Simple Summary
The tubers of Gastrodia elata, used in traditional medicine for over two thousand years, are highly susceptible to fungal infections, making tuber rot a common issue during its cultivation. This study investigated a significant outbreak of brown rot disease affecting G. elata f. elata in Hunan Province, China. Typical symptoms include initial brown spots on the tuber surface that expand and darken, with the spots spreading inward and causing the surrounding tissue to transition from brown to black. In this study, the fungal pathogen causing brown rot disease on G. elata was isolated and examined in detail for morphological characteristics, including color, sporulation structures, and spore size. Its identification was further supported by phylogenetic analysis. The findings indicated that the fungal pathogen causing brown rot disease on G. elata f. elata was Clonostachys rosea.
Abstract
Gastrodia elata, commonly known as Tian Ma, is a perennial mycoheterotrophic orchid. Qianyang Tian Ma (QTM), a geographical indication agricultural product from Hongjiang City, Hunan Province, China, is primarily characterized by the red variety, G. elata f. elata. A severe outbreak of tuber brown rot disease was documented in QTM during the harvesting season in Hunan. The fungal pathogen associated with the disease was isolated on potato saccharose agar (PSA) and identified through morphological and phylogenetic analyses. Pathogenicity tests were performed on healthy tubers of G. elata f. elata. The results showed that the representative isolate, named TMB, produced white hyphal colonies with a ring structure, broom-like phialides, partially curved ellipsoidal conidia, and orange–yellow spherical ascocarps on PSA. Phylogenetic analysis of the ITS, LSU, rpb2 and tub2 sequences using Bayesian and maximum-likelihood methods identified the isolate TMB as Clonostachys rosea, based on morphological and phylogenetic data. Pathogenicity tests revealed typical disease symptoms on healthy G. elata tubers 15 days post-inoculation with the isolate TMB. C. rosea is known to cause diseases in economically important crops, but there are no reports of its occurrence on G. elata f. elata in China. This study provides valuable insights into the occurrence, prevention, and control of brown rot disease in G. elata f. elata.
1. Introduction
Gastrodia elata Bl., commonly known as Tian Ma, is a perennial herbaceous plant in the Orchidaceae family with highly degenerated roots and leaves, relying on the mycelium of the parasitic fungus Armillaria mellea for nutrition after seed germination due to its inability to absorb soil nutrients or perform photosynthesis [1,2]. It is distributed from the Russian Far East through China, Japan, Korea, Southeast Asia, and countries south of the Himalayas, extending to India, New Guinea, Australia, New Caledonia, Sri Lanka, and Madagascar. In China, G. elata is known by various names such as Chi Jian, Du Yao Zhi, He Li Cao, and Bai Long Pi and is recognized as a traditional medicinal plant [3]. It is widely distributed across the Yunnan, Sichuan, Guizhou, Shaanxi, and Jilin provinces, as well as the eastern and northwestern regions of Liaoning, predominantly growing at altitudes ranging from 400 to 3200 m in sparse forests, forest clearings, forest edges, and shrub fringes [4]. Based on quality and color, G. elata can be classified into nine synonyms: G. elata f. elata, G. elata f. viridis, G. elata f. flavida, G. elata f. glauca, G. elata f. alba, G. elata f. pilifera, G. elata var. gracilis, G. elata var. pallens, and G. elata var. viridis [2]. The tuber of G. elata, which has been used medicinally for over two thousand years, is known for containing various chemical compounds, with the primary active ingredients being identified as gastrodin, polysaccharides, p-hydroxybenzyl alcohol (gastrodigenin), and others [2,3,5]. Gao et al. (2022) reported that the active components of G. elata alleviated depression in CUMS-induced mice by reducing hippocampal neuronal apoptosis, enhancing 5-HT and DA levels, lowering Ca2+ concentration and MAO activity, and modulating the expression of BDNF and NMDAR compared to controls [6]. Gan et al. (2024) found that G. elata polysaccharide significantly alleviated motor dysfunction, inhibited α-synuclein accumulation, and reduced dopaminergic neuron loss in mice with Parkinson’s disease by increasing the Bcl-2/Bax ratio, decreasing cleaved-caspase-3 levels, and reducing GFAP, TNF-α, IL-1β, and IL-6 levels through the TLR4/NF-κB pathway [7]. Wu et al. (2024) demonstrated that G. elata polysaccharide protected against myocardial injury in sleep-deprived mice by inhibiting ferroptosis via SIRT6, as evidenced by reduced ROS levels, improved oxidative stress markers, and the modulation of key ferroptosis-related proteins, with these effects reversed by the SIRT6 inhibitor OSS_128167 [8]. Fu et al. (2023) demonstrated that Gas-miR2-3p, a specific miRNA derived from G. elata, alleviated neuroinflammation by downregulating inflammatory factors such as TNF-α, IL-6, and IL-1β and by inhibiting the activation of the NF-κB signaling pathway [9]. Yu X et al. (2024) revealed that p-hydroxybenzaldehyde (p-HBA), one of the main active components of G. elata, enhanced antioxidant effects, delayed aging, and helped in the treatment and prevention of Alzheimer’s disease by reducing reactive oxygen species (ROS), lowering lipofuscin levels, and inhibiting β-amyloid aggregation [10]. Additionally, G. elata is highly nutritious, with its tubers being rich in protein, starch, and dietary fiber while being low in fat and also containing high levels of trace elements such as cobalt, iron, manganese, zinc, and selenium, which are essential for maintaining health and supporting metabolism [5,11,12].
