Biochemical, Histological, and Multi-Omics Analyses Reveal the Molecular and Metabolic Mechanisms of Cold Stress Response in the Chinese Soft-Shelled Turtle (Pelodiscus sinensis)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
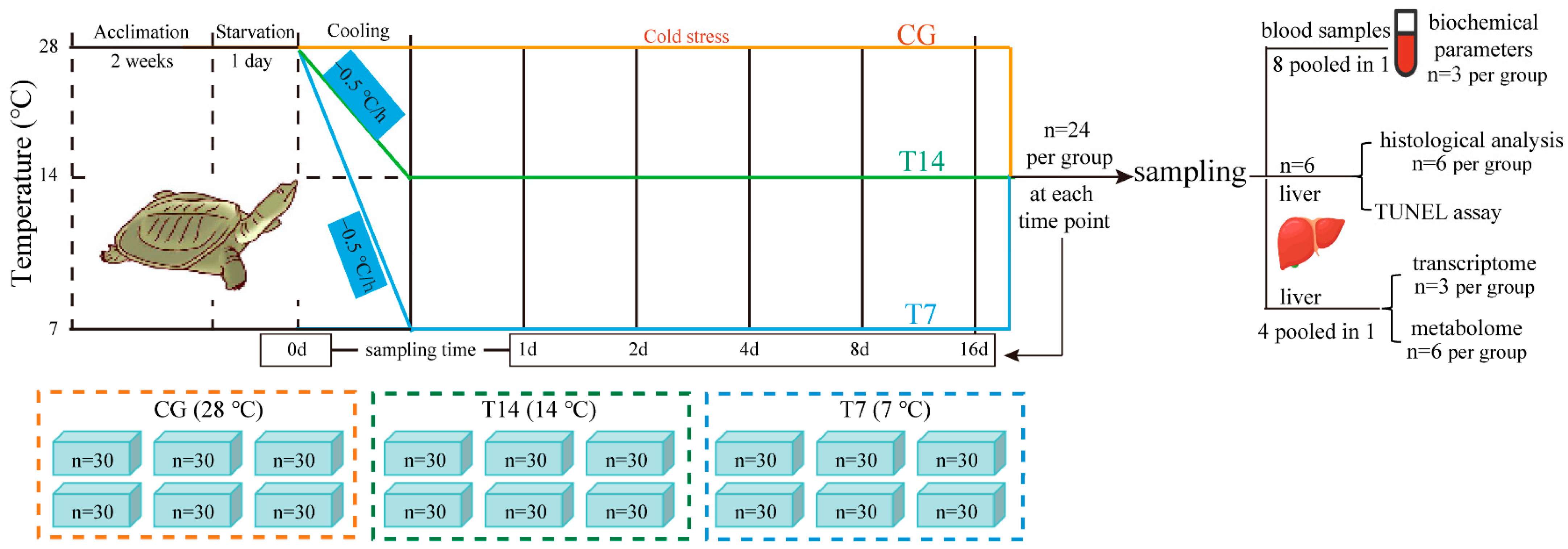
2.1. Experimental Animals and Design
2.2. Sample Collection
2.3. Plasma Parameters
2.4. Histological Analysis and TUNEL Assay
2.5. Transcriptomic Analysis of Liver Samples
2.6. Metabolomic Analysis of Liver Samples
2.7. Joint Analysis of Transcriptome and Metabolome
2.8. Validation of Transcriptomic Results by qRT-PCR
2.9. Statistical Analysis
3. Results
3.1. Plasma Biochemical Parameters During Cold Stress
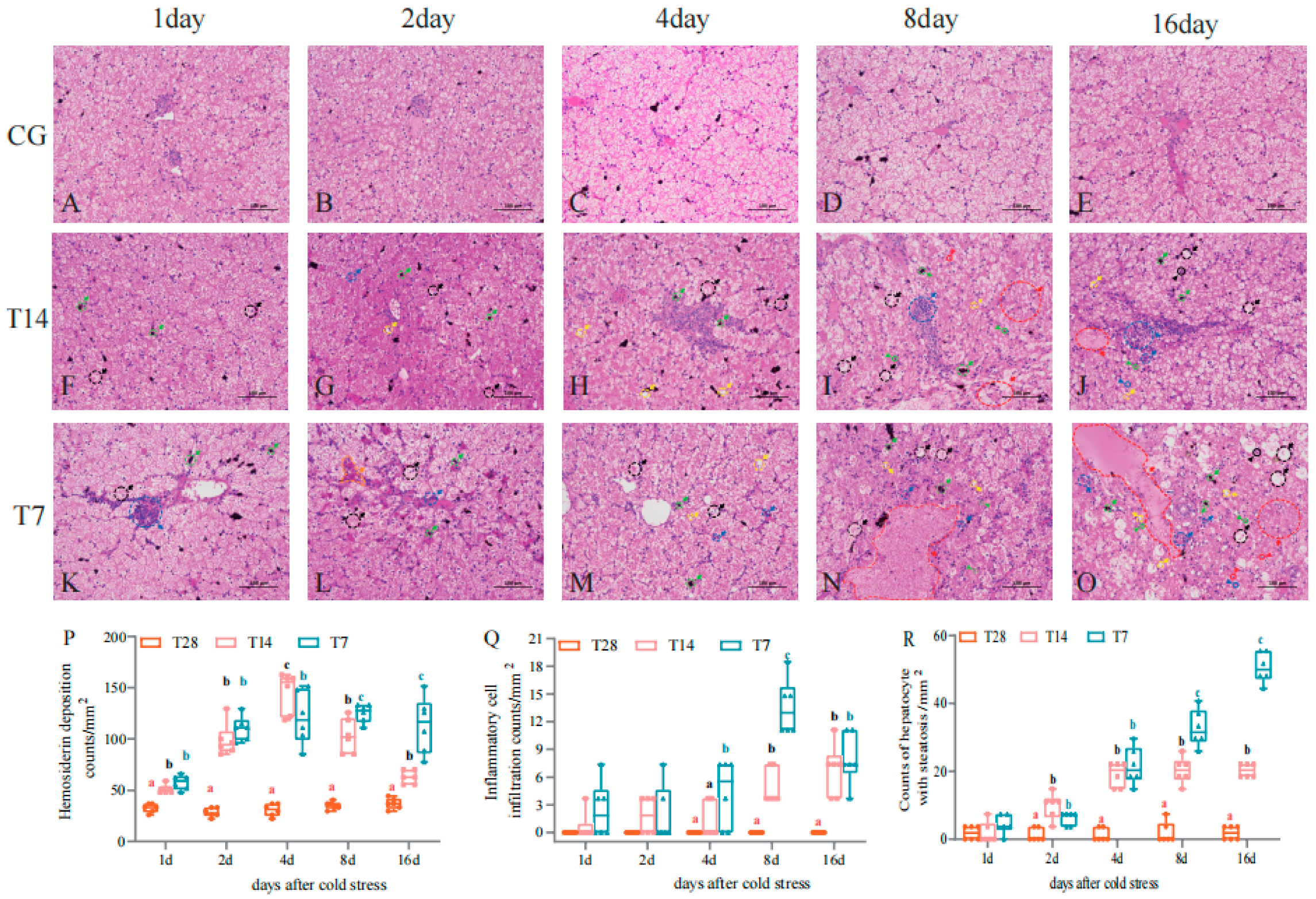
3.2. Cold Stress Induced the Damage of Hepatic Morphology
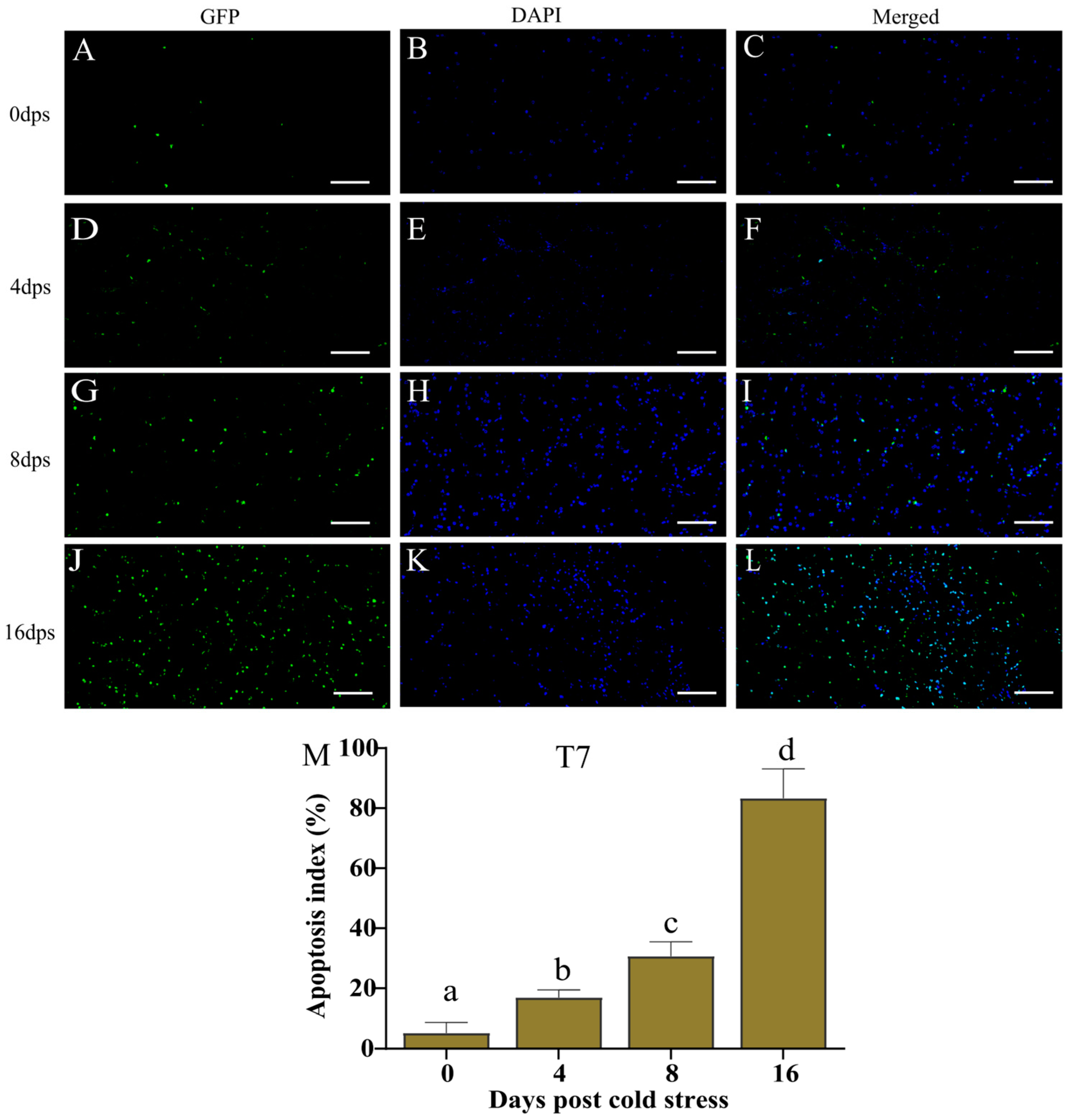
3.3. Cold Stress Intensified Cell Apoptosis
3.4. Effect of Cold Stress on Hepatic Transcriptome
3.5. Effect of Cold Stress on Hepatic Metabolome
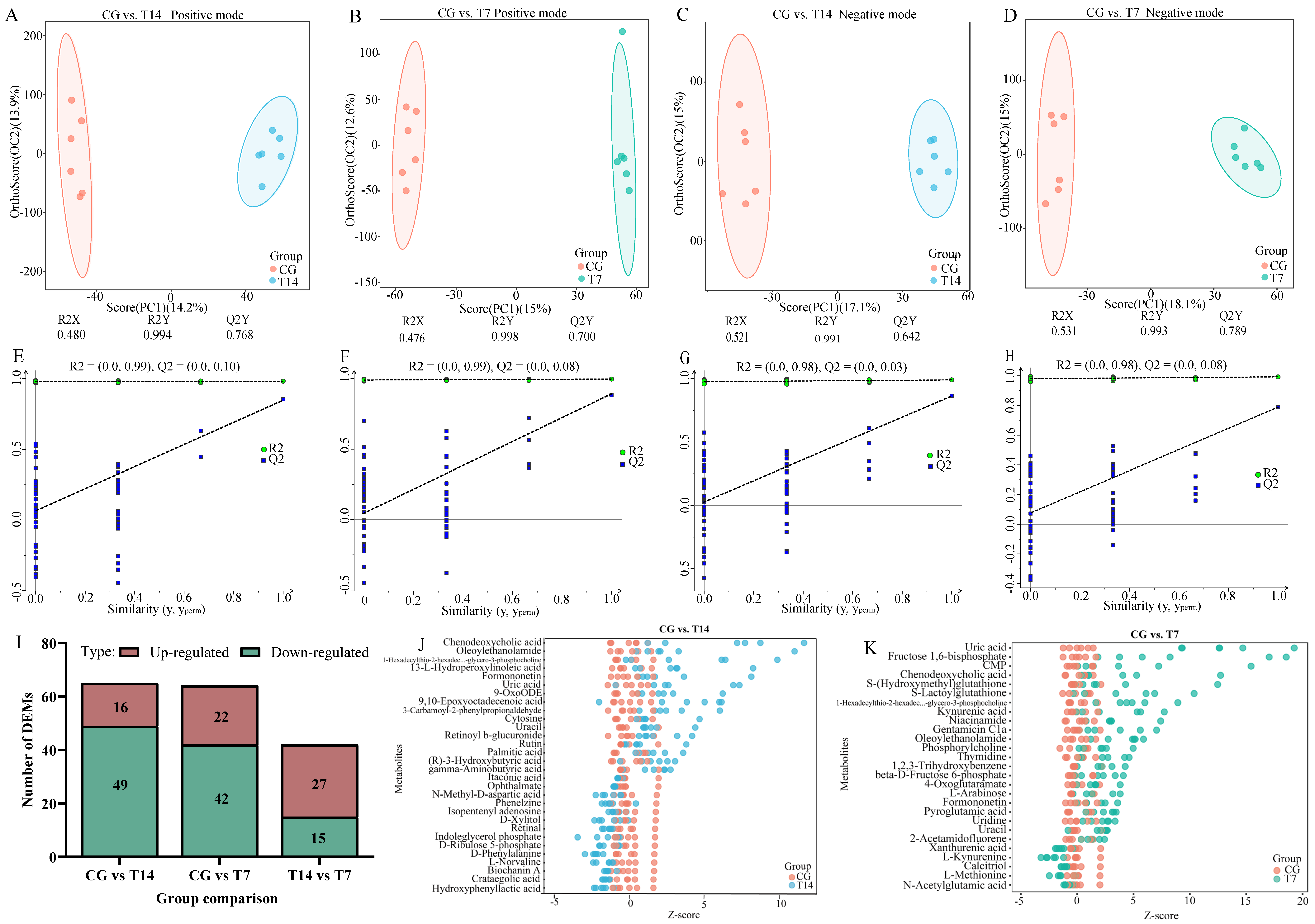
3.5.1. Assessment of Metabolic Profiles
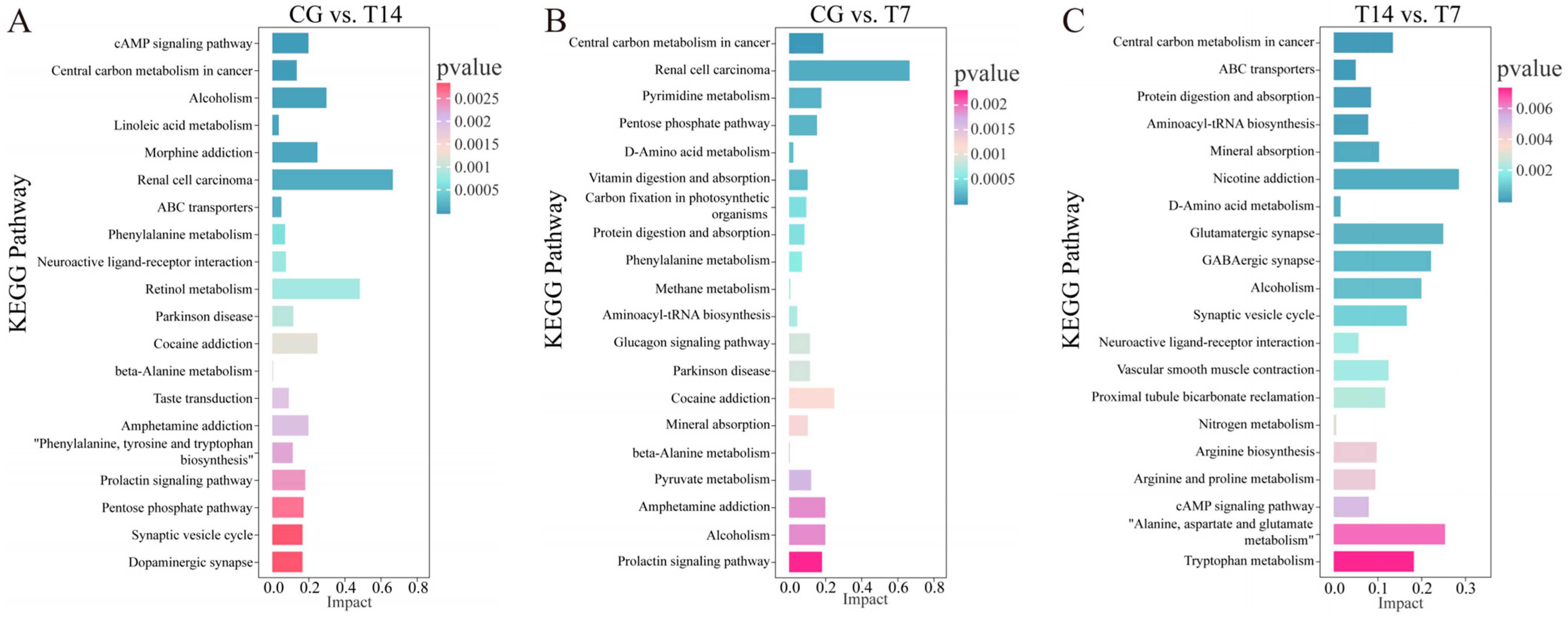
3.5.2. Identification and Enrichment Analysis of DEMs
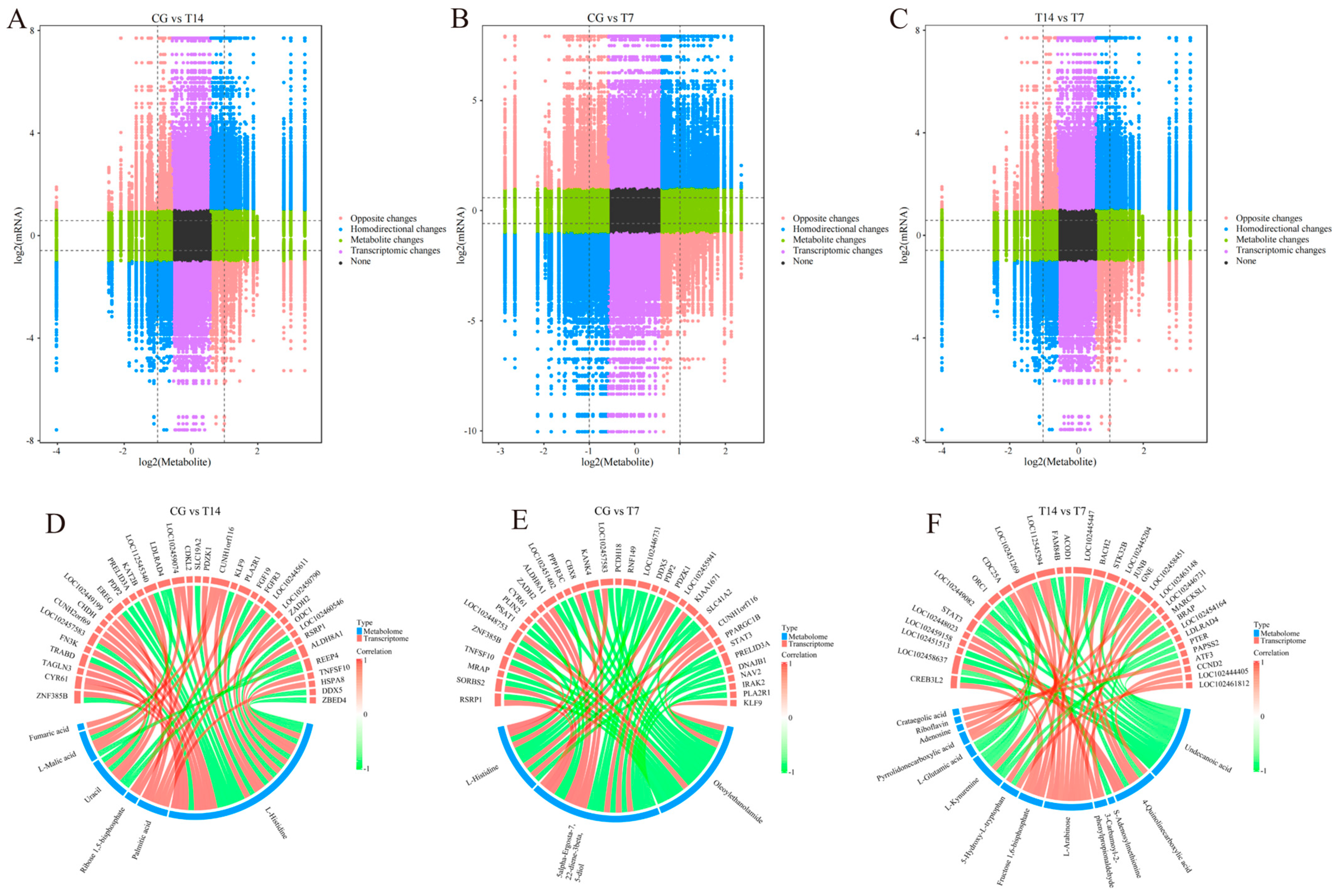
3.6. Joint Analysis of DEGs and DEMs
4. Discussion
4.1. Energy Metabolism Responding to Cold Stress
4.2. Tissue Damage and Cellular Apoptosis of the Liver Caused by Cold Stress
4.3. Key Signaling Pathways Responding to Cold Stress
4.3.1. Pyrimidine Metabolism
4.3.2. Amino Acid Metabolism and Pyruvate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, W.A. Climate change and extreme weather: A review focusing on the continental United States. J. Air Waste Manag. Assoc. 2021, 71, 1186–1209. [Google Scholar] [CrossRef]
- Song, F.F.; Zhang, G.J.; Ramanathan, V.; Leung, L.R. Trends in surface equivalent potential temperature: A more comprehensive metric for global warming and weather extremes. Proc. Natl. Acad. Sci. USA 2022, 119, e2117832119. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Zhang, X.; Francis, J.; Jung, T.; Kwok, R.; Overland, J.; Ballinger, T.J.; Bhatt, U.S.; Chen, H.W.; Coumou, D.; et al. Divergent consensuses on Arctic amplification influence on midlatitude severe winter weather. Nat. Clim. Chang. 2020, 10, 20–29. [Google Scholar] [CrossRef]
