Simple Summary
The genus Siphonaria Sowerby, 1823 is a group of typical pulmonates living on intertidal rocks. The fluctuation in the intertidal environment gives rise to variations in the shells of the Siphonaria species, leading to significant uncertainty in species identification using traditional morphological classification methods. In this study, we analyzed 245 Siphonaria specimens collected from the Chinese coast based on both morphological and molecular evidence. Our results revealed that five Siphonaria species were identified, i.e., Siphonaria japonica Donovan, 1824; Siphonaria sirius Pilsbry, 1894; Siphonaria atra Quoy & Gaimard, 1833; and two new species (Siphonaria petasus sp. nov. and Siphonaria floslamellosa sp. nov.). Key features for identifying these species were also provided in this study.
Abstract
The genus Siphonaria is a group of limpet-shaped pulmonates living on intertidal rocks along the Chinese coast. Due to the fluctuating intertidal environment, the morphological characteristics of Siphonaria shells are reshaped, resulting in morphologically divergent evolution within species and inaccuracy of taxonomic identification without molecular evidence. Large-scale identifications on Siphonaria animals combining morphological and molecular evidence are considerably absent. In this study, we sampled 245 Siphonaria specimens along the coast of China (from Guangxi to Shandong) and conducted the identification based on morphological characteristics and DNA barcoding data including 16S ribosomal RNA, 12S ribosomal RNA, Cytochrome c oxidase subunit I, and Histone 3. The results indicated five Siphonaria species, i.e., Siphonaria japonica Donovan, 1824; Siphonaria sirius Pilsbry, 1894; Siphonaria atra Quoy & Gaimard, 1833; and two new species (Siphonaria petasus sp. nov. and Siphonaria floslamellosa sp. nov., Zang, Ma & Wang). We conducted a detailed comparison of these species and found many morphological differences, with the most distinctive differences being the radial ribs and siphonal ridge. We created a new identification key based on these features, providing new insights into the phylogeny and taxonomy of the genus Siphonaria. In addition, the divergence time indicated that species of the Siphonaria genus underwent rapid diversification during the late Pliocene to Pleistocene (3.46–1.53 Mya), with climate likely being an important driver of this diversification.
1. Introduction
The genus Siphonaria G. B. Sowerby I, 1823 is one of the oldest extant marine pulmonates, commonly known as “false limpets” [1]. These animals use a specialized mantle cavity as a pulmonary sac for breathing [2]. To date, 116 Siphonaria species have been described worldwide [3], but only 4 species are recorded in China, i.e., Siphonaria japonica Donovan, 1824; S. atra Quoy & Gaimard, 1833; S. sirius Pilsbry, 1894; and S. laciniosa Linnaeus, 1758, with the validity of S. laciniosa still in doubt [4].
Early monographs described Siphonaria primarily based on shell morphology [5]. However, Siphonaria inhabits intertidal rocky shores worldwide, except the polar regions [6]. This broad distribution and fluctuating intertidal environment make their morphology variable and large-scale systematic taxonomy studies are quite challenging [7]. Some studies focused on local diversity of Siphonaria in specific regions. For example, Jenkins analyzed the morphological features and taxonomic status of various Siphonaria species from Australia [8,9,10]. Chambers and McQuaid found that both larval developmental modes existed in two subgenera of Siphonaria, contradicting a previous hypothesis that species within each subgenus exhibit a single developmental mode [11,12,13,14,15]. With the development of scanning electron microscope (SEM) technology, anatomical studies of Siphonaria soft tissues have gained increasing attention. The microscopical features of the gonads, radula, and other parts have provided important clarification for the taxonomy and individual identification of Siphonaria species [4,16].
Previous studies on Siphonaria species have shown significant variation in shell morphology at both species and geographic levels [17,18,19,20,21]. The intertidal environment, with its complexity and diverse habitats, provides continuous driving force for the formation and maintenance of cryptic species. Moreover, the morphological characteristics of the radula can be influenced by biological developmental stages and predatory behavior, which may mislead the classification studies on taxonomy of Siphonaria species [22]. Therefore, species identification based solely on morphology may not be accurate and molecular evidence is a vital supplement. Teske et al. conducted a phylogenetic study of South African Siphonaria species using Cytochrome c oxidase subunit I (COI) and ATPSβ sequences, hypothesizing that the four Siphonaria species are actually four morphological forms of a single species [23]. Giribet et al. used COI and 16S ribosomal RNA (16S rRNA) to study the complex population structure of Siphonaria pectinata Linnaeus, 1758 [22]. Colgan et al. analyzed five Siphonaria species from southeastern Australia using Histone (h3) and ITS-2 sequences and found a high distributional differentiation of Siphonaria species in the region [24]. Dayrat et al. conducted the first study on the diversity of Siphonaria species in the Indo-West Pacific region, combining shell morphology and molecular data (12S ribosomal RNA (12S rRNA), COI, and 16S rRNA) and confirming 41 species in the genus [7]. As a result, integrative taxonomy, combining multiple lines of evidence, could play a more important role in the delimitation of Siphonaria species.
During 2011–2022, we collected Siphonaria specimens along the Chinese coast and conducted the species identification based on both morphological and molecular characteristics. Our aims were (a) to provide detailed morphological descriptions of the species of Siphonaria in Chinese waters; (b) to conduct a systematic taxonomic study of Chinese Siphonaria species using an integrative taxonomic approach.
2. Materials and Methods
2.1. Sampling
In this study, a total of 245 Siphonaria specimens were collected from 19 cities from six provinces (Shandong, Zhejiang, Fujian, Guangdong, Guangxi, and Hainan) along the coast of China (Figure 1, Table A1). All specimens were preserved in 95% ethanol and deposited in the Marine Biological Museum, Chinese Academy of Sciences. The specimens were initially identified based on morphological characteristics such as siphonal ridges, shell size, and radial ribs referring to Bouchet et al. [25] and the World Register of Marine Species database [26].
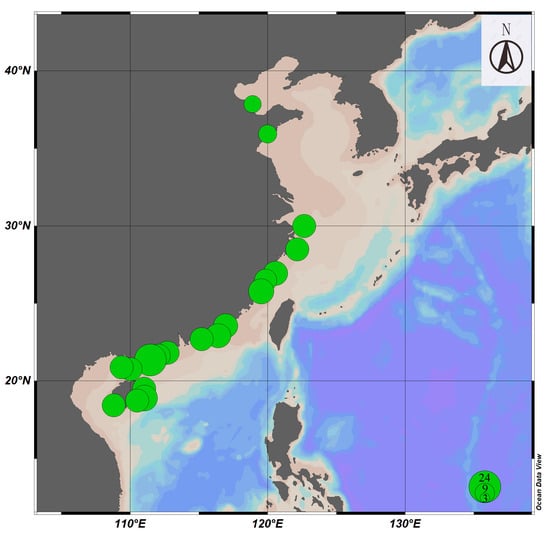
Figure 1.
The sampling map of Siphonaria species in this study. Note: The size of the circles represents the sample size.
2.2. Microscopic Observation
Shells were observed with a stereomicroscope (Zeiss Stemi SV11, Carl Zeiss AG, Jena, Germany). Morphological indices, including the shell color, size, shape, apex, siphonal notch, etc., were described. Imaging software ZEN 2.3 SP1 was used to capture the images.
2.3. DNA Extraction and PCR Amplification
About 30 mg adductor muscle of each specimen was used for DNA extraction using the TIANamp Marine Animal DNA Extraction Kit (DP324, Beijing Tiangen Biochemical Co., Ltd., Beijing, China). We selected three mitochondrial genes (COI, 12S rRNA, and 16S rRNA) and one nuclear gene (h3) for amplification, and all forward primers and reverse primers are shown in Table A2. PCR amplification was performed using the Biometra T100TM thermal cycler (Bio-Rad, Singapore). Reactions were carried out in a 30 μL system containing 15 μL of 2 × Canace® Gold PCR Master Mix (with Dye) (10102ES60, Yeasen Biotech Co., Ltd., Shanghai, China), 12 μL of double-distilled water, 1 μL of each primer, and 1 μL of template DNA. For specimens collected before 2016, Hieff Canace® Gold High Fidelity DNA Polymerase (10149ES10, Yeasen Biotech Co., Ltd., China) was used to perform amplification under the same conditions. The amplification parameters are listed in Table A4. The PCR products were detected by 1% agarose gel electrophoresis, and the resulting bands were sequenced using the 3730XL DNA Analyzer (Tsingke Biotechnology Co., Ltd., Beijing, China).