The tubers of G. elata are susceptible to infection by a variety of fungal species, with tuber rot being a prevalent issue during both cultivation and growth [13]. Li et al. (2022) identified Fusarium redolens as the pathogen causing tuber rot in G. elata in Guizhou Province, China, with infection rates ranging from 10% to 25% [14]. In 2018, root rot disease in G. elata from Gimcheon, Korea, was confirmed to be caused by F. oxysporum through morphological characteristics and molecular analysis [15]. Li et al. (2022) reported that Botrytis cinerea is responsible for causing gray mold on the flowers of G. elata [16]. Tang et al. (2022) used ITS and 16S rDNA gene analysis to identify microorganisms associated with tuber rot in G. elata, revealing that the genus Ilyonectria, particularly I. cyclaminicola and I. robusta, is closely related to the disease, with an isolation frequency of 42.0% [17]. These fungi not only cause tuber rot in G. elata but also inhibit the growth of the symbiotic fungus A. mellea. Overall, G. elata tuber diseases can be categorized into two main types: spot rot and wet rot. Spot rot is characterized by surface dehydration, wrinkling, and the formation of small black spots that progressively enlarge, resulting in a blackened appearance with internal necrosis along the central vascular bundles. Wet rot features water-soaked lesions without rust spots, leading to rapid tuber decay and a foul odor.
In Hunan Province, the renowned Qianyang Tian Ma (QTM), primary cultivar G. elata f. elata, was designated as a geographical indication agricultural product of Hongjiang City in 2020, with its geographical protection scope encompassing a broad area within Hongjiang City, covering 84 administrative villages in 16 townships and towns, including Anjiang Town, Taiping Township, Chatou Township, Maodu Township, Qiancheng Town, Jiangshi Town, Xuefeng Town, Tangwan Town, Tieshan Township, Qunfeng Township, Wanxi Township, Xima Township, Dachong Township, Shuping Township, Longchuantang Yao Township, Shendu Miao Township, as well as Xuefeng Mountain forest farm and Bamian Mountain farm [18]. In addition to local markets in Hunan, the product is also exported to nearby regions such as Guizhou and Guangxi, and even to Southeast Asia [19]. In recent years, the expansion and prolongation of G. elata cultivation have intensified problems related to continuous cropping systems. Among these issues, brown rot has emerged as a particularly severe disease. In its early stages, brown rot manifests as near-circular dark-brown lesions on QTM, which progressively enlarge, leading to significant reductions in both yield and quality.
This research investigates the pathogen responsible for brown rot disease in QTM, combining morphological observations with sequence analysis of genes such as ITS, LSU, rpb2, and tub2 to identify the pathogen species. Koch’s postulates are then applied to confirm the pathogenicity of the isolated fungus and verify it as the cause of brown rot in G. elata. This study provides a theoretical foundation for biological control methods and supports the development of QTM.
2. Materials and Methods
2.1. Materials
Diseased tubers of QTM were collected from the Xuefeng Mountains, Shuitian County of Hunan Province in southern China, located at an elevation of 700 m, 27.60° N, 110.20° E.
2.2. Fungal Isolation and Cultivation
In total, 12 diseased specimens were gathered from three different locations for laboratory testing. Tissues containing the infected sections of the specimens were sterilized using 75% ethanol for 1 min, followed by immersion in 2.5% hypochlorite for 45 s. After sterilization, the symptomatic samples were rinsed three times with sterile water, placed on potato saccharose agar plates, and incubated at 25 °C for 48 h [20]. The representative isolate, named TMB, was selected for study. The morphological characteristics of the colonies were documented, followed by the examination of pathogen morphology, color, sporulation structure, and measurement of spore size under a light microscope.
All the experiments were conducted in triplicate by default, with the spore size measured for 50 samples. Statistical significance was determined using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The results are presented as mean ± standard deviation (SD).
2.3. Molecular Phylogenetic Analyses
A 1 cm × 1 cm clot was inoculated into 50 mL of PSA liquid medium in a 250 mL flask and incubated at 25 °C and 150 r/min for 48 h. The hyphae were collected by centrifugation at 9800× g for 5 min, and the DNA extraction protocol was as follows: 200 mg of fresh mycelium was powdered using a mortar and pestle in liquid nitrogen, mixed with 500 µL of SDS extraction buffer (containing 100 mM Tris-HCl, 500 mM NaCl and 50 mM EDTA, pH8.5), and subsequently incubated at 65 °C for 10 min. Next, 150 µL of 5 mol/L KAc was added, followed by chilling on ice for 20–30 min. Centrifugation at 12,000 r/min for 10 min separated the supernatant, which was then mixed with 0.7 volumes of pre-cooled isopropanol for precipitation at 4 °C for 30 min. Genomic DNA precipitation was recovered by centrifugation. After air-drying, the DNA was dissolved in sterilized ddH2O. An equal volume of phenol and chloroform mixture was added, followed by centrifugation at 12,000 r/min for 8 min. The supernatant was treated by adding 2 volumes of pre-cooled ethanol, and precipitation was allowed to occur at 4 °C for 30 min. Genomic DNA was recovered post-centrifugation, and the precipitate was washed twice with 400 µL of 70% ethanol. Finally, the genomic DNA was lysed using 200 µL of sterile ddH2O.
The internal transcribed spacer (ITS) region (including the partial ITS1 sequence, the 5.8S, and ITS2 complete sequences), the large subunit (LSU) rRNA gene, the RNA polymerase II second largest subunit gene (rpb2), and the beta-tubulin gene (tub2) were amplified from strain TMB using the primer pairs ITS5/ITS4, LROR-2/LR7, rpb2-7cR/5f2, and Bt2a/Bt2b, respectively [21,22]. PCR amplification was conducted with a 25 μL reaction volume comprising 1 μL of template DNA, 9.5 μL of ddH2O, 12.5 μL of 2 × T5 Super PCR Mix, and 1 μL each of forward and reverse primers.
Subsequently, multiple sequence alignments were carried out using ClustalX 2.1 [20]. The ITS, LSU, rpb2, and tub2 sequences were combined via SequenceMatrix 1.8. The compatibility among the four genes was assessed using PAUP 4.0b10 (Swofford, 2002), indicating that the phylogenetic signals for the four loci were consistent (p = 0.05), suggesting they align well in the phylogenetic tree. The best-fit model and parameters were calculated with MrModelTest 2.3, and a phylogenetic analysis was conducted employing the Bayesian (MrBayes-3.2.7a) and maximum-likelihood (RAxML 1.0) methods [23]. Branches with bootstrap support values of at least 50% for maximum likelihood (ML) and Bayesian posterior probabilities (BPPs) of 0.70 or higher were considered significantly supported [24,25].