- Overland, J.E.; Ballinger, T.J.; Cohen, J.; Francis, J.A.; Hanna, E.; Jaiser, R.; Kim, B.M.; Kim, S.J.; Ukita, J.; Vihma, T.; et al. How do intermittency and simultaneous processes obfuscate the Arctic influence on midlatitude winter extreme weather events? Environ. Res. Lett. 2021, 16, 043002. [Google Scholar] [CrossRef]
- Cohen, J.; Pfeiffer, K.; Francis, J.A. Warm Arctic episodes linked with increased frequency of extreme winter weather in the United States. Nat. Commun. 2018, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Yuan, Y.; Ding, Y.H.; Li, K.X.; Fang, X.H.; Zhao, Y.H.; Sun, Y.; Zhu, J.; Ke, Z.J.; Wang, J.; et al. The 2020/21 extremely cold winter in China influenced by the synergistic effect of La Niña and warm Arctic. Adv. Atmos. Sci. 2022, 39, 546–552. [Google Scholar] [CrossRef]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J.C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredía, S.; de Verdal, H.; Gozlan, R.E. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- L’Honoré, T.; Farcy, E.; Blondeau-Bidet, E.; Lorin-Nebel, C. Inter-individual variability in freshwater tolerance is related to transcript level differences in gill and posterior kidney of European sea bass. Gene 2020, 741, 144547. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Zhong, D.B.; Li, S.; Zhang, Z.H.; Mo, H.L.; Wang, L.X. Acute temperature stresses trigger liver transcriptome and microbial community remodeling in largemouth bass (Micropterus salmoides). Aquaculture 2023, 573, 739573. [Google Scholar] [CrossRef]
- Huang, D.Y.; Zhu, J.; Xu, G.C.; Zhang, L.; Chen, X.R.; Wang, Y.L.; Ren, M.C.; Liang, H.L. Sodium chloride alleviates oxidative stress and physiological responses induced by extreme winter cold in genetically improved farmed tilapia (GIFT; Oreochromis niloticus). Sci. Total Environ. 2023, 15, 166800. [Google Scholar] [CrossRef]
