Development of DNA Markers for Acute Hepatopancreatic Necrosis Disease Tolerance in Litopenaeus vannamei through a Genome-Wide Association Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Samples
2.2. Bacterial Culture
2.3. Mortality Determination and Sample Collection of Shrimp Infected with VpAHPND
2.4. Sequencing and Variant Calling Analysis
2.5. Genome-Wide Association Analysis for AHPND Tolerance
3. Results
3.1. Determination of Shrimp Mortality after VpAHPND Infection
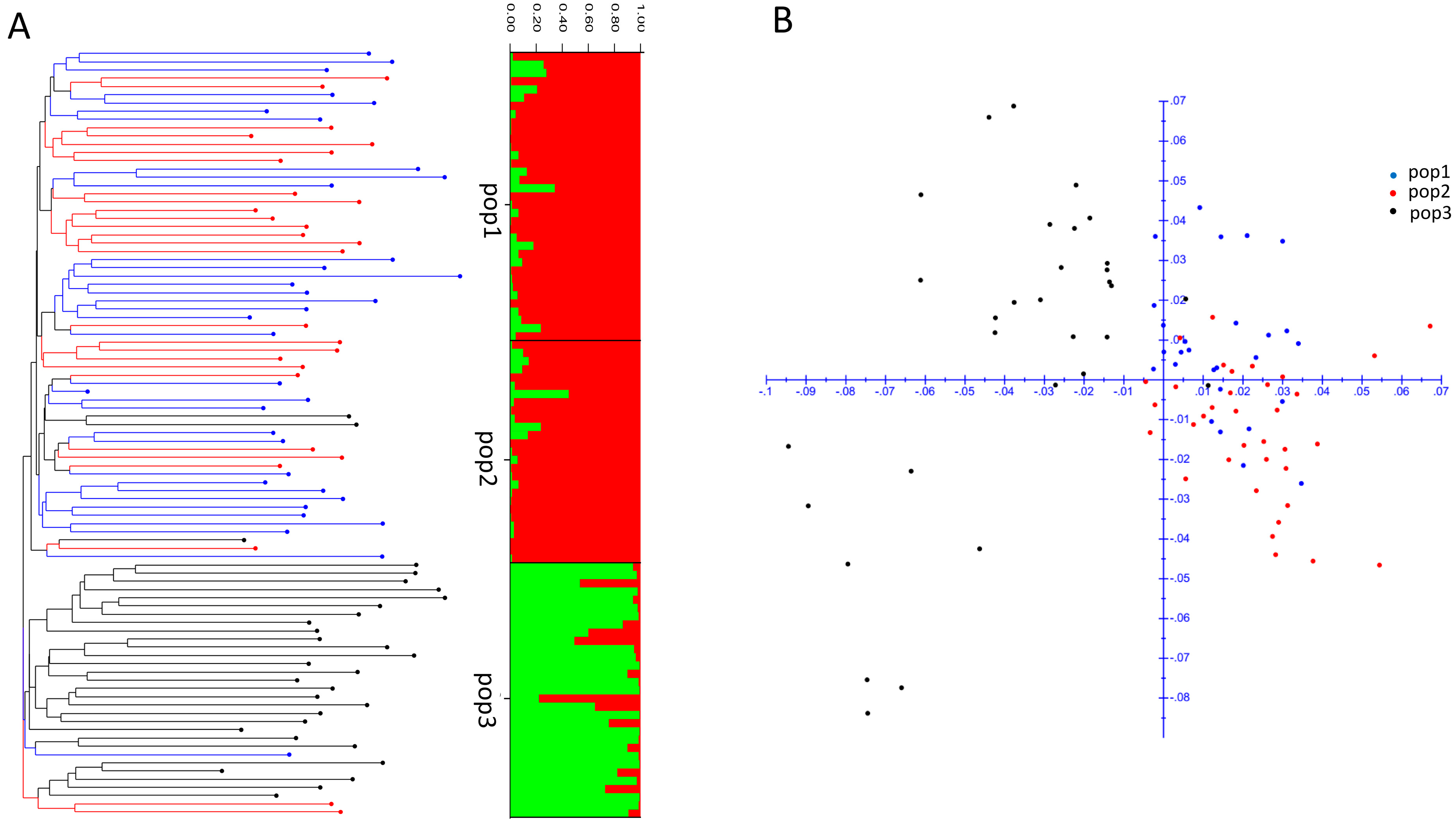
3.2. DArTSeq and Genetic Diversity among the Three Populations of Tested Shrimp
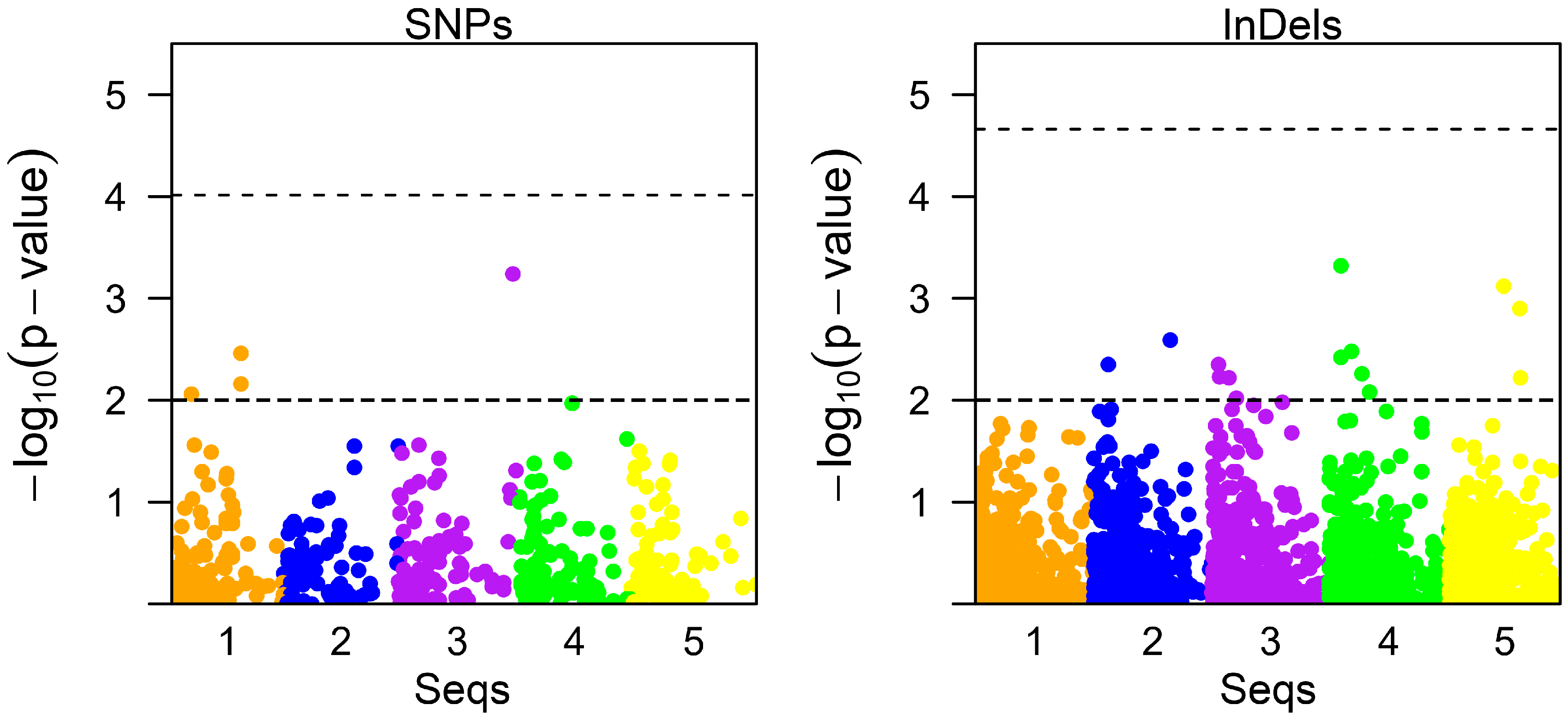
3.3. Association Analysis for AHPND Tolerance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Roy, S.; Behera, B.K.; Bossier, P.; Das, B.K. Acute hepatopancreatic necrosis disease (AHPND): Virulence, pathogenesis and mitigation strategies in shrimp aquaculture. Toxins 2021, 13, 524. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Zenger, K.R.; Khatkar, M.S.; Jones, D.B.; Khalilisamani, N.; Jerry, D.R.; Raadsma, H.W. Genomic selection in aquaculture: Application, limitations and opportunities with special reference to marine shrimp and pearl oysters. Front. Genet. 2019, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, M. Genomics in animal breeding from the perspectives of matrices and molecules. Hereditas 2023, 160, 20. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Shan, X.; Shi, Q. Research advances in the genomics and applications for molecular breeding of aquaculture animals. Aquaculture 2020, 526, 735357. [Google Scholar] [CrossRef]
- Yáñez, J.M.; Barría, A.; López, M.E.; Moen, T.; Garcia, B.F.; Yoshida, G.M.; Xu, P. Genome-wide association and genomic selection in aquaculture. Rev. Aquac. 2023, 15, 645–675. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Hamilton, A.; Tinch, A.E.; Guy, D.R.; Gharbi, K.; Steer, M.J.; Matika, O.; Bishop, S.C.; Houston, R.D. Genome wide association and genomic prediction for growth traits in juvenile farmed Atlantic salmon using a high density SNP array. BMC Genom. 2015, 16, 969. [Google Scholar] [CrossRef]
- Lyu, D.; Yu, Y.; Wang, Q.; Luo, Z.; Zhang, Q.; Zhang, X.; Xiang, J.; Li, F. Identification of growth-associated genes by genome-wide association study and their potential application in the breeding of Pacific white shrimp (Litopenaeus vannamei). Front. Genet. 2021, 12, 611570. [Google Scholar] [CrossRef]