2.4. Pathogenicity Testing
Pathogenicity assessments were performed on healthy QTM tubers to fulfill Koch’s postulates. Three tubers were initially surface-sterilized using 75% ethanol and then inoculated by spraying the entire tubers until run-off with a spore suspension of isolate TMB (1 × 106 conidia/mL), while three other tubers served as controls, sprayed with sterile water. After soaking, all the tubers were placed in sterile soil, kept in darkness, and incubated for 15 days at 25 °C.
3. Results
3.1. Investigation of Brown Rot Disease in QTM
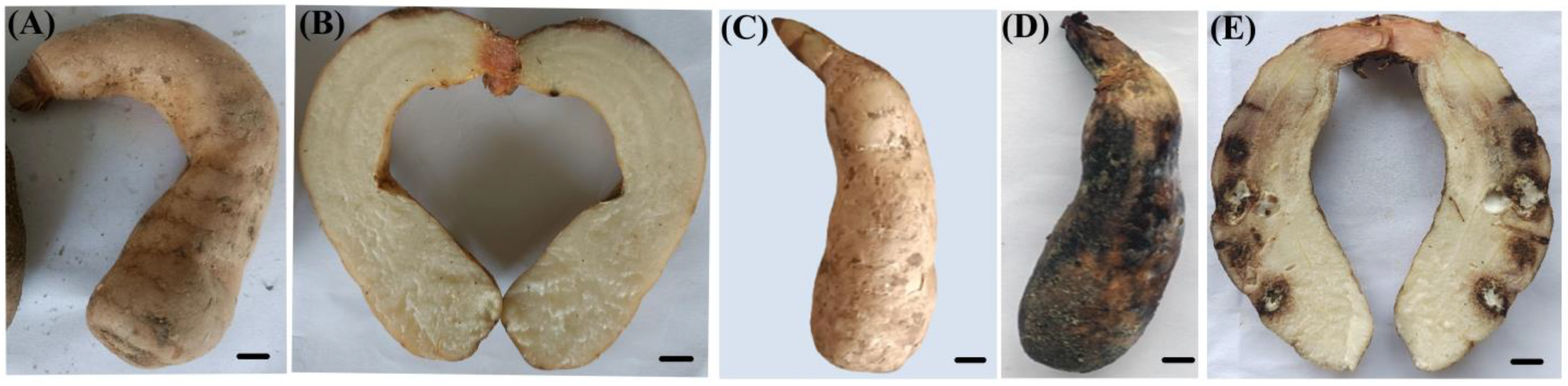
Qianyang Tian Ma (QTM), a geographically indicated agricultural product from Hongjiang City in Huaihua, Hunan Province, China, is renowned for its substantial economic and medicinal value. The survey on Qianyang Tian Ma from 2020 to 2023 showed that the crop failure rate ranged from 10% to 25%, while the rot rate ranged from 15% to 55%. Healthy QTM tubers have intact yellow outer skins, whereas infected ones display prominent brown spots on the outer skin in the infected areas, often accompanied by varying degrees of cavities or lesions. The pathogen infiltrates inward through the thin-walled tissues, causing the surrounding tissue to transition from brown to black, ultimately resulting in the entire tuber becoming blackened (Figure 1).

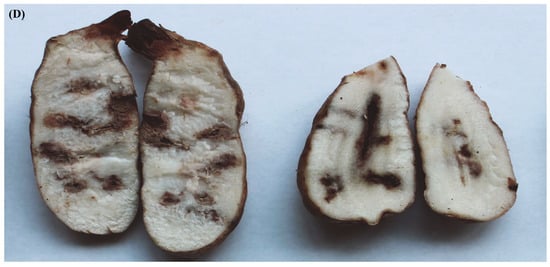
Figure 1.
Brown rot disease in QTM. (A) Pathogen-contaminated fungal material spreading disease to QTM (as indicated by the red arrows); (B) infected QTM; (C,D) internal anatomical sections of diseased QTM.