- Griffin, L.P.; Griffin, C.R.; Finn, J.T.; Prescott, R.L.; Faherty, M.; Still, B.M.; Danylchuk, A.J. Warming seas increase cold-stunning events for Kemp’s ridley sea turtles in the northwest Atlantic. PLoS ONE 2019, 14, e0211503. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Nie, M.; Song, H.; Xu, D.; You, F. Physiological responses to cold and starvation stresses in the liver of yellow drum (Nibea albiflora) revealed by LC-MS metabolomics. Sci. Total Environ. 2020, 715, 136940. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, M.J.; Xu, G.P.; Yu, H.; Jia, C.F.; Zhu, F.; Meng, Q.; Xu, D.F.; Du, S.R.; Zhang, D.C.; et al. Comprehensive analysis of histophysiology, transcriptome and metabolome tolerance mechanisms in black porgy (Acanthopagrus schlegelii) under low temperature stress. Sci. Total Environ. 2024, 927, 172318. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Shi, H.X.; Zhang, K.; Wei, Q.Z.; Niu, J.P.; Wang, J.L.; Hammond, S.K.; Liu, S. Cold stress provokes lung injury in rats co-exposed to fine particulate matter and lipopolysaccharide. Ecotoxicol. Environ. Saf. 2019, 168, 9–16. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Thiele, R.; Slater, M.J. Effects of extreme ambient temperature in European seabass, Dicentrarchus labrax acclimated at different salinities: Growth performance, metabolic and molecular stress responses. Sci. Total Environ. 2020, 735, 139371. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhao, J.; Wen, H.S.; Li, Y.; Li, J.F.; Li, L.M.; Tao, Y.X. The impact of acute thermal stress on the metabolome of the black rockfish (Sebastes schlegelii). PLoS ONE 2019, 14, e0217133. [Google Scholar] [CrossRef]
- Sha, J.; Feng, X.; Chen, Y.; Zhang, H.; Li, B.; Hu, X.; Fan, H. Dexmedetomidine improves acute stress-induced liver injury in rats by regulating MKP-1, inhibiting NF-κB pathway and cell apoptosis. J. Cell. Physiol. 2019, 234, 14068–14078. [Google Scholar] [CrossRef]
