Study on the Mechanism of Low-Intensity Pulsed Ultrasound in Ameliorating Glucose Metabolism Through Attenuation of Skeletal Muscle Atrophy in Mice with Type 1 Diabetes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Grouping and Treatments
2.3. FBG Analysis [18]
2.4. Oral Glucose Tolerance Tests (OGTTs) [18]
2.5. Grip Strength Test [18]
2.6. Quadriceps Femoris Muscle Contraction In Vivo and In Situ Test and Maximum Tensile Load of Intestine
2.7. Morphometric Analysis [17,18]
2.8. Serum Analysis
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
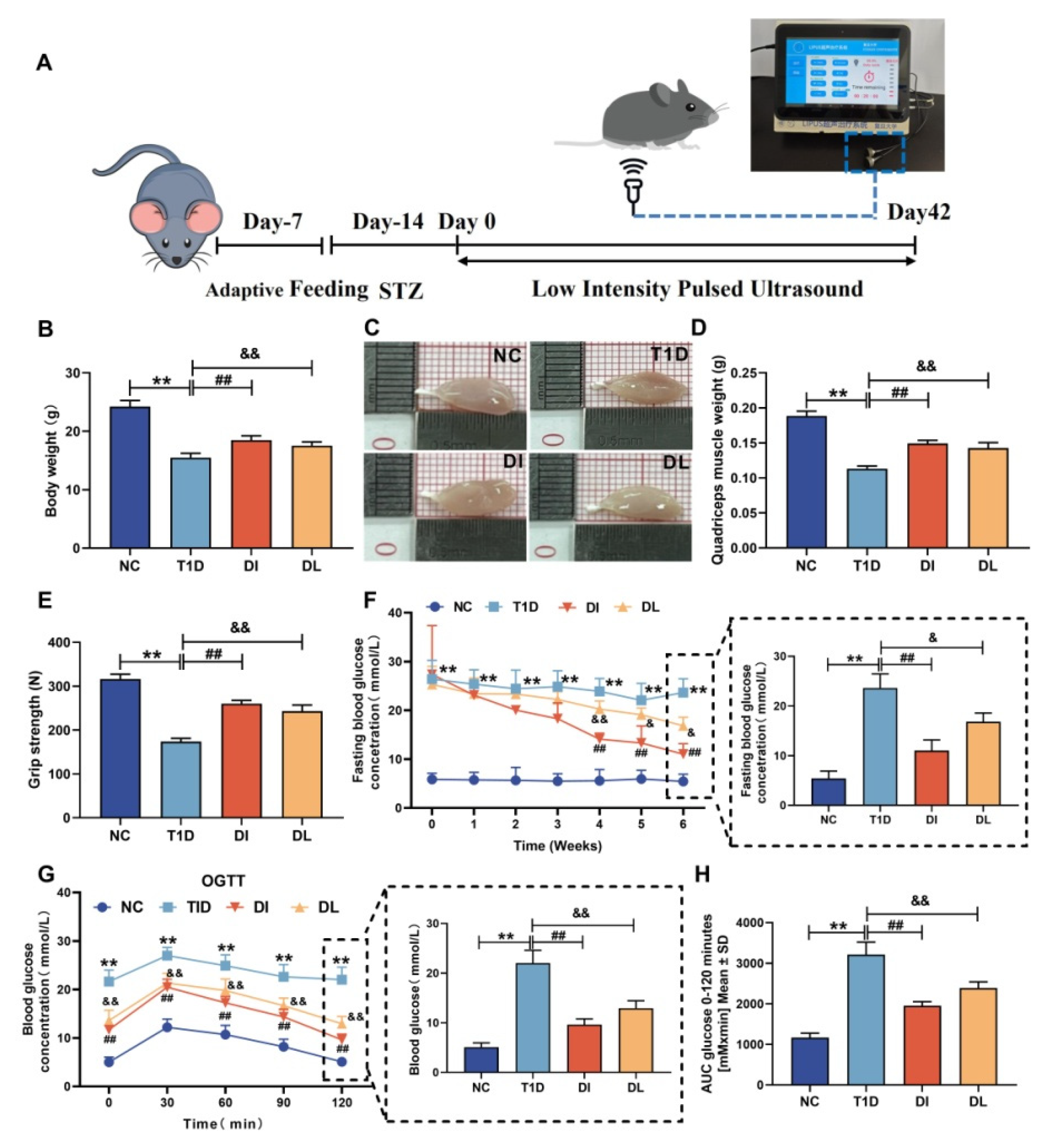
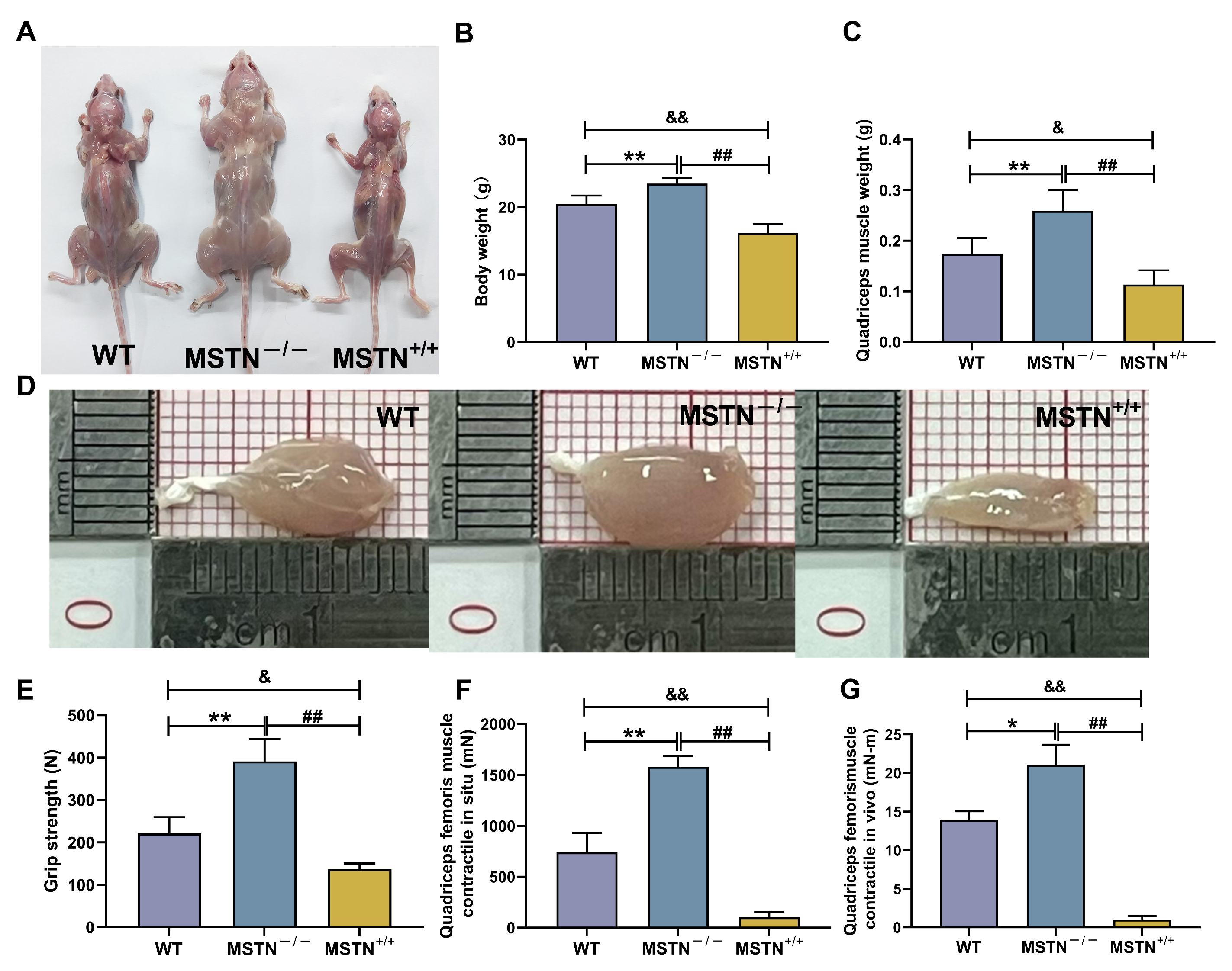
3.1. Physiological Characteristics of Mice
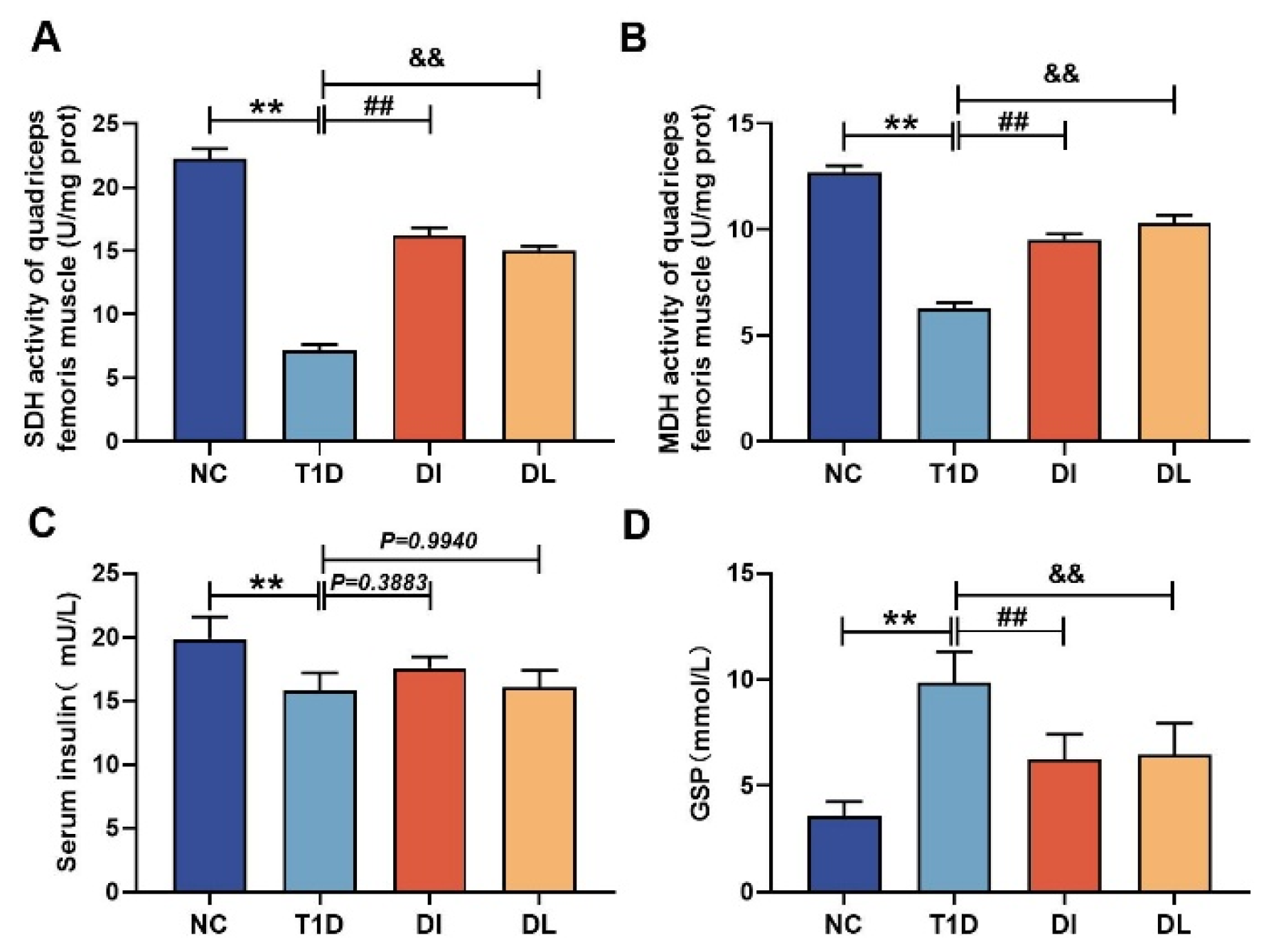
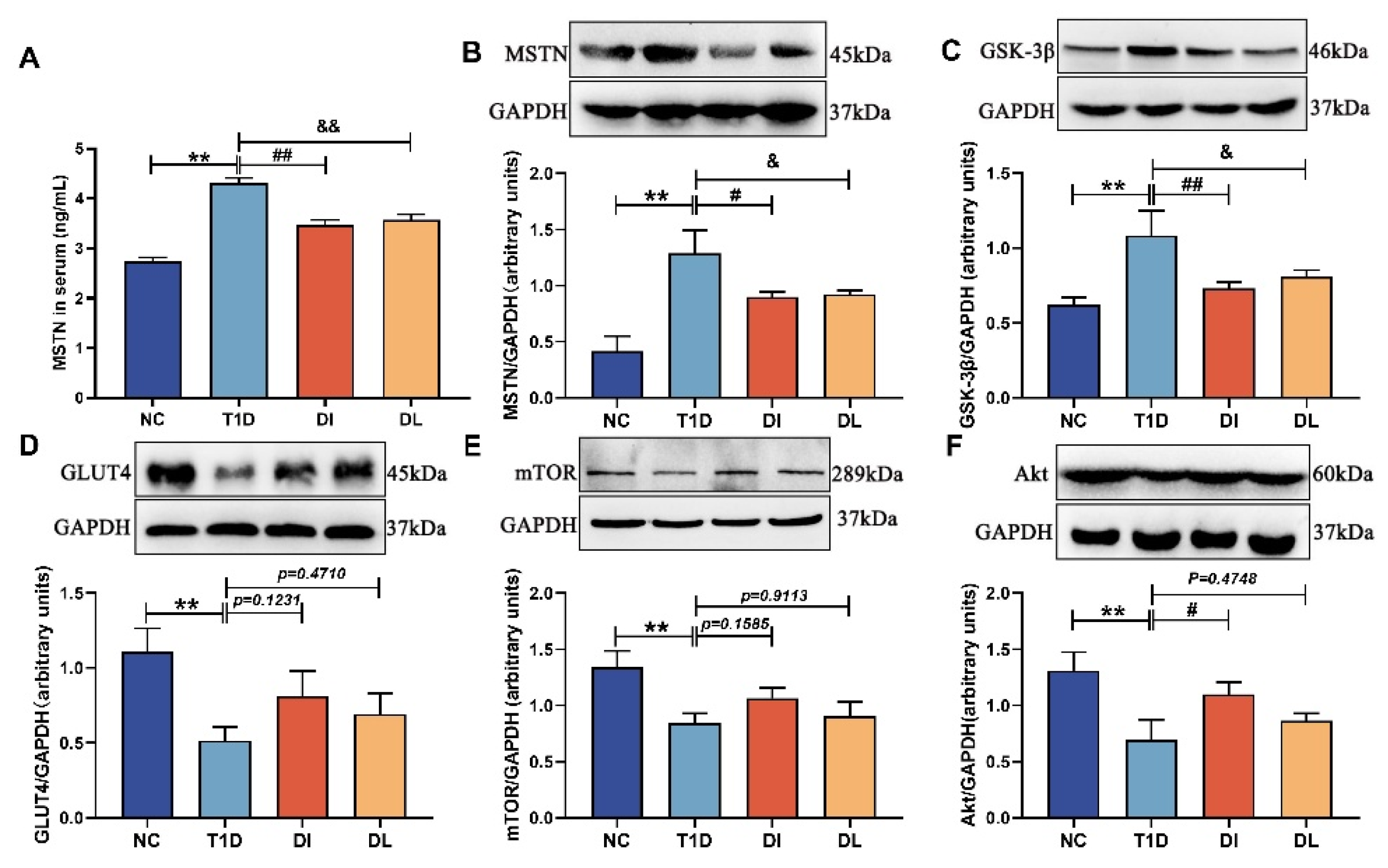
3.2. Serum Analysis
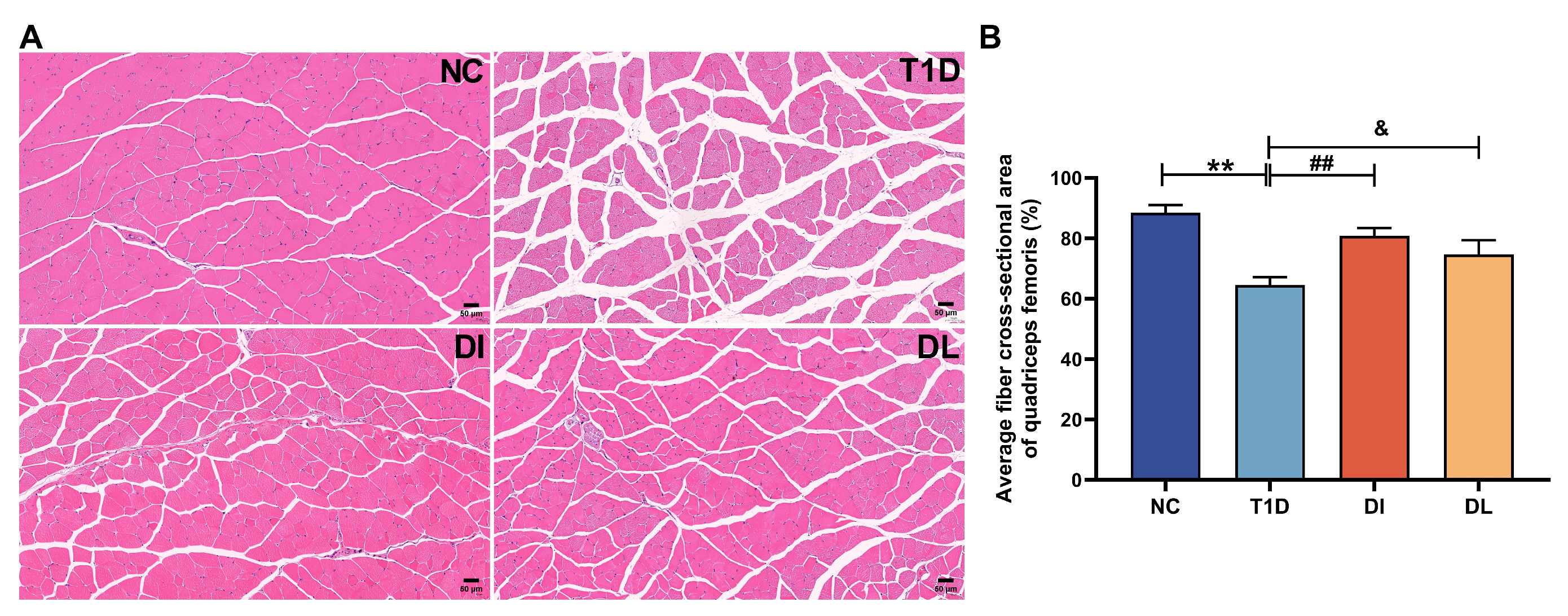
3.3. Morphological Analysis
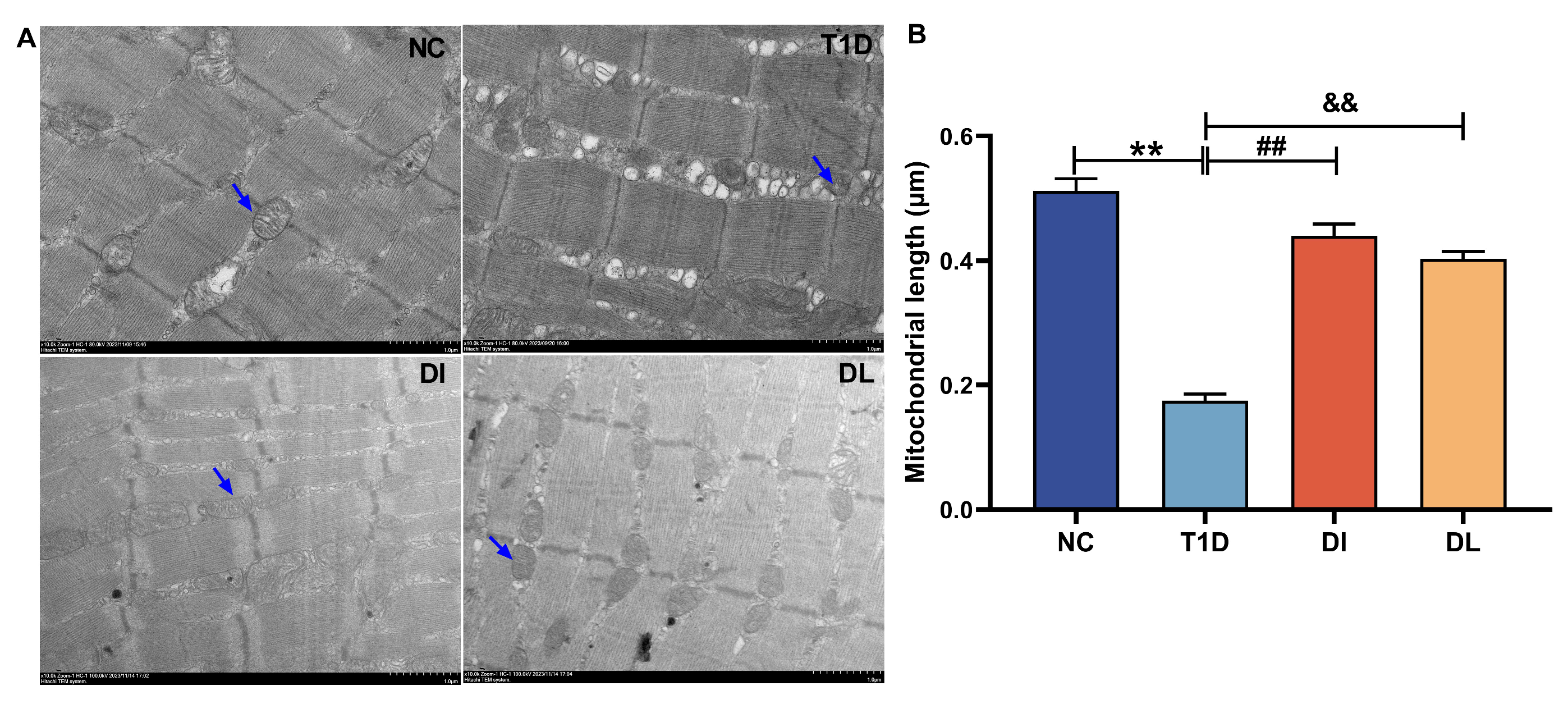
3.4. Electron Microscope Analysis
3.5. Western Blot Analysis
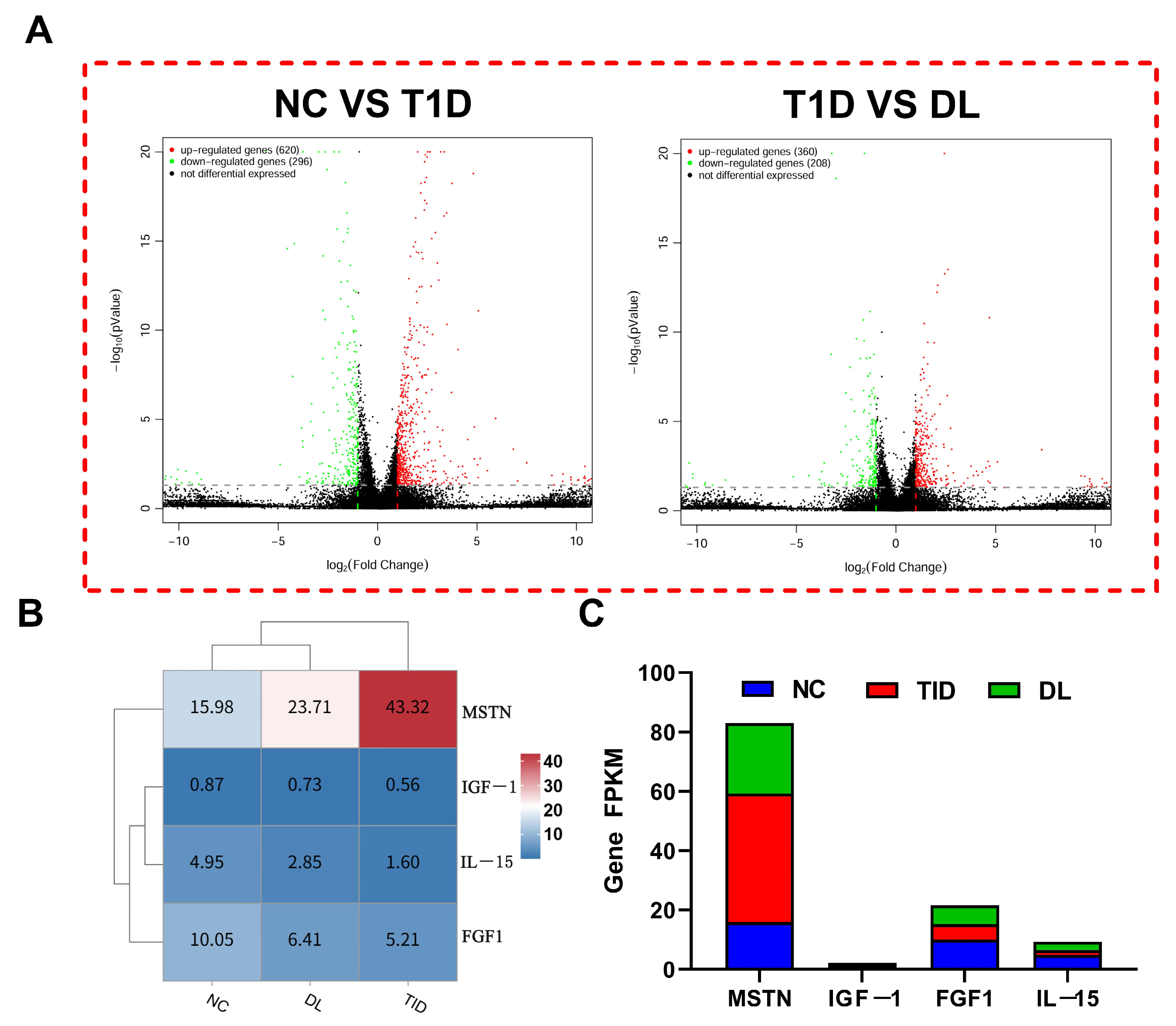
3.6. Transcriptome Sequencing Data Evaluation
3.7. The mRNA Expression Analysis
3.8. Physicochemical Characteristics of MSTN−/− and MSTN+/+ Mice
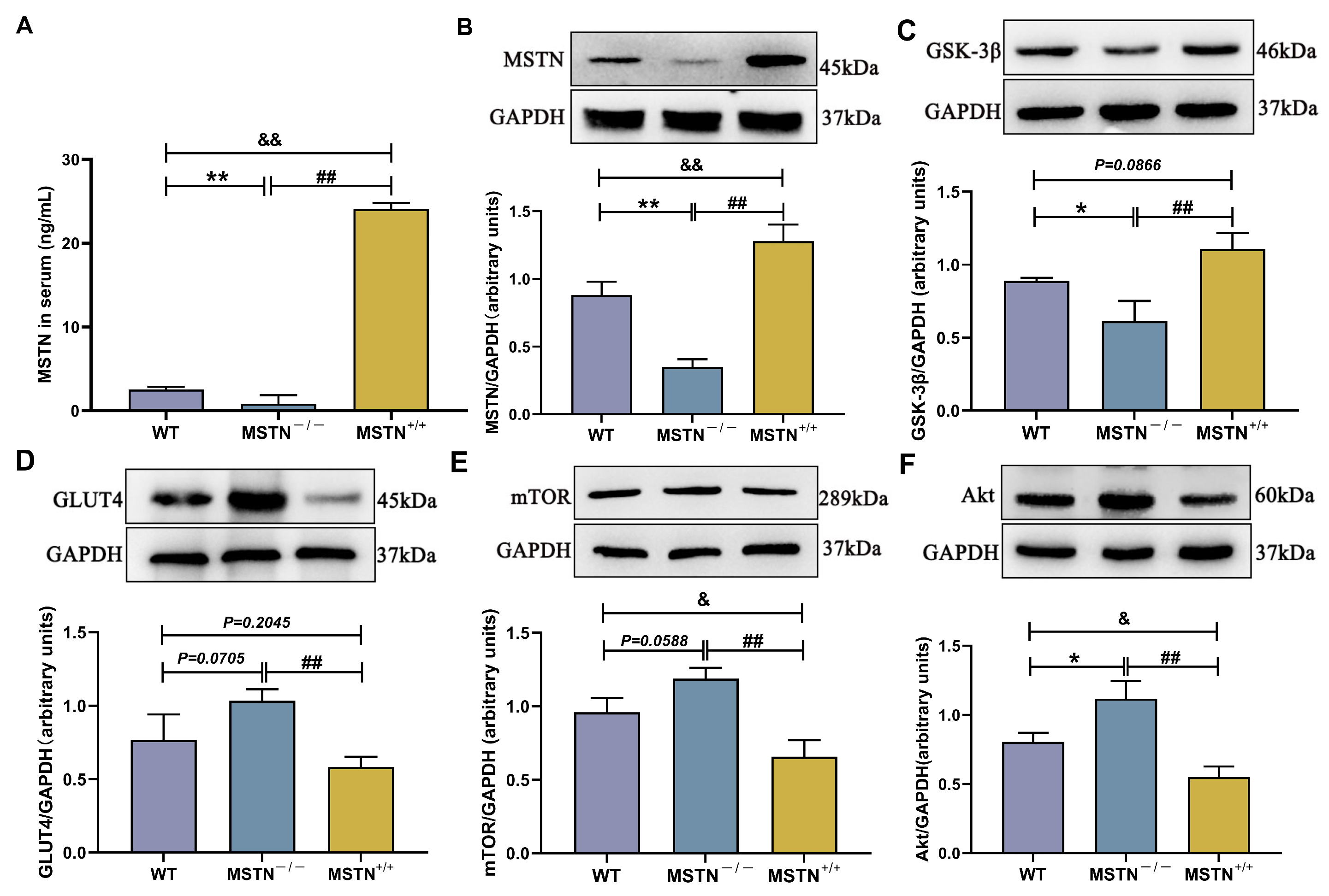
3.9. Protein Expression of MSTN−/− and MSTN+/+ Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, J.; Couper, J.J.; Mpundu-Kaambwa, C.; Giles, L.C.; Gent, R.; Coppin, B.; Peña, A.S. An extra 1,000 steps per day relates to improved cardiovascular health in children with type 1 diabetes. Diabetes Care 2016, 39, e108–e109. [Google Scholar] [CrossRef]