3.2. Morphological Characteristics of the Pathogen
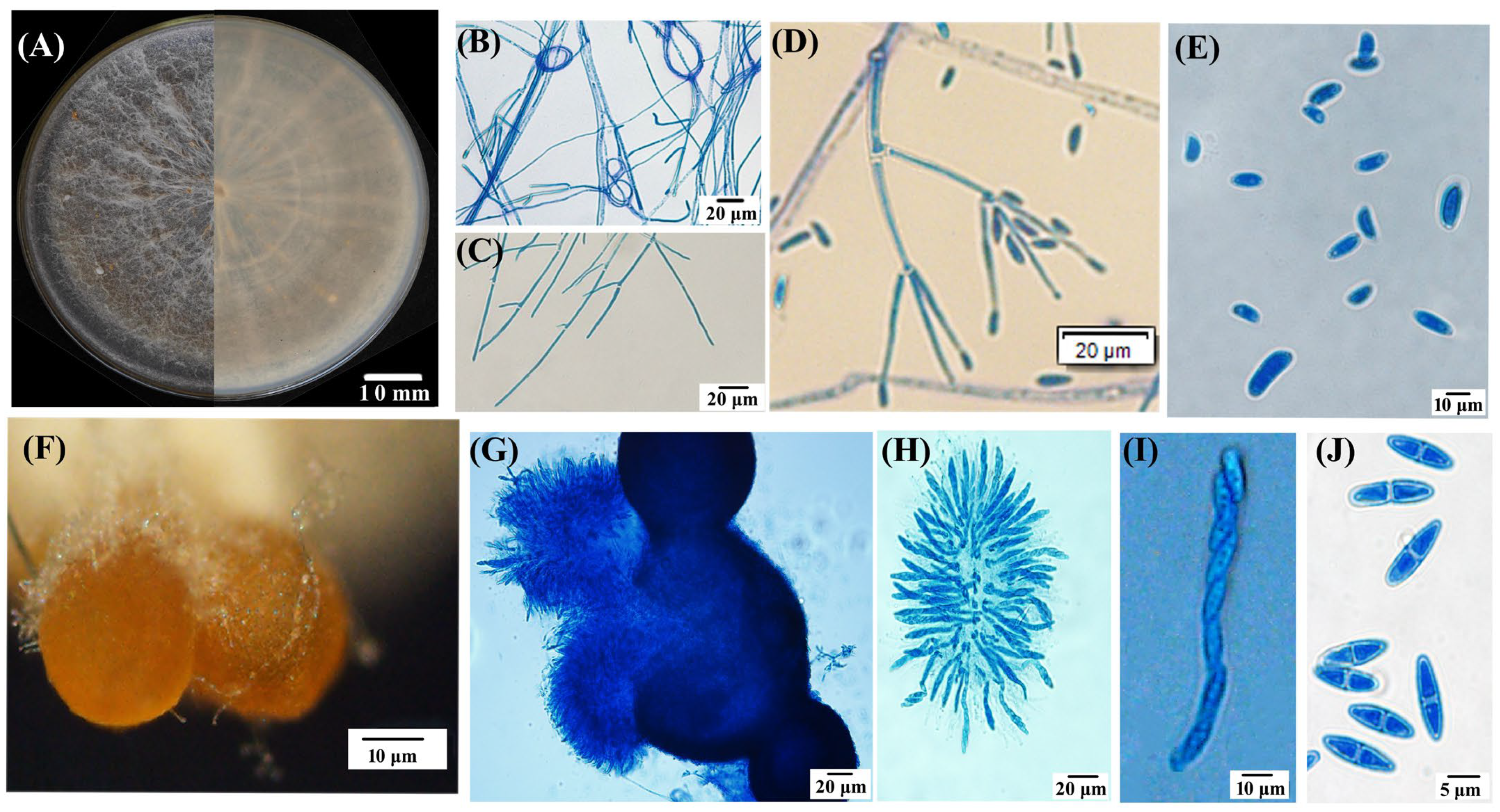
Nine out of the twelve fungal isolates separated from symptomatic lesions exhibited a similar morphology. The isolate TMB, as a representative, was used for this study. The isolate TMB cultured on a PSA medium for 11 days exhibited slow growth with concentric rings, smooth edges, a predominantly white color with a slight yellow pigment on the reverse side, sparse woolly mycelium, and a measured colony diameter of 6.1 ± 0.3 cm. After 21 days, the aerial mycelium decreased, and brown, granular ascocarp clusters began to appear (Figure 2A). The TMB mycelium possessed septate hyphae and a ring-like structure (Figure 2B,C). The PSA agar blocks covered with mycelium were submerged in sterile water for hydroponic culture, and after one week of incubation at 28 °C in darkness, secondary conidia developed on conidiophores that branched like brooms, exhibiting slightly swollen tips and small protrusions; the conidia were elliptical, slightly curved, and varied in size, 7.8–13.0 × 3.9–7.8 μm (x = (11.2 ± 2.2) × (5.4 ± 1.1) μm, n = 50) (Figure 2D,E). The ascocarps of TMB were approximately spherical, yellow–brown to brown in color, with a diameter ranging from 25.1 to 40.0 μm, often clustered together and attached to white mycelium at the base (Figure 2F). Upon maturation, the ascocarps increase in size, rupture, and release ascospore masses. Each ascus typically contains eight smooth, septate, spindle-shaped ascospores (6.8–10.7 × 2.4–3.9 μm (x = (9.41 ± 1.2) × (3.56 ± 3.12) μm, n = 50), as illustrated in Figure 2G–J.
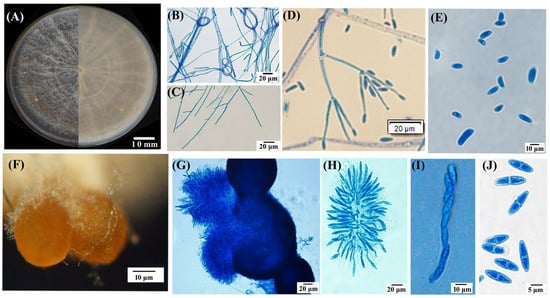
Figure 2.
Morphological characteristics of the representative isolate TMB. (A) Colonies cultured on PSA medium for 25 days, showing both front and reverse views; (B) hyphal ring structure; (C) hyphal morphology; (D) primary (Verticillium-like) conidiophores and conidia; (E) conidial morphology; (F) aggregated perithecia grown on a PSA plate; (G) perithecium structure; (H) asci; (I,J) ascospores. Scale bar: (A) = 10 mm; (B–D,G,H) = 20 μm; (E,F,I) = 10 μm; (J) = 5 μm.