- Joung, J.Y.; Cho, J.H.; Kim, Y.H.; Choi, S.H.; Son, C.G. A literature review for the mechanisms of stress-induced liver injury. Brain Behav. 2019, 9, e01235. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.S.; Yin, D.Q.; Li, Y.; Zhang, Y.R.; Cheng, J.H.; Zhang, K.; Ji, J.; Wang, T.; Jia, Y.Y.; et al. Integrated application of multiomics strategies provides insights into the environmental hypoxia response in Pelteobagrus vachelli muscle. Mol. Cell. Proteom. 2022, 21, 100196. [Google Scholar] [CrossRef] [PubMed]
- Auge, A.C.; Blouin-Demers, G.; Murray, D.L. Effects of temperature heterogeneity on freshwater turtle habitat selection at their northern range limit. J. Therm. Biol. 2023, 118, 103725. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, H.Q. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2024; p. 24. [Google Scholar]
- Ai, X.Q.; Lin, R.; Ali, Z.S.; Zhu, Q.J.; Ding, L.; Shi, H.T.; Hong, M.L. Seasonal changes in hepatic lipid metabolism and apoptosis in Chinese soft-shelled turtle (Pelodiscus sinensis). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 280, 109883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, B.; Yuan, L.; Niu, C. Acute cold stress improved the transcription of pro-inflammatory cytokines of Chinese soft-shelled turtle against Aeromonas hydrophila. Dev. Comp. Immunol. 2015, 49, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017, 18, 529. [Google Scholar] [CrossRef]
- Ji, L.; Chen, C.; Zhu, J.; Hong, X.; Liu, X.; Wei, C.; Zhu, X.; Li, W. Integrated time-series biochemical, transcriptomic, and metabolomic analyses reveal key metabolites and signaling pathways in the liver of the Chinese soft-shelled turtle (Pelodiscus sinensis) against Aeromonas hydrophila infection. Front. Immunol. 2024, 15, 1376860. [Google Scholar] [CrossRef]
- Houghton, S.G.; Cockerill, F.R. Real-time PCR: Overview and applications. Surgery 2006, 139, 1–5. [Google Scholar] [CrossRef]
- Xv, Z.C.; Chen, S.X.; Song, G.L.; Hu, H.; Lin, S.M.; Long, Y. Biochemical, histological and transcriptomic analyses for the immunological organs provide insights into heat stress-induced disease susceptibility in Largemouth Bass. Sci. Total Environ. 2024, 912, 168758. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Yu, Z.X.; Xu, Y.; Zhang, Y.B.; Mu, C.M.; Liu, P.; Li, J. Integrated transcriptomic and metabolomic responses in the hepatopancreas of kuruma shrimp (Marsupenaeus japonicus) under cold stress. Ecotoxicol. Environ. Saf. 2020, 206, 111360. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Peck, M.A. Climate change effects on fishes and fisheries: Towards a cause-and-effect understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef]
- Javed, M.; Usmani, N. Stress response of biomolecules (carbohydrate, protein and lipid profiles) in fish Channa punctatus inhabiting river polluted by Thermal Power Plant effluent. Saudi J. Biol. Sci. 2015, 22, 237–242. [Google Scholar] [CrossRef]
- Pinto, D.; Pellegrin, L.; Nitz, L.F.; da Costa, S.T.; Monserrat, J.M.; Garcia, L. Haematological and oxidative stress responses in Piaractus mesopotamicus under temperature variations in water. Aquac. Res. 2019, 50, 3017–3027. [Google Scholar] [CrossRef]
- Chang, C.H.; Zhou, X.W.; Wang, Y.C.; Lee, T.H. Differential effects of hypothermal stress on lactate metabolism in fresh water- and seawater-acclimated milkfish, Chanos chanos. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020, 248, 110744. [Google Scholar] [CrossRef] [PubMed]
- Umminger, B.L. Relation of whole blood sugar concentrations in vertebrates to standard metabolic rate. Comp. Biochem. Physiol. A Physiol. 1977, 56, 457–460. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z. Physiological responses to cold stress in the gills of discus fish (Symphysodon aequifasciatus) revealed by conventional biochemical assays and GC-TOF-MS metabolomics. Sci. Total Environ. 2018, 640–641, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Utaal, M.S. Serum lipids and lipoproteins: A brief review of the composition, transport and physiological functions. Int. J. Sci. Rep. 2019, 5, 309. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, H.P.; He, F.F.; Li, Y.J.; He, R.; Zhang, K.; Fan, J.H.; Yang, Z.J.; Yang, W.B. Novel paraffin/ethylene propylene diene monomer phase change latex with excellent stability and low viscosity. Sol. Energy Mater. Sol. Cells 2019, 200, 109957. [Google Scholar] [CrossRef]
- Wang, X.N.; Meng, Z.; Ma, A.J.; Liu, Z.F. Genotype × ammonia interaction effects on plasma physiological indexes in turbot (Scophthalmus maximus) cultured under acute ammonia stress for different periods of times. Ecotoxicol. Environ. Saf. 2022, 242, 113863. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yi, M.M.; Lu, M.X. Review on the fish health assessment. Acta Hydrobiol. Sin. 2019, 43, 226–232. [Google Scholar]
- Sachi, I.T.C.; Bonomo, M.M.; Sakuragui, M.M.; Modena, P.Z.; Paulino, M.G.; Carlos, R.M.; Fernandes, J.B.; Fernandes, M.N. Biochemical and morphological biomarker responses in the gills of a Neotropical fish exposed to a new flavonoid metal-insecticide. Ecotoxicol. Environ. Saf. 2021, 208, 111459. [Google Scholar] [CrossRef]
- Batts, K.P. Iron overload syndromes and the liver. Mod. Pathol. 2007, 20, S31–S39. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, N.C.; Xue, M.Y.; Xiao, Z.D.; Zhang, M.J.; Meng, Y.; Fan, Y.D.; Hu, X.W.; Qiu, J.Q.; Zhang, Q.H.; et al. Pathological characteristics of Chinese soft-shelled turtle (Pelodiscus sinensis) with white abdominal disease. Aquac. Rep. 2023, 31, 101670. [Google Scholar] [CrossRef]
- Liu, A.; Pirozzi, I.; Codabaccus, B.M.; Stephens, F.; Francis, D.S.; Sammut, J.; Booth, M.A. Effects of dietary choline on liver lipid composition, liver histology and plasma biochemistry of juvenile yellowtail kingfish (Seriola lalandi). Br. J. Nutr. 2021, 125, 1344–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Xing, G.D.; Qian, Y.; Sun, X.F.; Zhong, J.F.; Chen, K.L. Dihydromyricetin attenuates heat stress-induced apoptosis in dairy cow mammary epithelial cells through suppressing mitochondrial dysfunction. Ecotoxicol. Environ. Saf. 