- de Almeida Júnior, R.F.; de Souza, K.S.; Galdino, O.A.; da Silva Junior, A.A.; Arrais, R.F.; Machado, P.R.; Farias, K.J.; de Rezende, A.A. Chloroquine as a promising adjuvant therapy for type 1 diabetes mellitus. Sci. Rep. 2020, 1, 12098. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.P.; Riddell, M.C.; Hawke, T.J. Effects of type 1 diabetes mellitus on skeletal muscle: Clinical observations and physiological mechanisms. Pediatr Diabetes 2011, 12, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human skeletal muscle disuse atrophy: Effects on muscle protein synthesis, breakdown, and insulin resistance-a qualitative review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.K.; Rebalka, I.A.; D’Souza, D.M.; Hawke, T.J. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World J. Diabetes 2015, 17, 1323–1336. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscle as a secretory organ. Compr. Physiol. 2013, 3, 1337–1362. [Google Scholar] [CrossRef]
- Powers, S.K.; Lynch, G.S.; Murphy, K.T.; Reid, M.B.; Zijdewind, I. Disease-induced skeletal muscle atrophy and fatigue. Med. Sci. Sports Exerc. 2016, 48, 2307–2319. [Google Scholar] [CrossRef]
- Duarte-Clíments, G.; Sánchez-Gómez, M.B.; Palenzuela-Luis, N.; González-Abreu, J.; Guzmán-Fernández, C.J.; Ramos-Santana, S.; Gómez-Salgado, J.; Rodríguez-Gómez, J.Á.; Romero-Martín, M. Relationship between the self-concept and physical activity towards the prevention of chronic illnesses. Medicine 2020, 99, e20884. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Whittemore, R.; Grey, M. Physical activity in adults with type 1 diabetes. Diabetes Educ. 2016, 42, 108–115. [Google Scholar] [CrossRef]
- Brennan, M.C.; Brown, J.A.; Ntoumanis, N.; Leslie, G.D. Barriers and facilitators of physical activity participation in adults living with type 1 diabetes: A systematic scoping review. Appl. Physiol. Nutr. Metab. 2021, 46, 95–107. [Google Scholar] [CrossRef]
- Gejl, M.; Gjedde, A.; Brock, B.; Møller, A.; van Duinkerken, E.; Haahr, H.L.; Hansen, C.T.; Chu, P.L.; Stender-Petersen, K.L.; Rungby, J. Effects of hypoglycaemia on working memory and regional cerebral blood flow in type 1 diabetes: A randomised, crossover trial. Diabetologia 2018, 61, 551–561. [Google Scholar] [CrossRef] [PubMed]
- McGuire, B.; Dadah, H.; Oliver, D. The effects of acute hyperglycaemia on sports and exercise performance in type 1 diabetes: A systematic review and meta-analysis. J. Sci. Med. Sport. 2024, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Poolman, R.W.; Agoritsas, T.; Siemieniuk, R.A.; Harris, I.A.; Schipper, I.B.; Mollon, B.; Smith, M.; Albin, A.; Nador, S.; Sasges, W.; et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: A clinical practice guideline. BMJ 2017, 356, j576. [Google Scholar] [CrossRef]
- Xia, P.; Shi, Y.; Wang, X.; Li, X. Advances in the application of low-intensity pulsed ultrasound to mesenchymal stem cells. Stem Cell Res. Ther. 2022, 13, 214. [Google Scholar] [CrossRef]
- Padilla, F.; Puts, R.; Vico, L.; Raum, K. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics 2014, 54, 1125–1145. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Hsu, K.Y.; Kuo, C.H.; Lee, S.D.; Chen, S.C.; Chen, W.J.; Ueng, S.W. Using low-intensity pulsed ultrasound to improve muscle healing after laceration injury: An in vitro and in vivo study. Ultrasound Med. Biol. 2010, 36, 743–751. [Google Scholar] [CrossRef]
- Tang, L.; An, S.; Zhang, Z.; Fan, X.; Guo, J.; Sun, L.; Ta, D. MSTN is a key mediator for low-intensity pulsed ultrasound preventing bone loss in hindlimb-suspended rats. Bone 2021, 143, 115610. [Google Scholar] [CrossRef]
- Tang, L.; Li, N.; Jian, W.; Kang, Y.; Yin, B.; Sun, S.; Guo, J.; Sun, L.; Ta, D. Low-intensity pulsed ultrasound prevents muscle atrophy induced by type 1 diabetes in rats. Skelet. Muscle 2017, 7, 29. [Google Scholar] [CrossRef]
- Sala, D.; Zorzano, A. Differential control of muscle mass in type 1 and type 2 diabetes mellitus. Cell Mol. Life Sci. 2015, 72, 3803–3817. [Google Scholar] [CrossRef]
- Li, C.; Gao, Q.; Jiang, H.; Liu, C.; Du, Y.; Li, L. Changes of macrophage and CD4+ T cell in infammatory response in type 1 diabetic mice. Sci. Rep. 2022, 12, 14929. [Google Scholar]
- Kakleas, K.; Soldatou, A.; Karachaliou, F.; Karavanaki, K. Associated autoimmune diseases in children and adolescents with type 1 diabetes mellitus (T1DM). Autoimmun. Rev. 2015, 14, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Sun, J.S.; Liao, S.Y.; Tai, P.A.; Li, T.C.; Chen, M.H. Low-intensity pulsed ultrasound stimulates matrix metabolism of human annulus fibrosus cells mediated by transforming growth factor β1 and extracellular signal-regulated kinase pathway. Connect. Tissue Res. 2015, 56, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.J.; Cirone, J.M.; Morris, T.R.; Nuss, C.A.; Huegel, J.; Waldorff, E.I.; Zhang, N.; Ryaby, J.T.; Soslowsky, L.J. Pulsed electromagnetic field therapy improves tendon-to-bone healing in a rat rotator cuff repair model. J. Orthop. Res. 2017, 35, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, S.C.; Piston, D.W. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E1043–E1055. [Google Scholar] [CrossRef]
- Liu, H.Y.; Cao, S.Y.; Hong, T.; Han, J.; Liu, Z.; Cao, W. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM). J. Biol. Chem. 2009, 284, 27090–27100. [Google Scholar] [CrossRef]
- Hulmi, J.J.; Silvennoinen, M.; Lehti, M.; Kivelä, R.; Kainulainen, H. Altered REDD1, myostatin, and Akt/mTOR/FoxO/MAPK signaling in streptozotocin-induced diabetic muscle atrophy. Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E307–E315. [Google Scholar] [CrossRef]
- Sartori, R.; Gregorevic, P.; Sandri, M. TGFβ and BMP signaling in skeletal muscle: Potential significance for muscle-related disease. Trends Endocrinol. Metab. 2014, 9, 464–471. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Davies, M.V.; Koniaris, L.G.; Haynes, P.; Esquela, A.F.; Tomkinson, K.N.; McPherron, A.C.; Wolfman, N.M.; Lee, S.J. Induction of cachexia in mice by systemically administered myostatin. Science 2002, 296, 1486–1488. [Google Scholar] [CrossRef]
- Cleasby, M.E.; Jarmin, S.; Eilers, W.; Elashry, M.; Andersen, D.K.; Dickson, G.; Foster, K. Local overexpression of the myostatin propeptide increases glucose transporter expression and enhances skeletal muscle glucose disposal. Am. J. Physiol.-Endocrinol. Metab. 2014, 306, E814–E823. [Google Scholar] [CrossRef]
- Chang, L.; Graham, P.H.; Hao, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. Emerging roles of radioresistance in prostate cancer metastasis and radiation therapy. Cancer Metastasis Rev. 2014, 33, 469–496. [Google Scholar] [CrossRef]
- Ni, J.; Cozzi, P.; Hao, J.; Beretov, J.; Chang, L.; Duan, W.; Shigdar, S.; Delprado, W.; Graham, P.; Bucci, J.; et al. Epithelial cell adhesion molecule (EpCAM) is associated with prostate cancer metastasis and chemo/radioresistance via the PI3K/Akt/mTOR signaling pathway. Int. J. Biochem. Cell Biol. 2013, 45, 2736–2748. [Google Scholar] [CrossRef]
- Chang, L.; Graham, P.H.; Hao, J.; Ni, J.; Bucci, J.; Cozzi, P.J.; Kearsley, J.H.; Li, Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013, 4, e875. [Google Scholar] [CrossRef]
- Kotake, H.; Ogura, Y.; Yamada, S.; Inoue, K.; Watanabe, S.; Ichikawa, D.; Sugaya, T.; Ohata, K.; Natsuki, Y.; Hoshino, S.; et al. Mechanism for exercise-mediated prevention against muscle wasting on extensor digitorum longus muscle in Spontaneously Diabetic Torii fatty rats. J. Physiol. Sci. 2023, 73, 5. [Google Scholar] [CrossRef]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef]
- Lee, Y.S.; Huynh, T.V.; Lee, S.J. Paracrine and endocrine modes of myostatin action. J. Appl. Physiol. 2016, 120, 592–598. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Hu, J.; Quan, H.; Lee, S.K.; Korivi, M.; Wang, L.; Li, T.; Li, W. Aerobic exercise attenuates high-fat diet-induced glycometabolism impairments in skeletal muscle of rat: Role of EGR-1/PTP1B signaling pathway. Nutr. Metab. 2024, 21, 113. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020, 472, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
| Sample | Reads Count | Bases Count | N Bases Count | Average Length | Q10 Ratio | Q20 Ratio | Q30 Ratio | GC Ratio |
|---|---|---|---|---|---|---|---|---|
| NC | 43,210,200 | 6,367,262,475 | 351,293 | 147.36 | 99.99% | 99.31% | 96.75% | 48.3% |
| T1D | 46,391,874 | 6,842,194,008 | 367,889 | 147.49 | 99.99% | 99.31% | 96.80% | 49.91% |
| DL | 45,324,868 | 6,683,378,913 | 334,016 | 147.46 | 100% | 99.35% | 96.95% | 49.87% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Yu, Y.; Cao, M.; Pang, F.; Sun, L.; Wang, C.; Fan, X.; Tang, L. Study on the Mechanism of Low-Intensity Pulsed Ultrasound in Ameliorating Glucose Metabolism Through Attenuation of Skeletal Muscle Atrophy in Mice with Type 1 Diabetes. Biology 2025, 14, 1343. https://doi.org/10.3390/biology14101343
Ma Z, Yu Y, Cao M, Pang F, Sun L, Wang C, Fan X, Tang L. Study on the Mechanism of Low-Intensity Pulsed Ultrasound in Ameliorating Glucose Metabolism Through Attenuation of Skeletal Muscle Atrophy in Mice with Type 1 Diabetes. Biology. 2025; 14(10):1343. https://doi.org/10.3390/biology14101343
Chicago/Turabian StyleMa, Zhanke, Yanan Yu, Mengshu Cao, Fang Pang, Lijun Sun, Chenghui Wang, Xiushan Fan, and Liang Tang. 2025. "Study on the Mechanism of Low-Intensity Pulsed Ultrasound in Ameliorating Glucose Metabolism Through Attenuation of Skeletal Muscle Atrophy in Mice with Type 1 Diabetes" Biology 14, no. 10: 1343. https://doi.org/10.3390/biology14101343
APA StyleMa, Z., Yu, Y., Cao, M., Pang, F., Sun, L., Wang, C., Fan, X., & Tang, L. (2025). Study on the Mechanism of Low-Intensity Pulsed Ultrasound in Ameliorating Glucose Metabolism Through Attenuation of Skeletal Muscle Atrophy in Mice with Type 1 Diabetes. Biology, 14(10), 1343. https://doi.org/10.3390/biology14101343