3.3. Phylogenetic Analyses
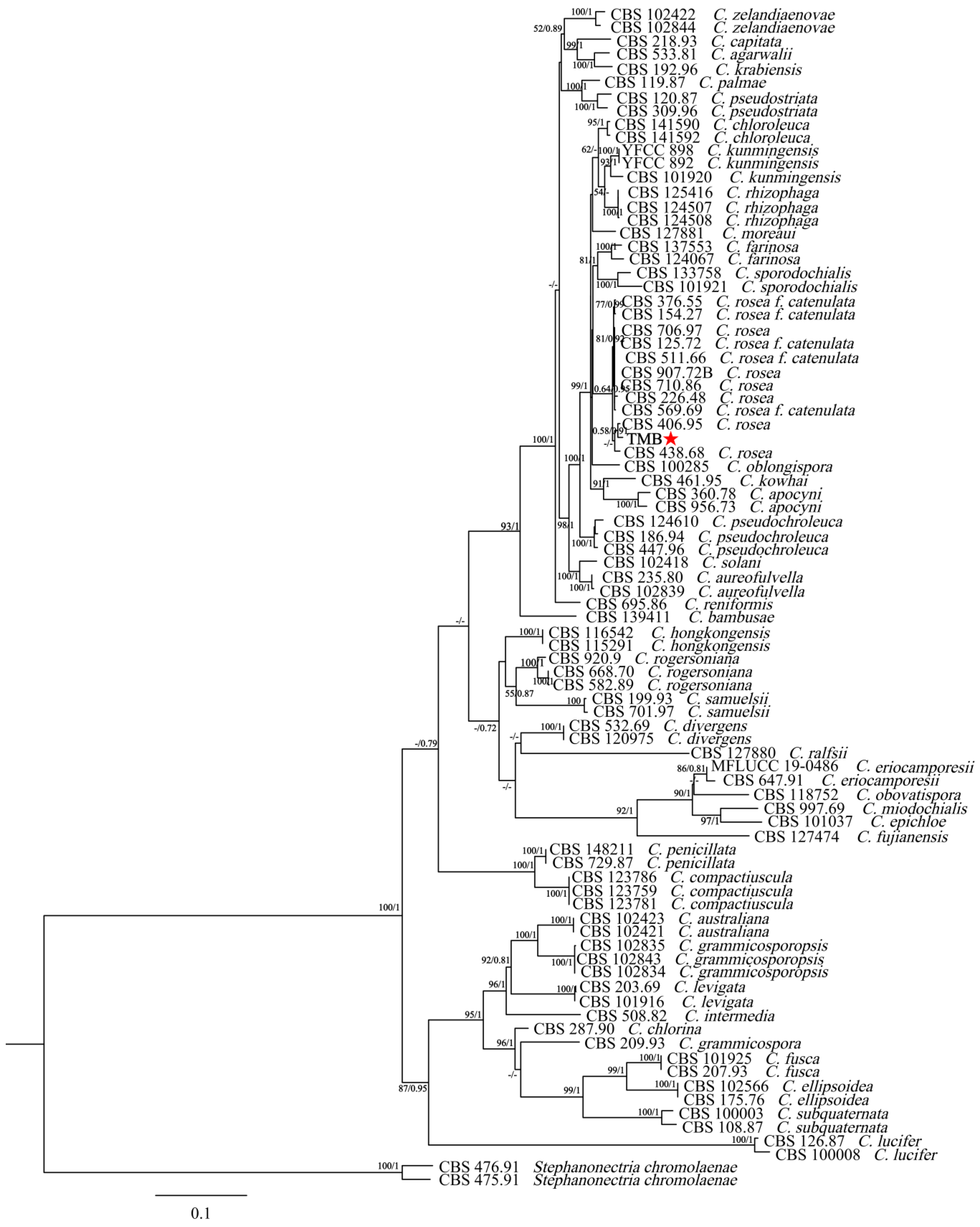
The new sequence data for the TMB isolate have been deposited in GenBank under the following accession numbers: ITS (PP994883), LSU (PP994885), rpb2 (PQ009939), and tub (PQ009940). The species, specimens, and GenBank accession numbers of sequences used in this study are detailed in Table 1. The ITS + nLSU + rpb2 + tub2 dataset included sequences from 86 fungal specimens representing 43 species. The dataset had an aligned length of 2535 characters that were parsimony informative. The maximum-likelihood (ML) analysis tree was generated using RAxML1.3.1 with the GTR+I+G model parameters determined by MrModelTest, based on 1000 bootstrap replicates. The Bayesian inference (BI) analysis was conducted using MrBayes v3.2.7a for 2 million generations, yielding an average standard deviation of split frequencies of 0.006685. The result indicated good convergence of the analysis, suggesting that the posterior probability distribution is reliable and the phylogenetic tree generated is robust. Figure 3 illustrates that the dataset, comprising 42 species of the genus Clonostachys, included the isolate TMB, which, along with C. rosea, formed a separate clade from the other species with strong support (100% ML bootstrap and 1.00 BPP). The genetic distance between the isolate TMB and C. rosea was the shortest among other Clonostachys species. Additionally, TMB had the smallest genetic distance with CBS 406.95 C. rosea, and the node at which the isolate TMB and CBS 406.95 C. rosea belonged to the same branch had a maximum-likelihood support rate (ML/BI) over 50%, with a Bayesian posterior probability of 0.91. Morphological and phylogenetic analyses revealed that the isolate TMB, distinguished by its unique characteristics and phylogenetic profile, was classified as Clonostachys rosea.

Table 1.
Species, specimens, and GenBank accession number of sequences used in this study.
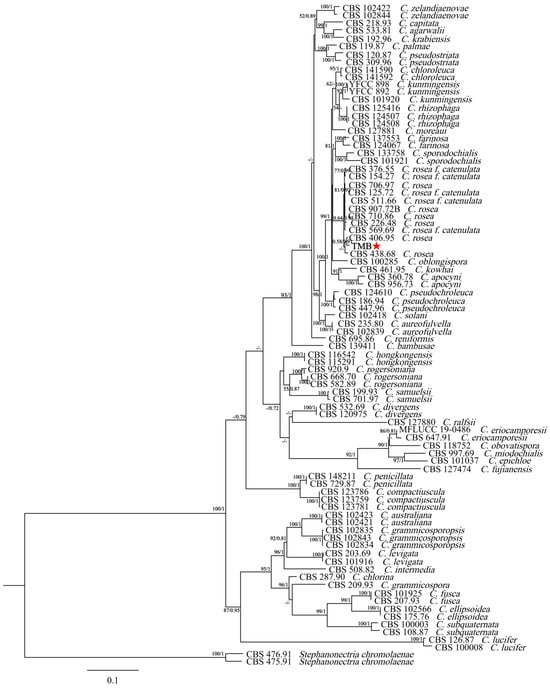
Figure 3.
A phylogenetic tree was generated using Bayesian inference and maximum-likelihood analyses of the combined ITS + LSU + rpb2 + tub2 dataset. Branch support values (ML/BI) are indicated for nodes with more than 50% bootstrap support and Bayesian posterior probabilities (BPPs) exceeding 0.70. Isolates in this study are highlighted in bold type and marked with a red asterisk.
3.4. Pathogenicity Results
After 15 days of cultivation, the G. elata tubers were removed from the culture bottles. As depicted in Figure 4, the infected tubers lost their normal fresh color, shifting from light yellow to dark brown. Compared to the control group, the affected areas were notably dull, showing yellow–brown or dark-brown discoloration. Mucilage secreted on the surface of the tubers adhered to the surrounding soil particles. After cutting open the tubers, as shown in Figure 4, brown patches with a central white mycelium were visible inside. The natural disease symptoms were consistent with those induced under laboratory conditions through inoculation. In the control group, no disease symptoms were observed. Isolates reisolated from the inoculated tubers displayed morphological characteristics identical to the original isolate TMB and were confirmed to be molecularly identical. Therefore, it can be concluded that TMB (Clonostachys rosea) causes rot in G. elata tubers.
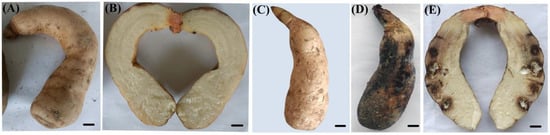
Figure 4.
Pathogenicity test of the isolate TMB on G. elata tubers. (A,B) The control tuber was inoculated with sterile water; (C) healthy tuber before inoculation with conidia of C. rosea; (D,E) healthy tuber inoculated with conidia of C. rosea after 15 days. Scale bar = 1 cm.
4. Discussion
The cultivation area for Qianyang Tian Ma (QTM) is located in the Xuefeng Mountain region of Hunan. The Xuefeng Mountain region in Hunan, situated within the Xuefeng Mountain Range (25°95′ N–29°40′ N, 109°41′ E–112°68′ E), represents the largest mountain range in Hunan and constitutes a major geographic boundary between the second and third topographic steps in China, extending from the Hunan–Guangxi border in the south to Yiyang in the north and oriented in a northeast–southwest direction [26]. The main peak of the Xuefeng Mountain Range, Subao Peak, reaches an elevation of 1934 m and has an annual average temperature of 10–19 °C. July temperatures average approximately 18 °C, providing optimal environmental conditions for the cultivation of G. elata. Huaihua City has a long history of G. elata cultivation, with extensive planting in counties such as Tongdao, Jingzhou, Huitong, Hongjiang, Zhongfang, and Xupu along the Xuefeng Mountain Range, where local residents continue to employ traditional planting techniques and practices [27].
This study focused on investigating the pathogenic fungus responsible for QTM tuber brown rot disease. The morphological characteristics of the representative isolate TMB were analogous to the features of Clonostachys rosea (Link) Schroers, Samuels, Seifert & W. Gams (1999: 373) [28]. Further molecular identification and validation using Koch’s postulates confirmed that the isolated representative TMB identified as Clonostachys rosea was responsible for the decaying QTM tubers. C. rosea was initially documented as a pathogen causing root rot in G. elata in 2020, specifically in Chuncheon City, Korea (latitude 37°52′ N, longitude 127°44′ E) [29], characterized by a temperate monsoon climate with typically lower annual precipitation, generally below 800 mm, and shorter rainy seasons [30,31]. In contrast, the QTM studied in Huaihua City, Hunan (latitude 25°52′22″–29°01′25″ N, longitude 108°47′13″–111°06′30″ E), grows in a subtropical monsoon climate, characterized by mean temperatures above 0 °C in the coldest months, annual precipitation exceeding 800 mm, and longer rainy seasons [32,33]. The annotated genome sequence of Korean G. elata (GenBank accession number MN026874) revealed a chloroplast genome 74 bp shorter than that of Chinese G. elata, with significant sequence variants including 495 SNPs and 75 InDels, in which insertions occurred in seven protein-coding genes (clpP, matK, rps12, rpl2, rpl16, ycf, and ycf2) and deletions in four genes (clpP, rpl16, rps12, and ycf2) [34]. Additionally, while Lee et al. (2020) reported C. rosea as the pathogen responsible for root rot in Korean G. elata, their study did not include any experimental images, such as those showing the characteristics of C. rosea, the infection process, or the pathogen testing results [29]. In contrast, our study on brown rot in QTM provides a detailed account of the entire experimental procedure and a systematic analysis of the isolate TMB identification, thereby offering a more comprehensive and accurate scientific basis for understanding brown rot in G. elata.
The colony morphology, ascocarps, conidia, and ascospores of the isolate TMB are similar to those described for C. rosea by Schroers et al. [35]. The colony reverse of the isolate TMB on PDA medium appears pale orange, with perithecia containing asci bundles densely crowded on a well-developed stroma, occasionally solitary and arising directly from the mycelium, forming after 21 d of incubation at 25 °C. Compared to the type strain CBS 710.86, the primary (Verticillium-like) conidiophores of the isolate TMB had more branching, with two to three levels of branching, and were dispersed at slightly less-acute angles. The primary (Verticillium-like) conidia of isolate TMB range from 7.8–13.0 × 3.9–7.8 μm, whereas those of the type strain CBS 710.86 range from 4.2–6.6 × 2.0–3.4 μm, indicating that TMB conidia are larger and more elongated than those of CBS 710.86. The ascospores of isolate TMB measure 6.8–10.7 × 2.4–3.9 μm, while those of CBS 710.86 range from 7.4–14.4 × 2.2–4.8 μm, making TMB ascospores shorter and thicker compared to those of CBS 710.86. Compared to isolate CBS 406.95 (after 7 days of cultivation), the isolate TMB displays fewer conidiophore branches, and secondary conidiophores have not yet been observed. Our ML and Bayesian results indicate that C. rosea grouped with C. farinosa, C. sporodochialis, and C. oblongispora, consistent with the phylogenetic tree of the genus Clonostachys constructed by Wang et al. [21]. The isolate TMB clustered with C. rosea, forming a distinct branch with the shortest genetic distance among Clonostachys species. Moreover, TMB had the closest genetic distance to C. rosea CBS 406.95, with their shared branch showing a maximum-likelihood support rate (ML/BI) over 50% and a Bayesian posterior probability of 0.91. There were several base pair differences between the isolate TMB and CBS 406.95 in the sequences, including three base pair differences in the ITS sequences, three base pair differences in the LSU sequences, two base pair differences in the tub2 gene, and four base pair differences in the rpb2 gene.
Currently, C. rosea is extensively used in scientific research and agricultural practices as a biocontrol agent against pests and fungal diseases. However, there are relatively few reports on its pathogenicity in plants, underscoring the need for further research to ensure its safe and effective use. G. elata, which primarily grows underground, is exposed to soil-borne pathogens over extended periods, making it an important model for studying these pathogens. The current research reports indicate that C. rosea can infect a range of hosts (as shown Table 2), including barley (Hordeum vulgare L. var. nudum Hook. f.) [36], faba bean (Vicia faba) in Tarom County, northwestern Iran [37], sugar beet (Beta vulgaris) [38], avocado (Persea americana) [39], and others. These findings suggest that C. rosea has a broad host range but also emphasize the need for a more thorough understanding of its pathogenic mechanisms. Despite its significant potential as a biocontrol agent, its potential pathogenicity needs further evaluation to prevent any adverse effects on agricultural production. Future research should focus on elucidating the pathogenic mechanisms of C. rosea, its ecological adaptability, and its effectiveness across different crops. Such studies are crucial for optimizing its use as a biocontrol agent and ensuring its safe and effective application in agriculture.

Table 2.
Plant pathogenic diseases induced by Clonostachys rosea.
5. Conclusions
To manage disease risks effectively, G. elata cultivation should be conducted in uncultivated lands with minimal indigenous fungal populations, implemented with regular crop rotation and avoidance of consecutive planting with susceptible crops. Before planting, the soil should undergo deep tillage. In our experimental study, we found that C. rosea exhibits high spore production and strong reproductive capabilities, which facilitates its potential transmission during the asexual reproduction of G. elata. Therefore, it is recommended to select seed tubers that are free from wounds and disease spots to minimize the risk of infection. Additionally, sexual reproduction in G. elata carries a lower risk of disease and allows the plant to inherit beneficial traits from the parent. Thus, promoting sexual reproduction in G. elata could be an effective strategy for managing brown rot disease.
Author Contributions
Conceptualization, J.Z. and H.Y.; Data curation, H.Y., L.P. and K.L.; Formal analysis, T.H. and J.S.; Investigation, J.Z. and L.P.; Software, H.Y. and K.L.; Visualization, L.P., H.Y. and K.L.; Writing—original draft, H.Y., T.H., B.S. and J.S.; Validation, L.P. and K.L.; Writing—review and editing, T.H., B.S., J.S. and J.Z.; Resources, J.Z., L.P., B.S. and J.S.; Project administration, H.Y. and K.L.; Supervision, J.Z. and T.H.; Funding acquisition, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Foundation of Hunan Double First-rate Discipline Construction Projects of Bioengineering (SWGC-03), Science and Technology Plan Project of Huaihua City (No. 2021R3127).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the relevant data used in this study are included in this manuscript. The corresponding author can be contacted if any further information is needed.
Acknowledgments
We are grateful to the editors and anonymous reviewers for their valuable comments and reviews.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, W.J.; Li, B.F. The biological relationship of Gastrodia elata and Armillaria Melle. J. Integr. Plant. Biol. 1980, 22, 57–62, 111. [Google Scholar]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata Blume—An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia Committee of China. Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Medical Science and Technology Press: Beijing, China, 2020; pp. 121–122. [Google Scholar]
- Guo, Y.; Mo, K.; Wang, G.; Zhang, Y.; Zhang, W.; Zhou, J.; Sun, Z. Analysis of prediction and spatial-temporal changes of suitable distribution of Gastrodiae rhizoma under future climate conditions. Chin. J. Inf. TCM 2022, 29, 1–7. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, R.; Li, H.; Liao, X.; Tang, X.; Wang, X.; Su, Z. How steaming and drying processes affect the active compounds and antioxidant types of Gastrodia elata Bl. f. glauca S. Chow. Food Res. Int. 2022, 157, 111277. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wu, Y.; Yang, L.; Chen, F.; Li, L.; Li, Q.; Wang, Y.; Li, L.; Peng, M.; Yan, Y.; et al. Anti-depressant-like effect of fermented Gastrodia elata Bl. by regulating monoamine levels and BDNF/NMDAR pathways in mice. J. Ethnopharmacol. 2023, 301, 115832. [Google Scholar] [CrossRef]
- Gan, Q.X.; Peng, M.Y.; Wei, H.B.; Chen, L.L.; Chen, X.Y.; Li, Z.H.; An, G.Q.; Ma, Y.T. Gastrodia elata polysaccharide alleviates Parkinson’s disease via inhibiting apoptotic and inflammatory signaling pathways and modulating the gut microbiota. Food Funct. 2024, 15, 2920–2938. [Google Scholar] [CrossRef]
- Wu, Y.; Miao, Y.; Cao, Y.; Gong, Z. Gastrodin prevents myocardial injury in sleep-deprived mice by suppressing ferroptosis through SIRT6. Naunyn Schmiedebergs Arch. Pharmacol. 2024. Available online: https://link.springer.com/article/10.1007/s00210-024-03230-4#citeas (accessed on 1 August 2024). [CrossRef]
- Fu, J.; Lu, Z.T.; Wu, G.; Yang, Z.C.; Wu, X.; Wang, D.; Nie, Z.M.; Sheng, Q. Gastrodia elata specific miRNA attenuates neuroinflammation via modulating NF-κB signaling pathway. Int. J. Neurosci. 2023, 15, 1–11. [Google Scholar] [CrossRef]
- Yu, X.; Tao, J.; Xiao, T.; Duan, X. P-hydroxybenzaldehyde protects Caenorhabditis elegans from oxidative stress and β-amyloid toxicity. Front. Aging Neurosci. 2024, 16, 1414956. [Google Scholar] [CrossRef]
- Xie, M.; Tao, W.; Wu, F.; Wu, K.; Huang, X.; Ling, G.; Zhao, C.; Lv, Q.; Wang, Q.; Zhou, X.; et al. Anti-hypertensive and cardioprotective activities of traditional Chinese medicine-derived polysaccharides: A review. Int. J. Biol. Macromol. 2021, 185, 917–934. [Google Scholar] [CrossRef]
- He, D.; Yang, X.; Hu, J.; Chi, H.; Lu, N.; Liu, Y.; Hu, K.; Yang, S.; Wen, X. Determination of trace cobalt in Gastrodia elata Bl. by effervescent tablet-assisted hydrophobic deep eutectic solvent microextraction combined with micro spectrophotometry. Microchem. J. 2024, 196, 109627. [Google Scholar] [CrossRef]
- Qiao, M.; Jing, T.; Wan, Y.; Yu, Z. Analyses of multilocus sequences and morphological features reveal Ilyonectria species associated with black rot disease of Gastrodia elata. Plant Dis. 2024, 108, 382–397. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.; Li, J.; Huang, M.; Shao, X.; Yang, Z. Fusarium redolens causes black rot disease in Gastrodia elata grown in China. Crop Prot. 2022, 155, 105933. [Google Scholar] [CrossRef]
- Lee, S.A.; Bae, E.K.; An, C.; Kang, M.J.; Park, E.J. Fusarium oxysporum causes root rot on Gastrodia elata in Korea: Morphological, phylogenetic, and pathogenicity analyses. Korean J. Mycol. 2022, 50, 41–46. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Yang, Z.; Li, C. Botrytis cinerea causes flower gray mold in Gastrodia elata in China. Crop Prot. 2022, 155, 105923. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.Q.; Jiang, W.K.; Yuan, Q.S.; Wang, Y.H.; Guo, L.P.; Yang, Y.; Yang, Y.; Zhou, T. Isolation, identification, and pathogenicity research of brown rot pathogens from Gastrodia elata. China J. Chin. Mater. Medica 2022, 47, 2288–2295. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China Announcement No. 378. Available online: http://www.moa.gov.cn/nybgb/2021/202101/202109/t20210917_6376748.htm (accessed on 11 June 2024). (In Chinese)
- Hongjiang City Awarded the Title of “Hometown of Tianma,” Qianyang Tianma Recognized as Remarkable. Available online: https://m.voc.com.cn/xhn/news/202304/17984504.html (accessed on 5 April 2024).
- Zou, J.; Yao, H.; Lei, T.; Chen, Z.; Su, X.; Liu, S. Epicoccum sorghinum causing leaf spot on Polygonatum cyrtonema in China. Plant Dis. 2024, 18, 1398. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, D.X.; Luo, R.; Wang, Y.B.; Thanarut, C.; Dao, V.M.; Yu, H. Phylogeny and systematics of the genus Clonostachys. Front. Microbiol. 2023, 14, 1117753. [Google Scholar] [CrossRef]
- Moreira, G.M.; Abreu, L.M.; Carvalho, V.G.; Schroers, H.J.; Pfenning, L.H. Multilocus phylogeny of Clonostachys subgenus Bionectria from Brazil and description of Clonostachys chloroleuca sp. nov. Mycol. Prog. 2016, 15, 1031–1039. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Dyer, K.A. Bayesian estimation of positively selected sites. J. Mol. Evol. 2004, 58, 661–672. [Google Scholar] [CrossRef]
- Shao, H.Q.; Wang, Z.F. Spatial distribution characteristics and influencing factors of health and wellness tourism resources in Xuefeng mountain area of Hunan Province. J. Nat. Sci. Hunan Norm. Univ. 2022, 45, 44–54. Available online: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=202202288407147826 (accessed on 1 August 2024). (In Chinese).
- Xuefeng Tianma Flourishes Anew: Huaihua Celebrates the First Harvest Festival of Xuefeng Tianma. Available online: https://m.voc.com.cn/xhn/news/202311/18977820.html (accessed on 7 April 2024). (In Chinese).
- Schroers, H.J.; Samuels, G.J.; Seifert, K.A.; Gams, W. Classification of the mycoparasite Gliocladium roseum in Clonostachys as C. rosea, its relationship to Bionectria ochroleuca, and notes on other Gliocladium-like fungi. Mycologia 1999, 91, 365–385. [Google Scholar] [CrossRef]
- Lee, S.A.; Kang, M.J.; Kim, T.D.; Park, E.J. First report of Clonostachys rosea causing root rot of Gastrodia elata in Korea. Plant Dis. 2020, 104, 3069. [Google Scholar] [CrossRef]
- Chung, Y.S.; Yoon, M.B.; Kim, H.S. On climate variations and changes observed in South Korea. Clim. Change 2004, 66, 151–161. [Google Scholar] [CrossRef]
- Chae, H.; Lee, H.; Lee, S.; Cheong, Y.; Um, G.; Mark, B.; Patrick, N. Local variability in temperature, humidity and radiation in the BaekduDaegan Mountain protected area of Korea. J. Mt. Sci. 2012, 9, 613–627. [Google Scholar] [CrossRef]
- Yu, G.H.; Tian, Q.J.; Yang, Y.L.; Mo, H.W. Analysis on temporal and spatial variation of FPAR in Hunan province. Environ. Earth Sci. Res. J. 2015, 2, 1–8. Available online: https://iieta.org/sites/default/files/Journals/EESRJ/02.3_01.pdf (accessed on 1 August 2024).
- Li, Z.; You, Q.; