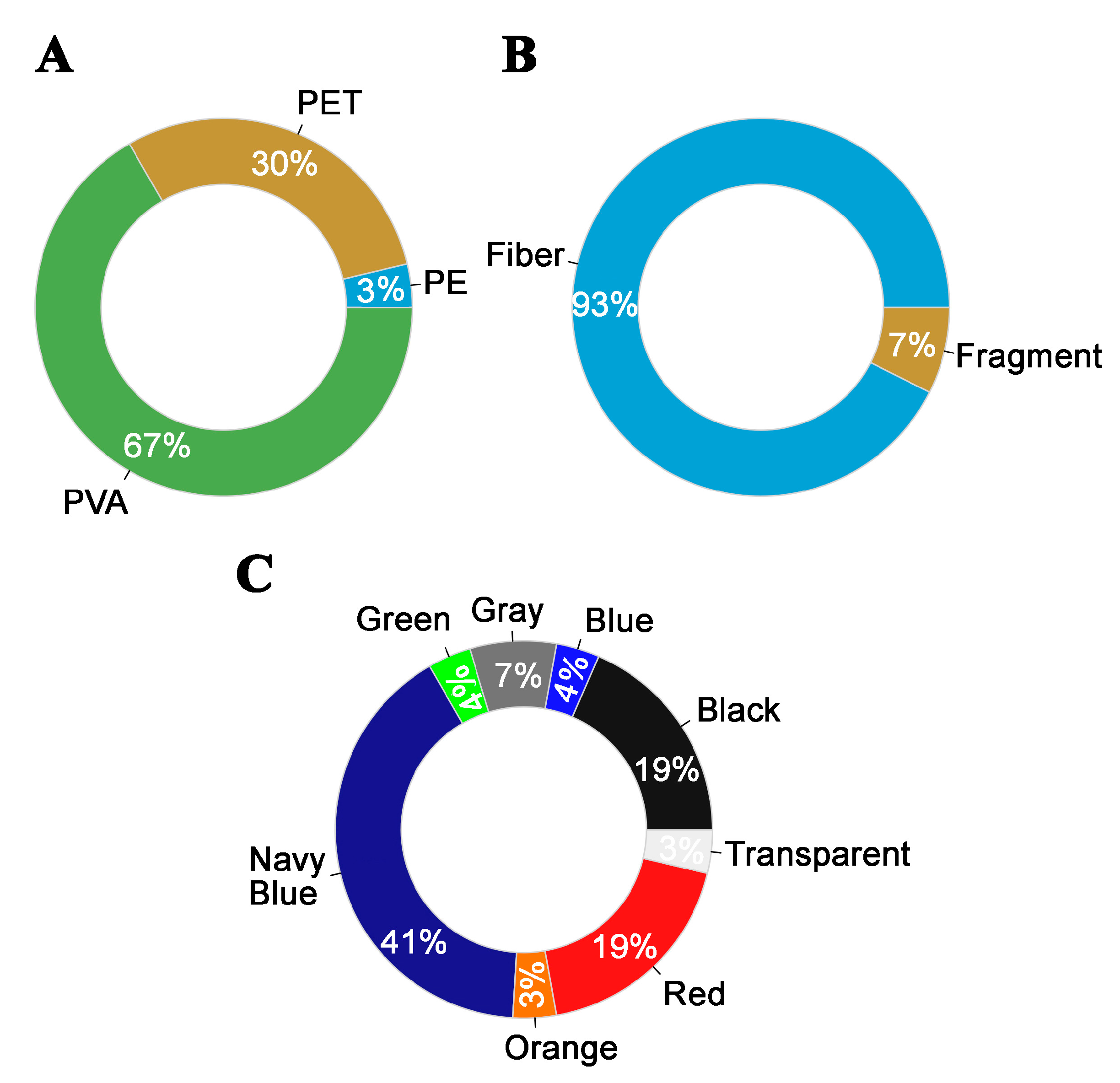
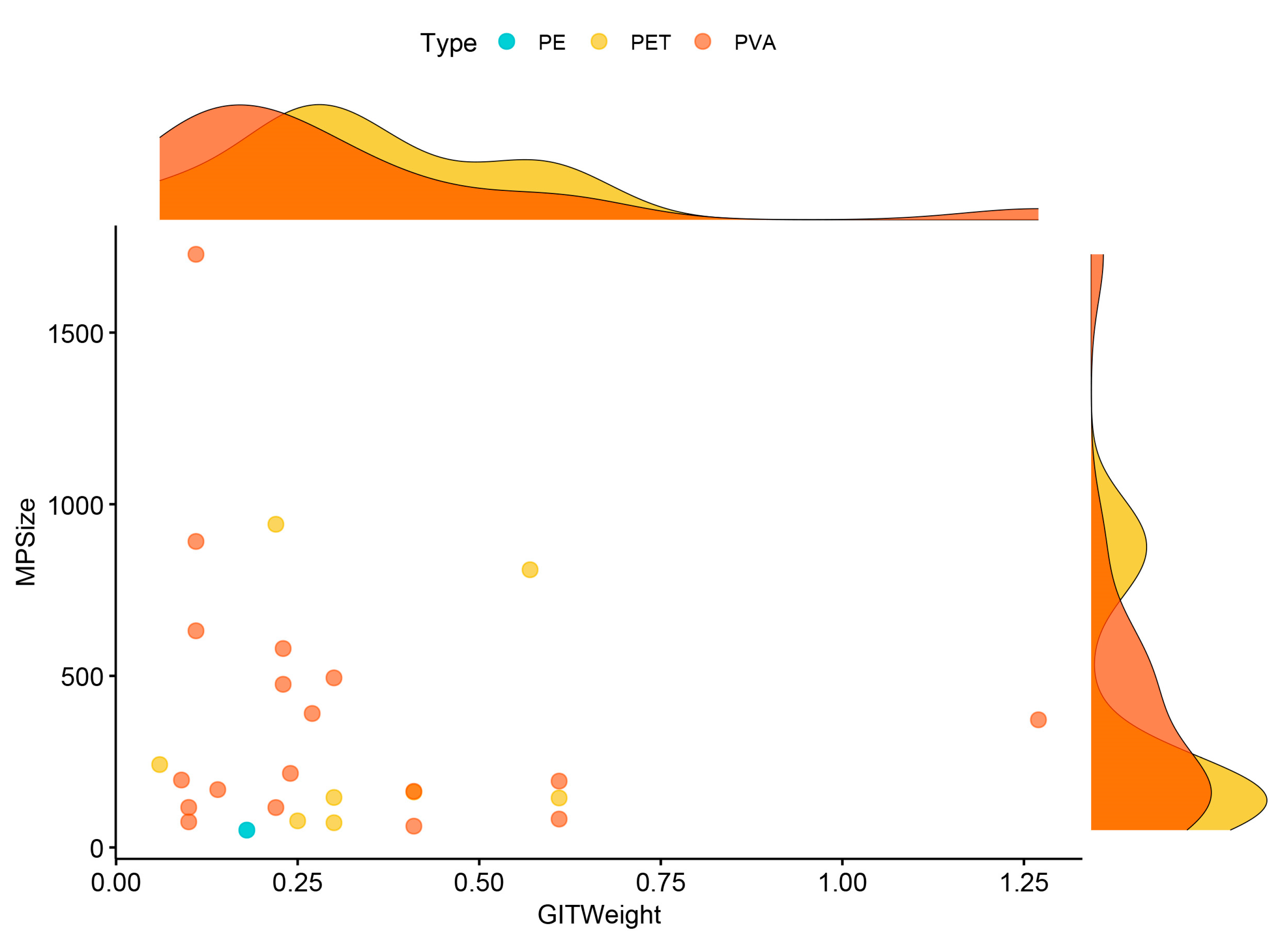
| MP Size (μm) | GIT Weight (g) | ||||
|---|---|---|---|---|---|
| N | Mean ± SE | Min–Max | Mean ± SE | Min–Max | |
| All items | 27 | 355.46 ± 73.26 | 50.53–1727.97 | 0.31 ± 0.05 | 0.06–1.27 |
| Type | N | Mean ± SE | Min–Max | Mean ± SE | Min–Max |
| PE | 1 | 50.53 | - | 0.18 | - |
| PET | 8 | 324.19 ± 122.35 | 71.96–941.59 | 0.34 ± 0.06 | 0.06–0.61 |
| PVA | 18 | 386.31 ± 95.86 | 62.09–1727.97 | 0.31 ± 0.07 | 0.09–1.27 |
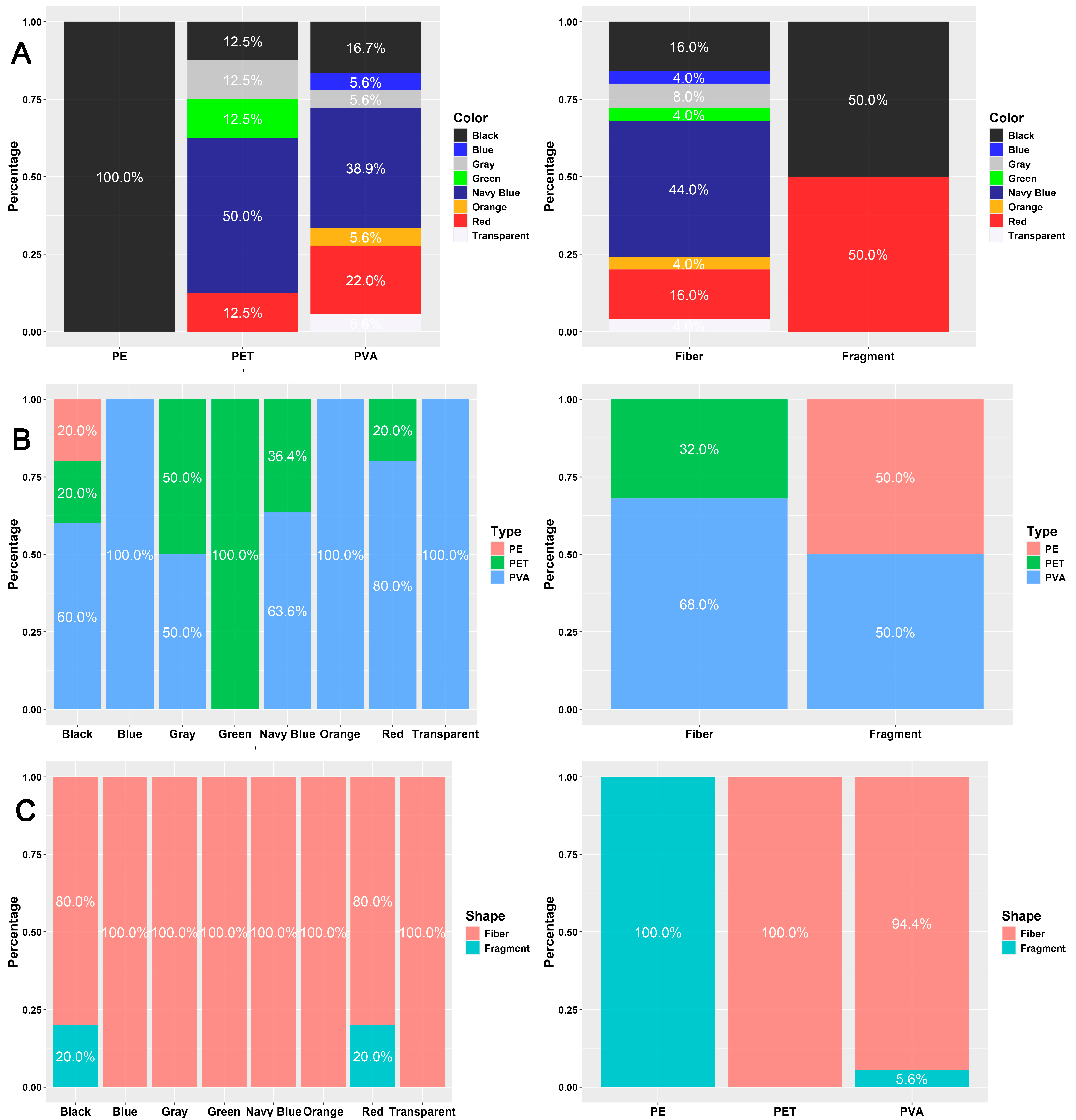
| Color | Mean ± SE | Min–Max | Mean ± SE | Min–Max | |
| Black | 5 | 92.27 ± 26.51 | 50.53–196.57 | 0.21 ± 0.06 | 0.09–0.41 |
| Blue | 1 | 193.44 | - | 0.61 | - |
| Gray | 2 | 318.59 ± 156.92 | 161.67–475.51 | 0.32 ± 0.09 | 0.23–0.41 |
| Green | 1 | 941.59 | - | 0.22 | - |
| Navy Blue | 11 | 298.04 ± 70.95 | 71.96–809.19 | 0.35 ± 0.11 | 0.06–1.27 |
| Orange | 1 | 494.21 | - | 0.30 | - |
| Red | 5 | 602.33 ± 317.94 | 82.74–1727.97 | 0.31 ± 0.09 | 0.1–0.61 |
| Transparent | 1 | 579.65 | - | 0.23 | - |
| Shape | Mean ± SE | Min–Max | Mean ± SE | Min–Max | |
| Fiber | 25 | 378.57 ± 77.32 | 62.09–1727.97 | 0.31 ± 0.05 | 0.06– 1.27 |
| Fragment | 2 | 66.63 ± 16.10 | 50.53–82.74 | 0.40 ± 0.21 | 0.18–0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dursun, C.; Demirci, N.; Candan, K.; Korkmaz, A.G.; Hastürk, E.B.; Yıldırım Caynak, E.; Ilgaz, Ç.; Kumlutaş, Y.; Gül, S. Characterization of Ingested Microplastics in a Regional Endemic Lizard Apathya cappadocica (Werner, 1902) from Türkiye. Biology 2025, 14, 1457. https://doi.org/10.3390/biology14101457
Dursun C, Demirci N, Candan K, Korkmaz AG, Hastürk EB, Yıldırım Caynak E, Ilgaz Ç, Kumlutaş Y, Gül S. Characterization of Ingested Microplastics in a Regional Endemic Lizard Apathya cappadocica (Werner, 1902) from Türkiye. Biology. 2025; 14(10):1457. https://doi.org/10.3390/biology14101457
Chicago/Turabian StyleDursun, Cantekin, Nagihan Demirci, Kamil Candan, Ahmet Gökay Korkmaz, Ecem Büşra Hastürk, Elif Yıldırım Caynak, Çetin Ilgaz, Yusuf Kumlutaş, and Serkan Gül. 2025. "Characterization of Ingested Microplastics in a Regional Endemic Lizard Apathya cappadocica (Werner, 1902) from Türkiye" Biology 14, no. 10: 1457. https://doi.org/10.3390/biology14101457
APA StyleDursun, C., Demirci, N., Candan, K., Korkmaz, A. G., Hastürk, E. B., Yıldırım Caynak, E., Ilgaz, Ç., Kumlutaş, Y., & Gül, S. (2025). Characterization of Ingested Microplastics in a Regional Endemic Lizard Apathya cappadocica (Werner, 1902) from Türkiye. Biology, 14(10), 1457. https://doi.org/10.3390/biology14101457