2021, 214, 112078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Li, L.L.; Li, C.J.; Liu, E.G.; Zhu, H.; Ling, Q.F. Heat stress-induced endoplasmic reticulum stress promotes liver apoptosis in largemouth bass (Micropterus salmoides). Aquaculture 2022, 546, 737401. [Google Scholar] [CrossRef]
- Hu, W.T.; Guo, W.L.; Meng, A.Y.; Sun, Y.; Wang, S.F.; Xie, Z.Y.; Zhou, Y.C.; He, C. A metabolomic investigation into the effects of temperature on Streptococcus agalactiae from Nile tilapia (Oreochromis niloticus) based on UPLC-MS/MS. Vet. Microbiol. 2017, 210, 174–182. [Google Scholar] [CrossRef]
- Kant, M.; Quintana, V.; Coskun, E.; Jaruga, P.; Lloyd, R.S.; Sweasy, J.B.; Dizdaroglu, M. Polymorphic variant Asp239Tyr of human DNA glycosylase NTHL1 is inactive for removal of a variety of oxidatively-induced DNA base lesions from genomic DNA. DNA Repair 2022, 117, 103372. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Lei, Z.; Ruilin, M.; Jing, W.; Leqiang, D. High temperature stress induced oxidative stress, gut inflammation and disordered metabolome and microbiome in tsinling lenok trout. Ecotoxicol. Environ. Saf. 2023, 266, 115607. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, N.H.; Harris, C.R.; Harris, R.A. Regulation of pyruvate metabolism in metabolic-related diseases. Rev. Endocr. Metab. Disord. 2014, 15, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Abusalamah, H.; Reel, J.M.; Lupfer, C.R. Pyruvate affects inflammatory responses of macrophages during influenza A virus infection. Virus Res. 2020, 286, 198088. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Cardaci, S.; Jerby, L.; MacKenzie, E.D.; Sciacovelli, M.; Johnson, T.I.; Gaude, E.; King, A.; Leach, J.D.; Edrada-Ebel, R.; et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat. Commun. 2015, 6, 6001. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.H.; Patrick, P.H.; Rytwinski, T.; Taylor, J.J.; Willmore, W.G.; Reesor, B.; Cooke, S.J. An updated review of cold shock and cold stress in fish. J. Fish Biol. 2022, 100, 1102–1137. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; Zhang, R.; Wang, X.W.; Zhu, H.; Tian, Z.H. Transcriptome analysis reveals molecular mechanisms responsive to acute cold stress in the tropical stenothermal fish tiger barb (Puntius tetrazona). BMC Genom. 2020, 21, 737. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.K.; Zhu, W.B.; Liang, Z.Y.; Feng, B.B.; Xie, X.D.; Li, Y.L.; Liu, Y.; Shi, X.L.; Fu, J.J.; Miao, L.H.; et al. High-temperature stress response: Insights into the molecular regulation of American shad (Alosa sapidissima) using a multi-omics approach. Sci. Total Environ. 2024, 916, 170329. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Shi, Q.; Chen, C.; Liu, X.; Zhu, J.; Hong, X.; Wei, C.; Zhu, X.; Li, W. Biochemical, Histological, and Multi-Omics Analyses Reveal the Molecular and Metabolic Mechanisms of Cold Stress Response in the Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Biology 2025, 14, 55. https://doi.org/10.3390/biology14010055
Ji L, Shi Q, Chen C, Liu X, Zhu J, Hong X, Wei C, Zhu X, Li W. Biochemical, Histological, and Multi-Omics Analyses Reveal the Molecular and Metabolic Mechanisms of Cold Stress Response in the Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Biology. 2025; 14(1):55. https://doi.org/10.3390/biology14010055
Chicago/Turabian StyleJi, Liqin, Qing Shi, Chen Chen, Xiaoli Liu, Junxian Zhu, Xiaoyou Hong, Chengqing Wei, Xinping Zhu, and Wei Li. 2025. "Biochemical, Histological, and Multi-Omics Analyses Reveal the Molecular and Metabolic Mechanisms of Cold Stress Response in the Chinese Soft-Shelled Turtle (Pelodiscus sinensis)" Biology 14, no. 1: 55. https://doi.org/10.3390/biology14010055
APA StyleJi, L., Shi, Q., Chen, C., Liu, X., Zhu, J., Hong, X., Wei, C., Zhu, X., & Li, W. (2025). Biochemical, Histological, and Multi-Omics Analyses Reveal the Molecular and Metabolic Mechanisms of Cold Stress Response in the Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Biology, 14(1), 55. https://doi.org/10.3390/biology14010055