- Medrano-Mendoza, T.; García, B.F.; Caballero-Zamora, A.; Yáñez, J.M.; Montoya-Rodríguez, L.; Quintana-Casares, J.C.; Durán-Auilar, M.; Campos-Montes, G.R. Genetic diversity, population structure, linkage disequilibrium and GWAS for resistance to WSSV in Pacific white shrimp (Litopenaeus vannamei) using a 50K SNP chip. Aquaculture 2023, 562, 738835. [Google Scholar] [CrossRef]
- Jones, D.B.; Nguyen, H.T.; Khatkar, M.S.; Simma, D.B.; Jerry, D.R.; Raadsma, H.W.; van der Steen, H.; Prochaska, J.; Zenger, K.R. The identification of a major sex QTL in the white-leg shrimp, Litopenaeus vannamei. Aquaculture 2020, 529, 735673. [Google Scholar] [CrossRef]
- Lyu, D.; Yu, Y.; Zhang, Q.; Luo, Z.; Wang, Q.; Xiang, J.; Li, F. Estimating genetic parameters for resistance to Vibrio parahaemolyticus with molecular markers in Pacific white shrimp. Aquaculture 2020, 527, 735439. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, Y.; Wang, Q.; Liu, F.; Luo, Z.; Zhang, C.; Zhang, X.; Huang, H.; Xiang, J.; Li, F. Identification of single nucleotide polymorphisms related to the resistance against acute hepatopancreatic necrosis disease in the Pacific white shrimp Litopenaeus vannamei by target sequencing approach. Front. Genet. 2019, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, Y.; Zhang, Q.; Zhang, X.; Yuan, J.; Huang, H.; Xiang, J.; Li, F. A novel candidate gene associated with body weight in the Pacific white shrimp Litopenaeus vannamei. Front. Genet. 2019, 10, 520. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, Z.; Wang, Q.; Zhang, Q.; Zhang, X.; Xiang, J.; Li, F. Development of high throughput SNP genotyping approach using target sequencing in Pacific white shrimp and its application for genetic study. Aquaculture 2020, 528, 735549. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Ge, H.; Wang, G.; Huang, S.; Yang, Z. SNP development in Penaeus vannamei via next-generation sequencing and DNA pool sequencing. Fishes 2021, 6, 36. [Google Scholar] [CrossRef]
- Sansaloni, C.; Petroli, C.; Jaccoud, D.; Carling, J.; Detering, F.; Grattapaglia, D.; Kilian, A. Diversity Arrays Technology (DArT) and next-generation sequencing combined: Genome-wide, high throughput, highly informative genotyping for molecular breeding of Eucalyptus. BMC Proc. 2011, 5, P54. [Google Scholar] [CrossRef]
- Mujyambere, V.; Adomako, K.; Olympio, O.S. Effectiveness of DArTseq markers application in genetic diversity and population structure of indigenous chickens in Eastern Province of Rwanda. BMC Genom. 2024, 25, 193. [Google Scholar] [CrossRef]
- Ladejobi, O.; Mackay, I.J.; Poland, J.; Praud, S.; Hibberd, J.M.; Bentley, A.R. Reference genome anchoring of high-density markers for association mapping and genomic prediction in European winter wheat. Front. Plant Sci. 2019, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- Mogga, M.; Sibiya, J.; Shimelis, H.; Lamo, J.; Yao, N. Diversity analysis and genome-wide association studies of grain shape and eating quality traits in rice (Oryza sativa L.) using DArT markers. PLoS ONE 2018, 13, e0198012. [Google Scholar] [CrossRef]
- Nowak, B.; Tomkowiak, A.; Bocianowski, J.; Sobiech, A.; Bobrowska, R.; Kowalczewski, P.Ł.; Bocianowska, M. The use of DArTseq technology to identify markers linked to genes responsible for seed germination and seed vigor in maize. Int. J. Mol. Sci. 2022, 23, 14865. [Google Scholar] [CrossRef]
- Curtolo, M.; Cristofani-Yaly, M.; Gazaffi, R.; Takita, M.A.; Figueira, A.; Machado, M.A. QTL mapping for fruit quality in Citrus using DArTseq markers. BMC Genom. 2017, 18, 289. [Google Scholar] [CrossRef] [PubMed]
- Guppy, J.L.; Jones, D.B.; Kjeldsen, S.R.; Le Port, A.; Khatkar, M.S.; Wade, N.M.; Sellars, M.J.; Steinig, E.J.; Raadsma, H.W.; Jerry, D.R.; et al. Development and validation of a RAD-Seq target-capture based genotyping assay for routine application in advanced black tiger shrimp (Penaeus monodon) breeding programs. BMC Genom. 2020, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data [Online]. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 15 January 2022).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Van der Auwera, G.A.; O’Connor, B.D. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra, 1st ed.; O’Reilly Media: Newton, MA, USA, 2020. [Google Scholar]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Perrier, X.; Flori, A.; Bonnot, F. Data Analysis Methods. In Genetic Diversity of Cultivated Tropical Plants; Science Publishers: Enfield, UK, 2003; pp. 43–76. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z. GAPIT version 3: Boosting power and accuracy for genomic association and prediction. Genom. Proteom. Bioinform. 2021, 19, 629–640. [Google Scholar] [CrossRef]
- Shim, H.; Chasman, D.I.; Smith, J.D.; Mora, S.; Ridker, P.M.; Nickerson, D.A.; Krauss, R.M.; Stephens, M. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS ONE 2015, 10, e0120758. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.M.; Farjana, N.; Afroz, R.; Hasan-Uj-Jaman; Saha, P.K.; Roy, H.S.; Rahman, M.A.; Farid, M.A. Genes expression in Penaeus monodon of Bangladesh; challenged with AHPND-causing Vibrio parahaemolyticus. Fish Shellfish. Immunol. Rep. 2023, 4, 100092. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Stevens, A.M.; Smith, S.A.; Taylor, D.P.; Kuhn, D.D. Strain and dose infectivity of Vibrio parahaemolyticus: The causative agent of early mortality syndrome in shrimp. Aquac. Res. 2017, 48, 3719–3727. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; Morales-Covarrubias, M.S. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl. Environ. Microbiol. 2015, 81, 1689–1699. [Google Scholar] [CrossRef]
- Sui, J.; Luan, S.; Cao, J.; Dai, P.; Meng, X.; Luo, K.; Chen, B.; Tan, J.; Fu, Q.; Kong, J. Genomic signatures of artificial selection in fecundity of Pacific white shrimp, Penaeus vannamei. Front. Genet. 2022, 13, 929889. [Google Scholar] [CrossRef]
- Fu, S.; Liu, J. Genome-wide association study identified genes associated with ammonia nitrogen tolerance in Litopenaeus vannamei. Front. Genet. 2022, 13, 961009. [Google Scholar] [CrossRef]
- Vu, N.T.T.; Zenger, K.R.; Silva, C.N.S.; Guppy, J.L.; Jerry, D.R. Population structure, genetic connectivity, and signatures of local adaptation of the giant black tiger shrimp (Penaeus monodon) throughout the Indo-Pacific region. Genome Biol. Evol. 2021, 13, evab214. [Google Scholar] [CrossRef]
- Farhadi, A.; Pichlmueller, F.; Yellapu, B.; Lavery, S.; Jeffs, A. Genome-wide SNPs reveal fine-scale genetic structure in ornate spiny lobster Panulirus ornatus throughout Indo-West Pacific Ocean. ICES J. Mar. Sci. 2022, 79, 1931–1941. [Google Scholar] [CrossRef]
- Santos, C.A.; Andrade, S.C.S.; Freitas, P.D. Identification of SNPs potentially related to immune responses and growth performance in Litopenaeus vannamei by RNA-seq analyses. PeerJ 2018, 6, e5154. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, W.; Ma, G.; Liang, L.; Shi, Q.; Tao, S. Patterns of insertion and deletion in Mammalian genomes. Curr. Genom. 2007, 8, 370–378. [Google Scholar] [CrossRef]
- Fernandes Júnior, G.A.; de Oliveira, H.N.; Carvalheiro, R.; Cardoso, D.F.; Fonseca, L.F.S.; Ventura, R.V.; de Albuquerque, L.G. Whole-genome sequencing provides new insights into genetic mechanisms of tropical adaptation in Nellore (Bos primigenius indicus). Sci. Rep. 2020, 10, 9412. [Google Scholar] [CrossRef] [PubMed]
- Uffelmann, E.; Huang, Q.Q.; Munung, N.S.; de Vries, J.; Okada, Y.; Martin, A.R.; Martin, H.C.; Lappalainen, T.; Posthuma, D. Genome-wide association studies. Nat. Rev. Methods Primers 2021, 1, 59. [Google Scholar] [CrossRef]
- Wang, M.; Jin, S.; Liu, S.; Fu, H.; Zhao, Y.; Jiang, L. Genome-wide association study of growth and sex traits provides insight into heritable mechanisms underlying growth development of Macrobrachium nipponense (Oriental River prawn). Biology 2023, 12, 429. [Google Scholar] [CrossRef]
- Evangelou, E.; Ioannidis, J. Meta-analysis methods for genome-wide association studies and beyond. Nat. Rev. Genet. 2013, 14, 379–389. [Google Scholar] [CrossRef]
- Zhao, R.; Kundu, P.; Saha, A.; Chatterjee, N. Heterogeneous transfer learning for building high-dimensional generalized linear models with disparate datasets. arXiv 2023, arXiv:2312.12786. [Google Scholar] [CrossRef]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellá, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef]
- Rakhra, G.; Rakhra, G. Zinc finger proteins: Insights into the transcriptional and post transcriptional regulation of immune response. Mol. Biol. Rep. 2021, 48, 5735–5743. [Google Scholar] [CrossRef] [PubMed]
- Almodóvar-Payá, A.; Villarreal-Salazar, M.; de Luna, N.; Real-Martínez, A.; Andreu, A.L.; Martín, M.A.; Arenas, J.; Lucia, A.; Vissing, J.; Krag, T.; et al. Preclinical research in glycogen storage diseases: A comprehensive review of current animal models. Int. J. Mol. Sci. 2020, 21, 9621. [Google Scholar] [CrossRef]
- Kumar, R.; Huang, J.Y.; Ng, Y.S.; Chen, C.Y.; Wang, H.C. The regulation of shrimp metabolism by the white spot syndrome virus (WSSV). Rev. Aquac. 2022, 14, 1150–1169. [Google Scholar] [CrossRef]
- Addison, W.N.; Pellicelli, M.; St-Arnaud, R. Dephosphorylation of the transcriptional cofactor NACA by the PP1A phosphatase enhances cJUN transcriptional activity and osteoblast differentiation. J. Biol. Chem. 2019, 294, 8184–8196. [Google Scholar] [CrossRef]
- Li, S.; Peng, W.; Chen, X.; Geng, X.; Sun, J. Identification and characterization of nascent polypeptide-associated complex alpha from Chinese mitten crab (Eriocheir sinensis): A novel stress and immune response gene in crustaceans. Fish Shellfish. Immunol. 2016, 48, 54–61. [Google Scholar] [CrossRef] [PubMed]

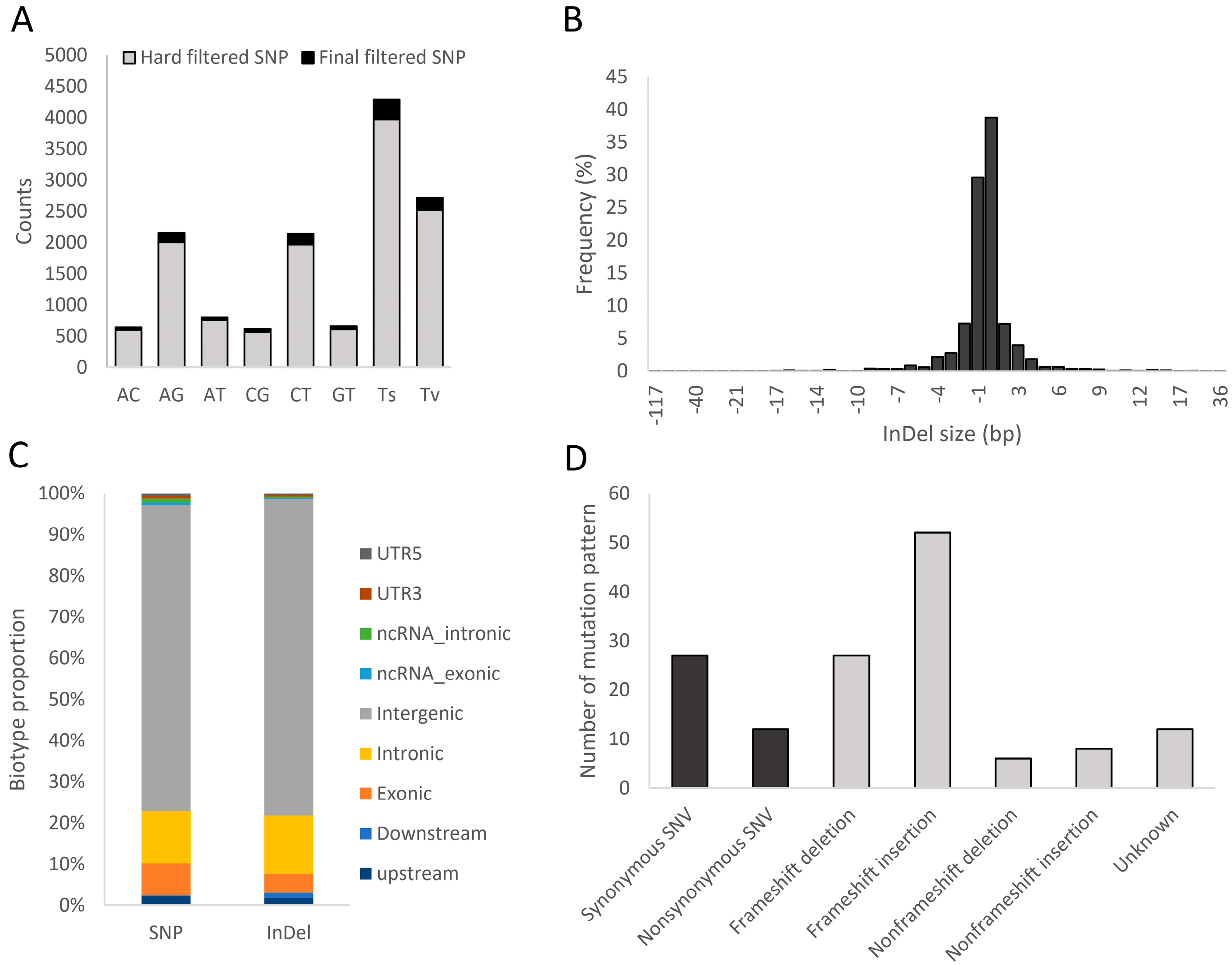
| Description | SNPs | InDels |
|---|---|---|
| Total variants | 108,983 | 17,212 |
| Hard-filtered variants | 53,083 | 15,557 |
| Final filtered variants | 516 | 2292 |
| SNP Id | Biotype | Genome Accession No. | Position | p-Value | MAF | Effect | PVE (%) | Gene Name |
|---|---|---|---|---|---|---|---|---|
| SNP2515 C > A | Intergenic | NW_020869786.1 | 872,951 | 0.0022 | 0.12 | −3.09 | 6.42% | - |
| SNP3827 A > C | Intergenic | NW_020870527.1 | 844,041 | 0.0033 | 0.19 | −2.31 | 6.43% | - |
| SNP6128 A > T | Intronic | NW_020872696.1 | 875,105 | 0.0040 | 0.05 | −3.72 | 9.29% | Uncharacterized (XM_027350909) |
| SNP2501 T > C | Exonic (Synonymous) | NW_020869780.1 | 528,035 | 0.0097 | 0.18 | −2.14 | 7.07% | Zinc finger protein 239-like (XM_027364481) |
| Candidate Markers | Ins/Del Gap (nt) | Biotype | Accession No. | Position | p-Value | MAF | Effect | PVE (%) | Gene Name |
|---|---|---|---|---|---|---|---|---|---|
| Indel0773 | Ins 2 | Intergenic | NW_020868718.1 | 1,222,723 | 2.4 × 10−4 | 0.17 | −2.53 | 9.88% | - |
| Indel3486 | Del 1 | Intergenic | NW_020870028.1 | 97,508 | 4.8 × 10−4 | 0.31 | −2.17 | 4.78% | - |
| Indel4880 | Ins 2 | Intergenic | NW_020870775.1 | 455,677 | 7.5 × 10−4 | 0.11 | −2.87 | 13.25% | - |
| Indel4460 | Del 1 | Intergenic | NW_020870542.1 | 589,428 | 1 × 10−3 | 0.17 | −2.32 | 4.83% | - |
| Indel4461 | Ins 1 | Intergenic | NW_020870542.1 | 589,433 | 1 × 10−3 | 0.17 | −2.32 | 5.19% | - |
| Indel1142 | Ins 1 | Intergenic | NW_020868919.1 | 635,311 | 2.5 × 10−3 | 0.33 | −1.72 | 5.26% | - |
| Indel3675 | Ins 1 | Intergenic | NW_020870115.1 | 185,999 | 3.3 × 10−3 | 0.065 | 3.11 | 8.86% | - |
| Indel3485 | Ins 1 | Intergenic | NW_020870028.1 | 97,483 | 3.8 × 10−3 | 0.31 | −1.79 | 2.97% | - |
| Indel2533 | Ins 1 | Intergenic | NW_020869537.1 | 56,216 | 3.8 × 10−3 | 0.20 | 2.09 | 3.83% | - |
| Indel3811 | Ins 1 | Intergenic | NW_020870177.1 | 120,506 | 4.5 × 10−3 | 0.12 | −2.45 | 10.66% | - |
| Indel0506 | Del 1 | Intronic | NW_020868577.1 | 271,787 | 5.5 × 10−3 | 0.065 | 2.93 | 10.73% | probable phosphorylase b kinase regulatory subunit alpha (XM_027376870) |
| Indel2406 | Ins 1 | Intergenic | NW_020869477.1 | 65,359 | 5.9 × 10−3 | 0.27 | −1.73 | 5.06% | - |
| Indel3386 | Del 1 | Exonic (Frameshift deletion) | NW_020869981.1 | 594,057 | 5.9 × 10−3 | 0.11 | −2.42 | 14.42% | nascent polypeptide-associated complex subunit alpha, muscle-specific form-like(XM_027366427) |
| Indel6073 | Del 1 | Intergenic | NW_020871916.1 | 143,341 | 6 × 10−3 | 0.65 | 2.95 | 11.85% | - |
| Indel6600 | Ins 6 | Intergenic | NW_020872438.1 | 335,379 | 8.2 × 10−3 | 0.19 | −1.88 | 5.92% | - |
| Indel2592 | Ins 1 | Intergenic | NW_020869574.1 | 204,883 | 9.5 × 10−3 | 0.30 | −1.68 | 8.24% | - |
| Indel2590 | Del 3 | Intergenic | NW_020869574.1 | 204,798 | 9.6 × 10−3 | 0.26 | −1.87 | 2.95% | - |
| Candidate Markers | Distance (kb) | Gene Name (RefSeq Accession No.) | Function |
|---|---|---|---|
| SNP2515 | Up 169.9 | uncharacterized LOC113812673 (XM_027364589) | - |
| Down 95.7 | uncharacterized LOC113812685 (XM_027364609) | - | |
| SNP3827 | Up 357.7 | vegetative cell wall protein gp1-like (XM_027371696) | Extracellular region |
| Indel0773 | Up 64.4 | proline-rich extensin-like protein EPR1 (XM_027353909) | Extracellular region |
| Down 70.7 | uncharacterized LOC113803169 (XM_027353894) | - | |
| Indel3486 | Down114.7 | Zinc finger protein 749-like (XM_027366949) | Transcription |
| Indel4880 | Up 29.2 | Mucin-2-like (XM_027374070) | Extracellular region |
| Indel4460 | Up 128 | Receptor-type guanylate cyclase Gyc76C-like (XM_027371820) | Immune response |
| Indel4461 | Up 128 | Receptor-type guanylate cyclase Gyc76C-like (XM_027371820) | Immune response |
| Indel1142 | Up 15.5 | probable Ras GTPase-activating protein (XM_027355753) | Tumorigenesis |
| Down 90.5 | uncharacterized LOC113804845 (XM_027355756) | - | |
| Indel3675 | Up 50.2 | putative neural-cadherin 2 (XM_027367808) | Cell-cell adhesion |
| Indel3485 | Down 114.8 | zinc finger protein 749-like (XM_027366949) | Transcription |
| Indel2533 | Up 30.5 | prolyl 4-hydroxylase subunit alpha-1-like (XM_027362215) | Collagen synthesis |
| Down 190.4 | basic salivary proline-rich protein 2-like (XM_027362206) | Extracellular region | |
| Indel3811 | Up 70.9 | nephrin-like (XM_027368425) | Secretory system |
| Indel2406 | Down 100.5 | uncharacterized histidine-rich protein DDB_G0274557-like (XM_027361657) | - |
| Indel6073 | Up 77.9 | Trnal-cag_17 | Translation |
| Down 10.2 | cell wall protein DAN4-like (XM_027379940) | Extracellular region | |
| Indel6600 | Up 190.5 | cell surface glycoprotein 1-like (XM_027381906) | Extracellular region |
| Indel2592 | Up 80.9 | Trnaf-gaa_48 | Translation |
| Down 49.9 | uncharacterized LOC113810924 (XM_027362580) | - | |
| Indel2590 | Up 80.9 | Trnaf-gaa_48 | Translation |
| Down 49.9 | uncharacterized LOC113810924 (XM_027362580) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whankaew, S.; Suksri, P.; Sinprasertporn, A.; Thawonsuwan, J.; Sathapondecha, P. Development of DNA Markers for Acute Hepatopancreatic Necrosis Disease Tolerance in Litopenaeus vannamei through a Genome-Wide Association Study. Biology 2024, 13, 731. https://doi.org/10.3390/biology13090731
Whankaew S, Suksri P, Sinprasertporn A, Thawonsuwan J, Sathapondecha P. Development of DNA Markers for Acute Hepatopancreatic Necrosis Disease Tolerance in Litopenaeus vannamei through a Genome-Wide Association Study. Biology. 2024; 13(9):731. https://doi.org/10.3390/biology13090731
Chicago/Turabian StyleWhankaew, Sukhuman, Phassorn Suksri, Ammara Sinprasertporn, Jumroensri Thawonsuwan, and Ponsit Sathapondecha. 2024. "Development of DNA Markers for Acute Hepatopancreatic Necrosis Disease Tolerance in Litopenaeus vannamei through a Genome-Wide Association Study" Biology 13, no. 9: 731. https://doi.org/10.3390/biology13090731
APA StyleWhankaew, S., Suksri, P., Sinprasertporn, A., Thawonsuwan, J., & Sathapondecha, P. (2024). Development of DNA Markers for Acute Hepatopancreatic Necrosis Disease Tolerance in Litopenaeus vannamei through a Genome-Wide Association Study. Biology, 13(9), 731. https://doi.org/10.3390/biology13090731