2.4. Phylogenetic Analyses
All sequences obtained in this study were compared with nucleotide sequences in GenBank using the Basic Local Alignment Search Tool (BLAST) to put the specimens into groups (5 groups were decided on in this study) [27]. To further clarify the systematic status of Siphonaria specimens in this study, a total of 50 sequences from 16 Siphonaria species were downloaded from the National Center for Biotechnology Information (NCBI, Table A3) to construct the phylogenetic trees, with Bullacta caurina W. H. Benson, 1842 selected as the outgroup [28]. Given the few h3 sequences available for Siphonaria species in GenBank, the phylogenetic analysis was conducted using COI, 12S rRNA, and 16S rRNA gene partial sequences. Fourteen specimens, of which the COI sequences were successfully obtained, were used for the phylogenetic analysis. The sequences of both those sequenced in this study (42 sequences) and downloaded from GenBank (48 sequences) were edited with BioEdit 7.2.5 and corrected to remove interference from degenerate bases [29]. The sequences were aligned using MAFFT 7 in the normal mode, and conserved regions with unambiguous positional homology were retained using Gblocks 0.91b with default parameters [30]. After alignment, sequences were concatenated, and the best-fit evolutionary model for each partition was selected using ModelFinder based on the corrected Akaike Information Criterion (details in Table A6) [31]. Phylogenetic analyses were conducted using two approaches. Bayesian inference (BI) was performed with MrBayes v.3.2.6, with two independent runs, each comprising four Markov Chain Monte Carlo chains running for 2 million generations and sampling every 1000 generations [32]. The initial 25% of trees were discarded as burn-in after running for 10 million generations. A maximum likelihood (ML) analysis was conducted using IQ-TREE 1.6.8 [33]. All software was integrated into PhyloSuite v. 1.2.2 [34]. Phylogenetic trees were visualized using FigTree v.1.4.3 [35].
2.5. Molecular Species Delimitation and Genetic Distance
Considering that only 14 sequences for COI were obtained in this study, the genetic distances among 5 Siphonaria groups (i.e., S. atra, S. sirius, S. japonica, S. petasus sp. nov., and S. floslamellosa sp. nov.) were calculated based on concatenations of 12S rRNA, 16S rRNA, and h3 to identify and uncover cryptic species using the Kimura-2-parameter model and analyzed with MEGA X [36,37]. Only specimens with 12S rRNA, 16S rRNA, and h3 successfully sequenced were used here and as a result, 73 concatenations (73 specimens, i.e., S. atra, 6; S. sirius, 30; S. japonica, 16; S. petasus sp. nov., 6; S. floslamellosa sp. nov., 15) from 219 sequences were used. The phylogenetic relationships of the 73 concatenations were analyzed by constructing a BI and ML tree using the methods described in 2.4. Research has proposed species delimitation methods based on genetic distances [38,39]. Following previous outcomes [40,41,42], we utilized two approaches to determine the molecular species boundaries of Siphonaria: ABGD (Automated Barcoding Gap Discovery) and ASAP (Assemble Species by Automatic Partitioning) [43,44]. The concatenated sequences were uploaded to the ABGD web interface [45] and analyzed using the following parameters: a P (prior limit to intraspecific diversity) range of 0.01 to 0.1 and a relative gap width (X) of 1.0. Transition/Transversion Bias (TS/TV, value = 2.0) was estimated using MEGA X, and a data analysis was performed with the Kimura 80 model [36]. The ASAP [46] used the same settings as those used for ABGD.
2.6. Haplotype Network
In total, 73 concatenations of 12S rRNA, 16S rRNA, and h3 sequences from 5 species groups were imported into DnaSP v6.12.03 [47]. To generate haplotype networks and visualize the phylogenetic relationships among haplotypes and lineages, PopArt 1.7 was used with the TCS algorithm mapping method [48,49].
2.7. Divergence Times
Due to the lack of definitive fossil or geographical record data, this study referred to previous research and selected an average substitution rate of 1.0% per million years to estimate the divergence times of Siphonaria species [24,50]. Again, 73 concatenations of 12S rRNA, 16S rRNA, and h3 sequences were used to estimate divergence times. Following Jung et al., we utilized two fossil records to estimate divergence times [51]. These fossil calibrations were based on the estimated ages of the most recent common ancestors as follows: (i) the genus Siphonaria (3.6–2.588 million years ago); (ii) S. pectinata (0.774–0.129 million years ago). Using the Model Finde in PhyloSuite, the TN93 model was selected for the BEAST analysis [31]. Bayesian MCMC methods in BEAST 2 were employed to estimate divergence times among major lineages using concatenated sequences of 16S rRNA, 12S rRNA, and h3 [52]. The analysis applied an uncorrelated log-normal relaxed clock model combined with a Yule speciation process. Two independent MCMC analyses were conducted, respectively, each running for 50 million generations with sampling every 1000 generations, with the first 25% of samples discarded as burn-in. Convergence of the runs was confirmed using Tracer 1.5 [53], ensuring effective sample sizes (ESSs) exceeding 200. The resulting divergence time tree was then visualized and beautified using Fig Tree v1.4.3.
3. Results
3.1. Morphological Descriptions of Siphonaria Species in China
This study identified five Siphonaria species groups based on morphological characteristics, three of which are known species and two of which are previously unreported. Based on past research and observations of samples, the morphologies of the three known species, i.e., S. japonica, S. atra, and S. sirius, are described as follows [54,55,56,57,58,59,60,61].
3.1.1. Siphonaria japonica
Description: Shell hard and thick, with surface pale yellow or brown; radial ribs more than 20, uniform in thickness; siphonal ridge underdeveloped, directly discernible from neither inside nor outside of the shell (Figure 2).
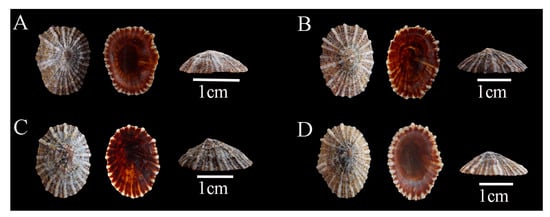
Figure 2.
Siphonaria japonica specimens collected in Guangdong, China. (A), WN-135; (B), WN-160; (C), WN-446; (D), WN-495.
3.1.2. Siphonaria sirius
Description: Shell flat, oval- or egg-shaped, relatively thick; shell margin smooth; radial ribs fewer than 20, vary in thickness, slightly raised above the shell surface; apex located at the center; siphonal ridge well developed, allowing direct identification (Figure 3).
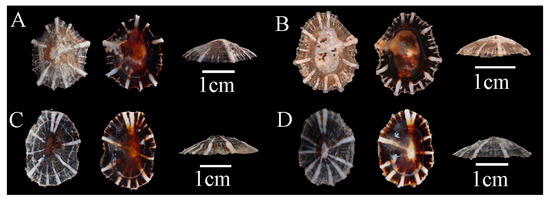
Figure 3.
Siphonaria sirius specimens collected in Guangdong, China. (A), WN-392; (B), WN-146; (C), WN-148; (D), WN-136.
3.1.3. Siphonaria atra
Description: Shell low, thick, and broad-elliptical; radial ribs fewer than 20, thickness variable, the ends protrude beyond shell margin, giving an irregularly serrated appearance; apex located at the center of the shell; siphonal ridge well developed (Figure 4).
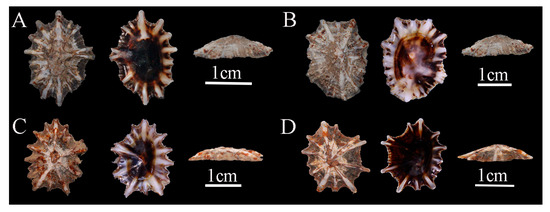
Figure 4.
Siphonaria atra specimens collected in Hainan, China. (A), WN-49; (B), WN-47; (C), WN-105; (D), WN-106.
3.2. Phylogenetic Relationship
Totally, 14 of COI (648 bp), 127 of 12S rRNA (317 bp), 142 of 16S rRNA (423 bp), and 116 of h3 (303 bp) sequences were obtained in this study (Table A5). Five species groups were decided from all 245 Siphonaria specimens by BLAST in this study, corresponding with the morphological results, of which three species were S. japonica, S. sirius, and S. atra. However, sequences of the other two species were quite different from the current sequences in GenBank, suggesting two species have not yet been recognized or studied. The maximum likelihood (ML) and Bayesian inference (BI) phylogenetic trees constructed based on 16S rRNA, 12S rRNA, and COI revealed similar topologies with most nodes showing high support values (Figure 5). All Siphonaria species were divided into two major clades. Clade A included seven known Siphonaria species and two species groups obtained in this study, i.e., S. japonica (WN-115, WN-116, and WN-120) and S. petasus sp. nov. (WN-93 and WN-132). Clade B comprises eight known Siphonaria species and three species groups obtained in this study, i.e., S. atra (WN-47, WN-105, and WN-106), S. sirius (WN-78, WN-136, and WN-137), and S. floslamellosa sp. nov. (WN-74, WN-110, and WN-114). Obviously, S. floslamellosa sp. nov. formed a distinct clade and clustered with S. szlandica. The monophyly of S. japonica (posterior probability = 100; bootstrap value = 100), S. petasus sp. nov. (posterior probability = 1; bootstrap value = 100), and S. floslamellosa sp. nov. (posterior probability = 1; bootstrap value = 100) was strongly supported.
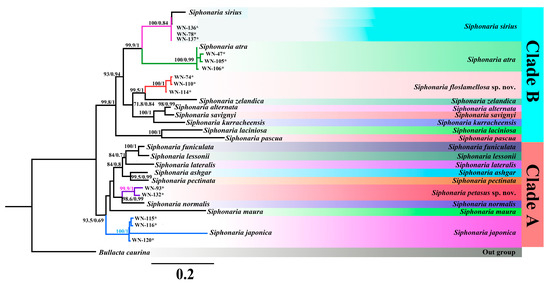
Figure 5.
BI tree and ML tree based on concatenated partial sequences of COI, 12S rRNA, and 16S rRNA of Siphonaria species. Maximum likelihood bootstrap support values were followed by Bayesian posterior probabilities, with only posterior probabilities larger than 50% shown. Species marked with asterisk (*) were sequenced in this study.
The K2P genetic distances calculated based on concatenated sequences of three fragments are shown in Table 1. The genetic distances between S. petasus sp. nov. and the other four species ranged from 11.7% (S. japonica) to 21.99% (S. sirius), with a mean of 19.17%. The genetic distances between S. floslamellosa sp. nov. and the other four species ranged from 16.74% (S. atra) to 21.96% (S. petasus sp. nov.), with a mean of 19.14%. Both ABGD and ASAP produced consistent species delimitation results, confirming five Siphonaria species groups in this study, including the monophyletic units of S. petasus sp. nov. and S. floslamellosa sp. nov. (Figure 6).

Table 1.
The K2P genetic distances among Siphonaria species based on concatenated sequences of 12S rRNA, 16S rRNA, and h3.
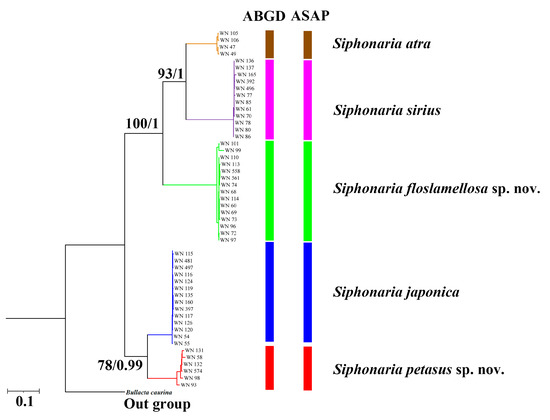
Figure 6.
BI tree and ML tree based on concatenated partial sequences of 16S rRNA, 12S rRNA, and h3 gene of Siphonaria specimens in this study for species delimitation. Maximum likelihood bootstrap support values were followed by Bayesian posterior probabilities, with only posterior probabilities and bootstrap values greater than 75% shown.
3.3. Haplotype Network
The haplotype network constructed by 73 Siphonaria samples (Figure 7) revealed that these samples were distinctly divided into five groups, strictly corresponding to the species delimitation. Among them, three known species (S. atra, S. japonica, and S. sirius) exhibited star-like network structures with clearly defined central haplotypes, while the two new species (S. petasus sp. nov. and S. floslamellosa sp. nov.) displayed complex network structures, with multiple haplotypes interconnected and no distinct central haplotypes identified.
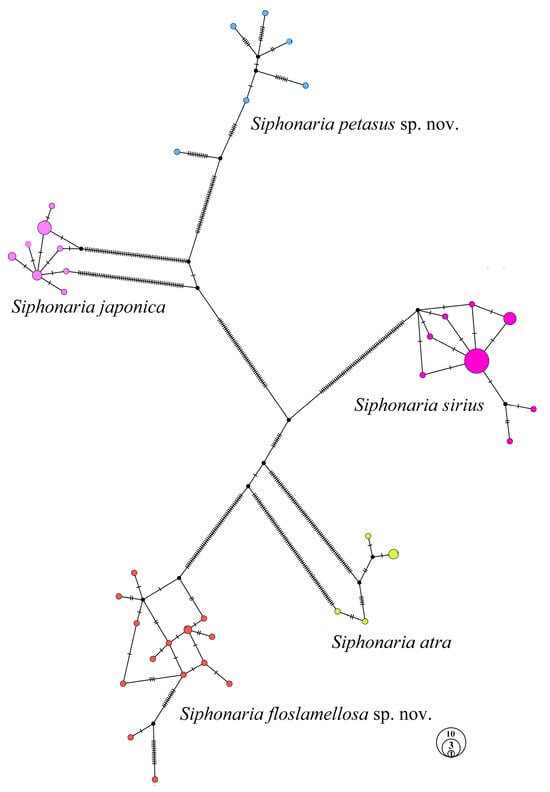
Figure 7.
Haplotype network of Siphonaria species based on concatenated partial sequences of 16S rRNA, 12S rRNA, and h3.
3.4. Divergence Time Estimation
The divergence time estimations (Figure 8) indicated that S. petasus sp. nov. and S. floslamellosa sp. nov. belonged to different clades, with their divergence occurring at approximately 3.46 Mya (CI95%: 2.94–4 Mya) during the late Pliocene. Their common ancestor diverged from Bullacta caurina, which also belongs to Tectipleura, approximately 15.08 million years ago (CI95%: 11.86–18.48 Mya) during the mid-Miocene. S. petasus sp. nov. formed a sister group with S. japonica, with an estimated divergence time of about 1.53 million years ago (CI95%: 1.17–1.94 Mya) during the early to middle Pleistocene. S. floslamellosa sp. nov. formed a sister group with the common ancestor species of S. atra and S. sirius, with their divergence estimated at approximately 2.52 million years ago (CI95%: 2–3.07 Mya) during the early Pleistocene.
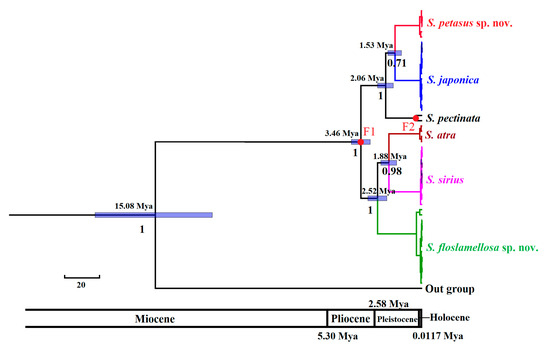
Figure 8.
Divergence times of Siphonaria species. Node numbers refer to divergence time, with posterior below nodes. Blue shading represents 95% confidence intervals of divergent time. F1: 3.6–2.588 Ma; F2: 0.774–0.129 Ma.
3.5. Two New Siphonaria Species in China
3.5.1. Siphonaria petasus sp. nov.
Taxonomy
Order—Siphonariida J. E. Gray, 1827.
Family—Siphonariidae J. E. Gray, 1827.
Genus—Siphonaria G. B. Sowerby, 1823.
Species—Siphonaria petasus sp. nov., Zang, Ma & Wang.
Etymology: The name of the species refers to a prominent apex.
Holotype: WN-570. The specimen was collected in May 2012, and deposited in the Marine Biological Museum of the Chinese Academy of Sciences.
Type locality: Wanning, Hainan, China.
Paratypes: WN-102, WN-103 and WN-574. Details are showen in Table 2.

Table 2.
Paratypes of Siphonaria petasus sp. nov.
Diagnosis: Shell thin and opaque; apex significantly elevated, slightly inclined to the left, protected by a conical calcareous cover; radial ribs 13 to 18, thickness variable, slightly raised above the surface; shell coloration pale yellow to reddish-brown, apex white.
Description: Shell thin and opaque, diverse coloration, textured, width 5 to 10 mm, length 12 to 16 mm; apex protected by a reinforced conical calcareous cover; radial ribs 13 to 18, thickness variable, extending from apex to shell margin, slightly raised above surface, accompanied by triangular spine-like projections; siphonal ridge distinct, radial ribs pale white; interspaces between ribs vary in color, pale yellow to black, secondary ribs inconspicuous; shell interior deep brown, with a smooth nacreous layer; siphonal notch lighter, pale yellow or white; attachment area of the soft tissues forms continuous muscle scars, interrupted at the siphonal notch, creating a “C”-shaped pattern (Figure 9).
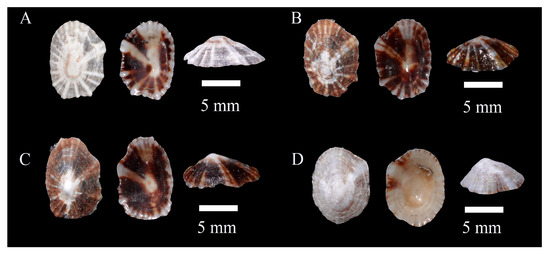
Figure 9.
The specimens of Siphonaria petasus sp. nov. (A), Holotype WN-570. (B), WN-102; (C), WN-103; (D), WN-574.
Habitat: Rocky areas of the mid- to low intertidal zones in the eastern and southern regions of Hainan.
Sampling locations: A total of 11 specimens were collected in this study from various locations in Hainan, China, including Wenchang, Sanya, Qionghai, and Wanning (Table A1).
3.5.2. Siphonaria floslamellosa sp. nov.
Taxonomy
Order—Siphonariida J. E. Gray, 1827.
Family—Siphonariidae J. E. Gray, 1827.
Genus—Siphonaria G. B. Sowerby, 1823.
Species—Siphonaria floslamellosa sp. nov., Zang, Ma & Wang.
Etymology: The species name is derived from the numerous and evenly thick radial ribs, giving the entire shell the appearance of a multi-layered flower.
Holotype: WN-114. The specimen was collected in May 2013, and deposited in the Marine Biological Museum of the Chinese Academy of Sciences.
Type locality: Sanya, Hainan, China.
Paratypes: WN-101, WN-96 and WN-97. Details are showen in Table 3.

Table 3.
Paratypes of Siphonaria floslamellosa sp. nov.
Diagnosis: Shell medium to moderately small, irregular, ovate- and cap-shaped, with white calcareous deposits; radial ribs 20 to 30, evenly thick, protrude above the shell surface, extend from apex to margin, ends extend beyond margin; inner shell surface covered with a smooth nacreous layer; right siphonal ridge well developed, muscle scars “C”-shaped, interrupted at the siphonal ridge.
Description: Shell medium to moderately small, irregular, ovate- and cap-shaped, width 7 to 11 mm, length 12 to 15 mm; shell thin and opaque, adorned with white calcareous deposits; radial ribs 20 to 30, white, interspaces pale yellow near apex and dark brown toward margin, evenly thick, accompanied by spine-like projections, extending from apex to beyond margin, forming clear ridge-like structures; secondary ribs slender, located between radial ribs; right siphonal ridge well developed; shell apex offset to the left rear, opposite the direction of water canal; inner shell surface covered with a smooth nacreous layer, deep brown; grooves between radial ribs like lighter white bands; muscle scars C-shaped, interrupted at the siphonal ridge (Figure 10).
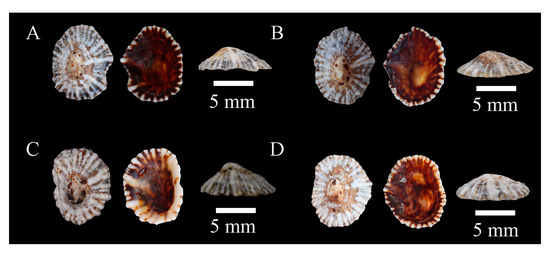
Figure 10.
Specimens of Siphonaria floslamellosa sp. nov. Holotype: (A), WN-114. Paratypes: (B), WN-101; (C), WN-96; (D): WN-97.
Habitat: Rocky areas of the mid- to low intertidal zones in the eastern and southern coasts of Hainan.
Sampling location: A total of 25 specimens were collected from various locations in Hainan, including Wenchang, Sanya, Qionghai, and Wanning (Table A1).
3.6. Identification Key to Siphonaria Species in China
To facilitate the identification of Siphonaria species in Chinese waters, key characteristics of five Siphonaria species in China were concluded (Table 4). The key identification for Siphonaria species in China was based on characteristics such as the apex, shell margin, radial ribs, and color distribution of the inner shell.

Table 4.
Key characteristics for five Siphonaria species in China.
Identification Key to Siphonaria species in China
- Radial ribs fewer than 20, varying in thickness, with wide interspaces; radial rib at the siphonal ridge distinctly wider than adjacent ribs······················································2Radial ribs more than 20, uniform in thickness, with narrow interspaces; radial rib at the siphonal ridge similar in width to adjacent rib··························································· 3
- Apex smooth and flat, low, without a covering············································································································································ 4Apex elevated, covered with a calcareous layer·····································S. petasus sp. nov.
- The siphonal ridge is underdeveloped; the surface of the shell is pale yellow or brown······················································································································ S. japonicaThe siphonal ridge is developed, it can be clearly identified both from the interior and exterior of the shell; the surface of the shell is white············· S. floslamellosa sp. nov.
- Radial ribs flush with shell surface, terminating at the shell edge; shell margin smooth··························································································································S. siriusRadial ribs significantly raised above shell surface, protruding beyond the shell edge; shell margin irregular and uneven···································································································································S. atra
4. Discussion
4.1. Morphological Analyses
Previous studies suggested that S. japonica could be clearly distinguished from S. sirius and S. atra by its underdeveloped siphonal ridge. Additionally, they proposed that the number of radial ribs could separate S. sirius (which typically has six white radial ribs) from S. atra (which has more than six radial ribs) [58]. However, this study found that the number of radial ribs is not a stable characteristic within individuals. Some S. sirius specimens possess as many as nine or even more radial ribs (Figure 3). Meanwhile, certain S. atra individuals have only 6–9 radial ribs (Figure 4). Therefore, relying solely on the number of radial ribs to distinguish them is not reliable. A comprehensive consideration of other morphological characteristics, such as the structure of the radial ribs and the shell margin, should be employed for accurate identification. Meanwhile, we found that the morphological characteristics of radial ribs have been underestimated in previous taxonomic studies. Features of the radial ribs, such as the number, thickness, color at specific locations, whether they extend beyond the shell margin, and whether they rise above the shell surface, can effectively distinguish Siphonaria species in China. Radial ribs are among the few traits in Siphonaria that can be directly observed with the naked eye without the aid of tools, making them highly efficient for taxonomic research.
In the morphological comparison, we noted that S. floslamellosa sp. nov. closely resembles Siphonaria acmaeoides Pilsbry, 1894. According to the description by Hirano [60,61], S. acmaeoides has a slender, nearly symmetrical shell, thin and opaque, with a calcareous layer covering the shell surface. The radial ribs on the shell surface are light yellow or dark brown, and the interior of the shell is dark brown, with lighter or white areas corresponding to the positions of the radial ribs. The rough radial ribs and the white calcareous covering make these two species appear to be very similar. However, based on the original description by Pilsbry (1894), S. acmaeoides has 9–16 radial ribs, and the siphonal ridge is undeveloped, whereas S. floslamellosa sp. nov. has 20–30 radial ribs, with a well-developed siphonal ridge. Additionally, S. acmaeoides was collected from Japan, while all samples of S. floslamellosa sp. nov. were collected from the coastal regions of Hainan Island, China (Wenchang, Qionghai, and Sanya). Therefore, based on morphological differences and distribution records, it can be concluded that S. floslamellosa sp. nov. and S. acmaeoides are two completely distinct species, and the taxonomic status of S. floslamellosa sp. nov. can be confirmed.
4.2. Phylogenetic Analyses and Species Delimitation
In this study, we constructed phylogenetic trees using maximum likelihood (ML) and Bayesian inference (BI) methods, based on a short-sequence concatenated dataset of the COI, 12S rRNA, and 16S rRNA genes (Figure 5). Both the ML and BI trees exhibited consistent topological structures, with clear definitions of the relationships among Siphonaria species and extremely high support values. The two species delimitation methods also yielded the same results, both supporting the distinct grouping of S. petasus sp. nov. and S. floslamellosa sp. nov. (Figure 6). Furthermore, the phylogenetic tree we generated showed branching patterns that are consistent with those of Dayrat, where Siphonaria is divided into two major clades, A and B, with S. petasus sp. nov. and S. floslamellosa sp. nov. assigned to clades A and B, respectively, both of which were strongly supported as monophyletic. A genetic analysis (Table 1) showed that the average K2P genetic distances of S. petasus sp. nov. and S. floslamellosa sp. nov. from other known Siphonaria species with records in China were 18.24% and 18.19%, respectively, which are close to the average interspecies genetic distances of 18.56%, further supporting the validity of these new species. Although the K2P genetic distances between S. petasus sp. nov. and S. japonica are the smallest (11.7%), there are obvious morphological differences between the two species (Table 4), ruling out the possibility of them being the same species. Unfortunately, due to the lack of fresh samples, we are unable to perform a further mitochondrial genome-based analysis of these species at this time.
It is worth noting that S. floslamellosa sp. nov. appears to have been previously studied by Wang [62]. Through sequence alignment, we found that S. floslamellosa sp. nov. is identical to the sequences (COI, 16S rRNA) uploaded by Wang to GenBank. However, the article did not explicitly name or describe the species based on morphological features, only noting that it differed molecularly from all known Siphonaria species. Therefore, we are unable to directly compare their morphological characteristics and can only infer that they represent the same species based on their highly similar molecular traits.
4.3. The Significance of DNA Barcoding in the Taxonomic Study of Marine Mollusca
A large number of reports have utilized DNA barcoding for the species identification of intertidal gastropods. For instance, Sun used COI to identify 45 species of Mesogastropoda along the coast of China, and all species were clearly distinguished [63]. Yu et al. used COI, 28S ribosomal RNA, and h3 to identify Nipponacmea species in China, revealing three species: N. radula, N. fuscoviridis, and N. nigrans [64]. However, earlier reports indicated that only N. schrenckii existed along the Chinese coast. Similarly, in this study, we found two different shell morphs of S. petasus sp. nov.; yet, their DNA sequences were completely identical (Figure 9). These studies demonstrate the significant role of DNA barcoding in the taxonomy of mollusks, as it not only helps researchers quickly and accurately distinguish closely related species, but also provides a thorough understanding of species diversity. This makes DNA barcoding an excellent tool for exploring the hidden diversity within intertidal ecosystems, which are known for their rich cryptic species.
4.4. Population History and Dynamics
The divergence time results (Figure 8) indicate that the divergence of the two major clades of the genus Siphonaria occurred during the late Pliocene. According to the studies of Lisiecki et al. and Zachos et al., there was a significant global temperature drop and glacial expansion during the Pliocene, laying the foundation for the transition into the Pleistocene ice ages [65,66]. Divergence within each of the two major clades of Siphonaria occurred during the Pleistocene. The fluctuations between glacial and interglacial periods during the Pleistocene were particularly intense, with the fluctuation cycles gradually extending from 40,000 years in the early Pleistocene to 100,000 years in the late Pleistocene [67]. During these alternating cycles, sea level changes caused the frequent fragmentation and restoration of intertidal habitats. The Qiongzhou Strait, affected by land bridge formation, periodically opened and closed, which may have directly influenced the isolation and gene flow of intertidal species, such as Siphonaria, and subsequently affected the evolutionary processes of Siphonaria species.
The haplotype network we obtained (Figure 7) shows that the three species, S. japonica, S. atra, and S. sirius, exhibit a center–periphery structure, with a central, highly common haplotype and several other haplotypes that differ by one to five steps. In contrast, the networks of S. petasus sp. nov. and S. floslamellosa sp. nov. display both star-like and complex structures. In the haplotype networks of these two species, there are many dispersed haplotypes with very small differences, mostly ranging from one to eight steps. This may be due to the fragmentation of intertidal habitats during the alternation between glacial and interglacial periods, which led to the Siphonaria populations undergoing multiple migrations and expansions. Different migration and expansion events superimposed ultimately resulted in the coexistence of star-like and complex branching network structures.
The distribution patterns of Siphonaria observed by Dayrat et al. in the Indo-Pacific region are similar to those observed in other gastropod groups, where the ranges of closely related species do not overlap. According to the study by Williams and Reid [68], this suggests that Siphonaria in the Indo-Pacific region follows an allopatric speciation pattern. However, in this study, we found that the distribution ranges of the four Siphonaria species in the Hainan Island region overlap. This may be due to the rise in sea level during interglacial periods, which led to the restoration of intertidal habitats and the disappearance of geographical barriers. Additionally, ocean currents may have caused the larvae of Siphonaria to exhibit migratory behavior in the same direction, ultimately leading to sympatric distributions of different Siphonaria species.
5. Conclusions
In this study, we described two new Siphonaria species collected in China, i.e., Siphonaria petasus sp. nov. and Siphonaria floslamellosa sp. nov. Through detailed morphological comparisons and a molecular phylogenetic analysis, we confirmed that these species exhibit clear evolutionary divergence from other congeners. We found that the characteristics of radial ribs were highly effective in distinguishing Siphonaria species. The number, thickness, and structural relationships of radial ribs with the shell surface and shell margin are of great significance in the taxonomy of Siphonaria species. By comparing the commonly found Siphonaria species in China, we have compiled a dichotomous key for the efficient identification of different Siphonaria species. The discovery of new species not only enhances the species diversity of the genus Siphonaria, but also provides new evidence for further studies on species differentiation. Due to the significant influence of environmental factors on the shells of marine mollusks, individuals of the same species can exhibit considerable morphological differences, whereas DNA barcoding remains consistently stable. Therefore, DNA barcode-based studies can improve the accuracy of species identification. Furthermore, as a newly developed method for species delimitation based on genetic distances, the applicability of ASAP was validated in this study. In the prospective research on taxonomy of mollusks, molecular methods should be integrated with morphological results.
Author Contributions
Conceptualization, G.Z. and J.W.; methodology, G.Z. and J.W.; validation, P.M. and G.Z.; formal analysis, G.Z.; investigation, J.W.; data curation, J.W.; writing—original draft preparation, G.Z.; visualization, Z.T. and Y.C.; writing—review and editing, P.M.; supervision, C.L.; project administration, H.W.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (nos. 42006080, 42076092, and 41776179), the National Key R&D Program of China (nos. 2022YFD2401301, 2022FY100304, and 2023YFD2400800), the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB42000000), the Earmarked Fund for Modern Agro-industry Technology Research System (CARS-47), and the Key R&D Program of Shandong Province (no. 2023CXGC010411).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Sampling collection information.
Table A1.
Sampling collection information.
| Species | Province | Locality | Quantity | Time |
|---|---|---|---|---|
| Siphonaria japonica | Shandong | Dongying | 1 | March 2022 |
| Qingdao | 1 | July 2022 | ||
| Zhejiang | Wenzhou | 5 | March 2013 | |
| Ningbo | 2 | May 2017 | ||
| Zhoushan | 2 | June 2017 | ||
| Fujian | Zhangzhou | 2 | August 2017 | |
| Fuzhou | 5 | May 2017 | ||
| Quanzhou | 1 | May 2017 | ||
| Ningde | 1 | March 2013 | ||
| Guangdong | Huizhou | 2 | April 2018 | |
| Shantou | 1 | April 2018 | ||
| Shantou | 1 | March 2019 | ||
| Yangjiang | 5 | March 2013 | ||
| Yangjiang | 11 | August 2017 | ||
| Yangjiang | 4 | April 2018 | ||
| Jiangmen | 27 | May 2017 | ||
| Jiangmen | 3 | August 2017 | ||
| Jiangmen | 19 | April 2018 | ||
| Siphonaria sirius | Zhejiang | Wenzhou | 14 | May 2017 |
| Zhoushan | 1 | May 2017 | ||
| Guangdong | Shantou | 1 | April 2018 | |
| Jiangmen | 30 | April 2018 | ||
| Jiangmen | 14 | August 2017 | ||
| Yangjiang | 28 | August 2017 | ||
| Hainan | Wenchang | 12 | May 2013 | |
| Wanning | 1 | May 2013 | ||
| Sanya | 3 | May 2013 | ||
| Siphonaria atra | Hainan | Sanya | 8 | January 2014 |
| Qionghai | 1 | May 2013 | ||
| Lingshui | 1 | January 2011 | ||
| Guangxi | Beihai | 1 | April 2018 | |
| Siphonaria petasus sp. nov. | Hainan | Wenchang | 2 | April 2018 |
| Wenchang | 4 | May 2013 | ||
| Sanya | 3 | May 2013 | ||
| Qionghai | 1 | May 2013 | ||
| Wanning | 2 | March 2012 | ||
| Siphonaria floelamellosa sp. nov. | Hainan | Wenchang | 5 | May 2013 |
| Qionghai | 2 | May 2013 | ||
| Wanning | 1 | May 2013 | ||
| Sanya | 17 | May 2013 |

Table A2.
Primers used for PCR amplification in this study.
Table A2.
Primers used for PCR amplification in this study.
| Marker | Primer Name | Primer Sequences | References |
|---|---|---|---|
| COI | LCO1490 | 5′-GGTCAACAAATCATAAAGATATTGG-3′ | [69] |
| HCO2198 | 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ | ||
| 16S rRNA | 16SF | 5′-GCCTGTTTATCAAAAACAT-3′ | [70] |
| 16SR | 5′-CCGGTCTGAACTCAGATCACG-3′ | ||
| h3 | H3F | 5′-ATGGCTCGTACCAAGCAGACVGC-3′ | [71] |
| H3R | 5′-ATATCCTTRGGCATRATRGTGAC-3′ | ||
| 12S rRNA | 12SF | 5′-AAACTAGGATTAGATACCCTATTAT-3′ | [7] |
| 12SR | 5′-GAGGGTGACGGGCGGTGTGT-3′ |

Table A3.
GenBank accession numbers of downloaded sequences.
Table A3.
GenBank accession numbers of downloaded sequences.
| Species | GenBank Accession Nos. | |||
|---|---|---|---|---|
| 12S rRNA | COI | 16S rRNA | h3 | |
| Siphonaria japonica Donovan, 1824 | KF001063 | KF000759 | KF000911 | LC384345 |
| Siphonaria asghar Biggs, 1958 | KF001053 | KF000749 | KF000901 | - |
| Siphonaria pectinata Linnaeus, 1758 | KF001067 | KF000763 | KF000915 | - |
| Siphonaria lateralis A. Gould, 1846 | KF001076 | KF000772 | KF000924 | - |
| Siphonaria funiculata Reeve, 1856 | KF001079 | KF000775 | KF000927 | - |
| Siphonaria lessonii Blainville, 1827 | KF001035 | KF000731 | KF000883 | - |
| Siphonaria normalis A. Gould, 1846 | KF001090 | KF000786 | KF000938 | - |
| Siphonaria maura G. B. Sowerby I, 1835 | KF001107 | KF000803 | KF000956 | - |
| Siphonaria pascua Rehder, 1980 | KF001109 | KF000805 | KF000957 | - |
| Siphonaria laciniosa Linnaeus, 1758 | KF001020 | KF000716 | KF000868 | - |
| Siphonaria zelandica Quoy & Gaimard, 1833 | KF001029 | KF000725 | KF000877 | - |
| Siphonaria kurracheensis Reeve, 1856 | KF001052 | KF000748 | KF000900 | - |
| Siphonaria savignyi Krauss, 1848 | KF001051 | KF000747 | KF000899 | - |
| Siphonaria alternata Say, 1826 | KF001108 | KF000804 | KF000955 | - |
| Siphonaria atra Quoy & Gaimard, 1833 | KF001023 | KF000719 | KF000871 | - |
| Siphonaria sirius Pilsbry, 1894 | KF001136 | KF000832 | KF000984 | LC384094 |
| Bullacta caurina W. H. Benson, 1842 | HQ833922 | - | HQ833986 | HQ834193 |

Table A4.
PCR amplification programs in this study.
Table A4.
PCR amplification programs in this study.
| PCR Master Mix | Marker | Program |
|---|---|---|
| 2 × Hieff Canace® Gold PCR Master Mix (with Dye) | COI | 94 °C 5 min, 32 × (94 °C 30 s 46 °C 30 s, 72 °C 50 s), 72 °C 8 min, 4 °C hold |
| 16S rRNA | 94 °C 5 min, 32 × (94 °C 30 s, 49 °C 30 s, 72 °C 50 s), 72 °C 8 min, 4 °C hold | |
| h3 | 94 °C 5 min, 32 × (94 °C 30 s, 52 °C 30 s, 72 °C 50 s), 72 °C 8 min, 4 °C hold | |
| 12S rRNA | 94 °C 5 min, 32 × (94 °C 30 s, 46 °C 30 s, 72 °C 50 s), 72 °C 8 min, 4 °C hold | |
| Hieff Canace® Gold High Fidelity DNA Polymerase | COI | 98 °C 3 min, 30 × (98 °C 10 s, 46 °C 20 s, 72 °C 23 s), 72 °C 5 min, 4 °C hold |
| 16S rRNA | 98 °C 3 min, 30 × (98 °C 10 s, 46 °C 20 s, 72 °C 15 s), 72 °C 5 min, 4 °C hold | |
| h3 | 98 °C 3 min, 30 × (98 °C 10 s, 50 °C 20 s, 72 °C 15 s), 72 °C 5 min, 4 °C hold | |
| 12S rRNA | 98 °C 3 min, 30 × (98 °C 10 s, 46 °C 20 s, 72 °C 15 s), 72 °C 5 min, 4 °C hold |

Table A5.
The GenBank accession numbers for the sequences obtained in this study.
Table A5.
The GenBank accession numbers for the sequences obtained in this study.
| Specimen | h3 | 12S rRNA | 16S rRNA | |
|---|---|---|---|---|
| Siphonaria petasus sp. nov. | WN-58 | PQ846807 | PQ834496 | PQ824544 |
| WN-93 | PQ846806 | PQ834497 | PQ824545 | |
| WN-98 | PQ846805 | PQ834498 | PQ824546 | |
| WN-131 | PQ846804 | PQ834499 | PQ824547 | |
| WN-132 | PQ846803 | PQ834500 | PQ824548 | |
| WN-574 | PQ846802 | PQ834501 | PQ824549 | |
| Siphonaria floslamellosa sp. nov. | WN-561 | PQ846808 | PQ834502 | PQ824551 |
| WN-558 | PQ846809 | PQ834503 | PQ824552 | |
| WN-114 | PQ846810 | PQ834507 | PQ824553 | |
| WN-113 | PQ846811 | PQ834508 | PQ824554 | |
| WN-110 | PQ846812 | PQ834509 | PQ824555 | |
| WN-101 | PQ846813 | PQ834510 | PQ824556 | |
| WN-99 | PQ846814 | PQ834504 | PQ824557 | |
| WN-97 | PQ846815 | PQ834505 | PQ824558 | |
| WN-96 | PQ846816 | PQ834506 | PQ824559 | |
| WN-74 | PQ846817 | PQ834511 | PQ824560 | |
| WN-73 | PQ846818 | PQ834512 | PQ824561 | |
| WN-72 | PQ846819 | PQ834513 | PQ824562 | |
| WN-69 | PQ846820 | PQ834514 | PQ824563 | |
| WN-68 | PQ846821 | PQ834515 | PQ824564 | |
| WN-60 | PQ846822 | PQ834516 | PQ824565 | |
| Siphonaria atra | WN-542 | PQ858636 | PQ870139 | PQ862295 |
| WN-539 | PQ858637 | PQ870140 | PQ862294 | |
| WN-106 | PQ858635 | PQ870138 | PQ862293 | |
| WN-105 | PQ858638 | PQ870141 | PQ862296 | |
| WN-49 | PQ858639 | PQ870142 | PQ862297 | |
| WN-47 | PQ858640 | PQ870143 | PQ862298 | |
| Siphonaria japonica | WN-498 | PQ858643 | PQ870159 | PQ862302 |
| WN-497 | PQ858641 | PQ870157 | PQ862299 | |
| WN-481 | PQ858644 | PQ870156 | PQ862300 | |
| WN-397 | PQ858645 | PQ870153 | PQ862301 | |
| WN-160 | PQ858646 | PQ870154 | PQ862303 | |
| WN-157 | PQ858642 | PQ870158 | PQ862304 | |
| WN-135 | PQ858647 | PQ870155 | PQ862305 | |
| WN-126 | PQ858648 | PQ870152 | PQ862306 | |
| WN-124 | PQ858649 | PQ870151 | PQ862307 | |
| WN-120 | PQ858650 | PQ870150 | PQ862308 | |
| WN-119 | PQ858651 | PQ870149 | PQ862309 | |
| WN-117 | PQ858652 | PQ870148 | PQ862310 | |
| WN-116 | PQ858653 | PQ870147 | PQ862311 | |
| WN-115 | PQ858654 | PQ870146 | PQ862312 | |
| WN-55 | PQ858655 | PQ870145 | PQ862313 | |
| WN-54 | PQ858656 | PQ870144 | PQ862314 | |
| Siphonaria sirius | WN-568 | PQ858666 | PQ870160 | PQ862315 |
| WN-562 | PQ858667 | PQ870161 | PQ862316 | |
| WN-559 | PQ858669 | PQ870162 | PQ862317 | |
| WN-556 | PQ858670 | PQ870163 | PQ862318 | |
| WN-496 | PQ858672 | PQ870164 | PQ862319 | |
| WN-392 | PQ858673 | PQ870165 | PQ862320 | |
| WN-165 | PQ858678 | PQ870166 | PQ862321 | |
| WN-164 | PQ858679 | PQ870167 | PQ862322 | |
| WN-185 | PQ858674 | PQ870168 | PQ862323 | |
| WN-182 | PQ858675 | PQ870169 | PQ862324 | |
| WN-181 | PQ858676 | PQ870170 | PQ862325 | |
| WN-180 | PQ858677 | PQ870171 | PQ862326 | |
| WN-140 | PQ858680 | PQ870172 | PQ862327 | |
| WN-139 | PQ858681 | PQ870173 | PQ862328 | |
| WN-138 | PQ858682 | PQ870174 | PQ862329 | |
| WN-137 | PQ858683 | PQ870175 | PQ862330 | |
| WN-136 | PQ858684 | PQ870176 | PQ862331 | |
| WN-107 | PQ858685 | PQ870177 | PQ862332 | |
| WN-104 | PQ858686 | PQ870178 | PQ862333 | |
| WN-95 | PQ858657 | PQ870179 | PQ862334 | |
| WN-94 | PQ858658 | PQ870180 | PQ862335 | |
| WN-86 | PQ858659 | PQ870181 | PQ862336 | |
| WN-85 | PQ858660 | PQ870182 | PQ862337 | |
| WN-80 | PQ858661 | PQ870183 | PQ862338 | |
| WN-78 | PQ858662 | PQ870184 | PQ862339 | |
| WN-77 | PQ858663 | PQ870185 | PQ862340 | |
| WN-70 | PQ858664 | PQ870186 | PQ862341 | |
| WN-51 | PQ858671 | PQ870189 | PQ862342 | |
| WN-56 | PQ858668 | PQ870188 | PQ862343 | |
| WN-61 | PQ858665 | PQ870187 | PQ862344 |

Table A6.
Phylogenetic tree partitions and evolutionary models selected of short segments of Siphonaria species.
Table A6.
Phylogenetic tree partitions and evolutionary models selected of short segments of Siphonaria species.
| Genes | Model |
|---|---|
| 12S rRNA | TIM2+F+R3 |
| 16S rRNA | TIM2+F+R3 |
| COI (p1) | HKY+F+R2 |
| COI (p2) | TIM2+F+I+G4 |
| COI (p3) | TVM+F+G4 |
| h3 (p1) | TN+F |
| h3 (p2) | K2P+R2 |
| h3 (p3) | K2P+R2 |
References
- Solem, A.; Trueman, E.; Clarke, M. Origin and Diversification of Pulmonate Land Snails; Academic Press: Cambridge, MA, USA, 1985; pp. 269–293. [Google Scholar]
- Yonge, C. The mantle cavity in Siphonaria alternata Say. J. Molluscan Stud. 1952, 29, 190–199. [Google Scholar] [CrossRef]
- MOLLUSCABASE. Available online: https://www.molluscabase.org/index.php (accessed on 18 October 2024).
- Kim, Y.; Park, J.; Hwang, U.W.; Park, J.-K. Taxonomic review of Korean Siphonaria Species (Mollusca, Gastropoda, Siphonariidae). ARPHA Prepr. 2024, 5, e139436. [Google Scholar]
- Hubendick, B. Phylogenie und Tiergeographie der Siphonariidae: Zur Kenntnis der Phylogenie in der Ordnung Basommatophora und des Ursprungs der Pulmonatengruppe; Almqvist och Wiksells boktryck.: Uppsala, Sweden, 1945. [Google Scholar]
- Hodgson, A.N. The biology of siphonariid limpets (Gastropoda: Pulmonata). In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2002; pp. 253–322. [Google Scholar]
- Dayrat, B.; Goulding, T.C.; White, T.R. Diversity of Indo-West Pacific Siphonaria (Mollusca: Gastropoda: Euthyneura). Zootaxa 2014, 3779, 246–276. Available online: https://www.researchgate.net/publication/262693679 (accessed on 15 October 2024). [CrossRef] [PubMed]
- Jenkins, B. Siphonaria funiculata Reeve (Siphonariidae, Pulmonata): A Redescription Making S. Virgulata Hedley A Geographical Variant of S. Funiculata. J. Malacol. Soc. Aust. 1981, 5, 1–15. [Google Scholar] [CrossRef]
- Jenkins, B. Redescriptions and relationship of Siphonaria zelandica Quoy and Gaimard to S. australis Quoy and Gaimard with a description of S. propria sp. nov.(Mollusca: Pulmonata: Siphonariidae). J. Malacol. Soc. Aust. 1983, 6, 1–35. [Google Scholar] [CrossRef]
- Jenkins, B. A new siphonariid (Mollusca: Pulmonata) from southwestern Australia. J. Malacol. Soc. Aust. 1984, 6, 113–123. [Google Scholar] [CrossRef]
- Chambers, R.J.; McQuaid, C.D. A review of larval development in the intertidal limpet genus Siphonaria (gastropoda, pulmonata). J. Molluscan Stud. 1994, 60, 415–423. [Google Scholar] [CrossRef]
- Chambers, R.J.; McQuaid, C.D. Notes on the taxonomy, spawn and larval development of South African species of the intertidal limpet Siphonaria (Gastropoda: Pulmonata). J. Molluscan Stud. 1994, 60, 263–275. [Google Scholar] [CrossRef]
- Coddington, J.A. Cladistic tests of adaptational hypotheses. Cladistics 1988, 4, 3–22. [Google Scholar] [CrossRef]
- Jablonski, D.; Lutz, R.A. Larval ecology of marine benthic invertebrates: Paleobiological implications. Biol. Rev. 1983, 58, 21–89. [Google Scholar] [CrossRef]
- Reid, D.G. A cladistic phylogeny of the genus Littorina (Gastropoda): Implications for evolution of reproductive strategies and for classification. Hydrobiologia 1990, 193, 1–19. [Google Scholar] [CrossRef]
- Gueller, M.; Zelaya, D.G.; Ituarte, C. How many Siphonaria species (Gastropoda: Euthyneura) live in southern South America? J. Molluscan Stud. 2016, 82, 80–96. [Google Scholar] [CrossRef]
- Angas, G.F. Descriptions of thirty-two new species of marine shells from the coast of New South Wales. Proc. Zool. Soc. Lond. 1867, 110–117. Available online: https://www.biodiversitylibrary.org/page/29533550 (accessed on 9 October 2024).
- Angas, G.F. A list of species of marine Mollusca found in Port Jackson Harbour, New South Wales, and on the adjacent coasts, with notes on their habits, etc. Proc. Zool. Soc. Lond. 1867, 185–223. Available online: https://biostor.org/reference/59673 (accessed on 9 October 2024).
- Clark, K.B.; Goetzfried, A. Zoogeographic influences on development patterns of North Atlantic Ascoglossa and Nudibranchia, with a discussion of factors affecting egg size and number. J. Molluscan Stud. 1978, 44, 283–294. [Google Scholar]
- Hanley, S. Description of new Pinnae. Proc. Zool. Soc. Lond. 1858, 26, 225–228. [Google Scholar] [CrossRef]
- Hubendick, B. On a small quantity of Siphonaria material from Queensland. Mem. Natl. Mus. Vic. 1955, 19, 126–136. [Google Scholar] [CrossRef]
- Giribet, G.; Kawauchi, G.Y. How many species of Siphonaria pectinata (Gastropoda: Heterobranchia) are there? J. Molluscan Stud. 2016, 82, 137–143. [Google Scholar]
- Teske, P.R.; Barker, N.P.; McQuaid, C.D. Lack of genetic differentiation among four sympatric southeast African intertidal limpets (Siphonariidae): Phenotypic plasticity in a single species? J. Molluscan Stud. 2007, 73, 223–228. [Google Scholar] [CrossRef]
- Colgan, D.J.; da Costa, P. Possible drivers of biodiversity generation in the Siphonaria of southeastern Australia. Mar. Biodivers. 2013, 43, 73–85. [Google Scholar] [CrossRef]
- Bouchet, P.; Rocroi, J.-P.; Hausdorf, B.; Kaim, A.; Kano, Y.; Nuetzel, A.; Parkhaev, P.; Schroedl, M.; Strong, E.E. Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 2017, 61, 1–526. [Google Scholar] [CrossRef]
- World Register of Marine Speices. Available online: https://www.marinespecies.org/index.php (accessed on 15 October 2024).
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0022283605803602 (accessed on 12 October 2024). [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene (accessed on 9 October 2024).
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 1999; pp. 95–98. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Bui Quang, M.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlic, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, a Graphical Viewer of Phylogenetic Trees. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 12 October 2024).
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide-sequences. J. Mol. Evol. 1980, 16, 111–120. Available online: https://link.springer.com/article/10.1007/bf01731581 (accessed on 12 October 2024). [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis Across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hebert, P.D.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Stoeckle, M.Y.; Zemlak, T.S.; Francis, C.M. Identification of birds through DNA barcodes. PLoS Biol. 2004, 2, e312. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Kong, L. Comparing the efficiency of single-locus species delimitation methods within Trochoidea (Gastropoda: Vetigastropoda). Genes 2022, 13, 2273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S. Description of a new species of Polycera Cuvier, 1816 (Gastropoda: Nudibranchia: Polyceridae) from the East China Sea. Nautilus 2023, 137, 79–90. [Google Scholar]
- Chen, Y.; Li, C.; Lu, R.; Wang, H. Morphological and Molecular Analysis Identified a Subspecies of Crassostrea ariakensis (Fujita, 1913) along the Coast of Asia. Genes 2024, 15, 644. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Automated Barcoding Gap Discovery. Available online: https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html (accessed on 19 October 2024).
- Assemble Species by Automatic Partitioning. Available online: https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html (accessed on 19 October 2024).
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. Available online: https://tinyurl.com/3hw35ev5 (accessed on 15 October 2024). [CrossRef]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Teske, P.R.; Papadopoulos, I.; Mmonwa, K.L.; Matumba, T.G.; McQuaid, C.D.; Barker, N.P.; Beheregaray, L.B. Climate-driven genetic divergence of limpets with different life histories across a southeast African marine biogeographic disjunction: Different processes, same outcome. Mol. Ecol. 2011, 20, 5025–5041. [Google Scholar] [CrossRef]
- Jung, P. Revision of the Strombina-Group (Gastropoda: Columbellidae), Fossil and Living: Distribution, Biostratigraphy, and Systematics; Birkhäuser: Basel, Switzerland, 1989. [Google Scholar]
- Bouckaert, R.; Heled, J.; Kuehnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. Available online: http://www.biomedcentral.com/1471-2148/7/214 (accessed on 15 October 2024). [CrossRef] [PubMed]
- Christiaens, J. The Limpets of Hong Kong with Descriptions of Seven New Species and Subspecies. 2002. Available online: https://tinyurl.com/2nvf5vwu (accessed on 15 October 2024).
- Iredale, T. Marine molluscs from Lord Howe Island, Norfolk Island, Australia and New Caledonia. Aust. Zool. 1940, 9, 429–443. Available online: http://creativecommons.org/licenses/by-nc-sa/3.0/ (accessed on 15 October 2024).
- Jenkins, B.; Köhler, F. Hidden in plain sight: Systematic review of Indo-West Pacific Siphonariidae uncovers extensive cryptic diversity based on comparative morphology and mitochondrial phylogenetics (Mollusca, Gastropoda). Megataxa 2024, 13, 1–217. [Google Scholar] [CrossRef]
- Liu, R. Checklist of Marine Biota of China Seas; China Science Press: Beijing, China, 2008; p. 1267. [Google Scholar]
- Pilsbry, H.A. Notices of new Japanese mollusks, II. Nautilus 1894, 8, 9–10. Available online: https://www.biodiversitylibrary.org/page/12596971 (accessed on 16 October 2024).
- Pilsbry, H.A. Notices of new Japanese land snails. Proc. Acad. Nat. Sci. Phila. 1900, 52, 381–384. Available online: https://www.jstor.org/stable/4062638 (accessed on 16 October 2024).
- Hirano, Y. Siphonaria (pulmonate limpet) survey of Japan-II. Periodicity of spawning activity in Siphonaria japonica. Publ. Seto Mar. Biol. Lab. 1980, 25, 335–342. [Google Scholar] [CrossRef][Green Version]
- Hirano, Y.; Inaba, A. Siphonaria (pulmonate limpet) survey of Japan-I. Observations on the behavior of Siphonaria japonica during breeding season. Publ. Seto Mar. Biol. Lab. 1980, 25, 323–334. [Google Scholar] [CrossRef]
- Jie, W. Taxonomy of Siphonaria Along the Coast of China and Molecular Phylogeny of Siphonarimorpha. Master’s Thesis, Xiamen University, Xiamen, China, 2015. [Google Scholar]
- Sun, Y.; Li, Q.; Kong, L.; Zheng, X. DNA barcoding of Caenogastropoda along coast of China based on the COI gene. Mol. Ecol. Resour. 2012, 12, 209–218. [Google Scholar] [CrossRef]
- Yu, S.-S.; Wang, J.; Wang, Q.-L.; Huang, X.-W.; Dong, Y.-W. DNA barcoding and phylogeographic analysis of Nipponacmea limpets (Gastropoda: Lottiidae) in China. J. Molluscan Stud. 2014, 80, 420–429. [Google Scholar] [CrossRef]
- Lisiecki, L.E.; Raymo, M.E. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 2005, 20, PA1003. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. Available online: http://www.jstor.org/stable/3083539 (accessed on 17 October 2024). [CrossRef] [PubMed]
- Jouzel, J.; Masson-Delmotte, V.; Cattani, O.; Dreyfus, G.; Falourd, S.; Hoffmann, G.; Minster, B.; Nouet, J.; Barnola, J.-M.; Chappellaz, J. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science 2007, 317, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.T.; Reid, D.G. Speciation and diversity on tropical rocky shores: A global phylogeny of snails of the genus Echinolittorina. Evolution 2004, 58, 2227–2251. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. Available online: https://www.mbari.org/wp-content/uploads/2016/01/Folmer_94MMBB.pdf (accessed on 17 October 2024).
- Banks, M.A.; Hedgecock, D.; Waters, C. Discrimination between closely related Pacific oyster species (Crassostrea) via mitochondrial DNA sequences coding for large subunit rRNA. Mol. Mar. Biol. Biotechnol. 1993, 2, 129–136. Available online: https://europepmc.org/article/med/8103411 (accessed on 17 October 2024).
- Vân Le, H.L.; Lecointre, G.; Perasso, R. A 28S rRNA-based phylogeny of the gnathostomes: First steps in the analysis of conflict and congruence with morphologically based cladograms. Mol. Phylogenetics Evol. 1993, 2, 31–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).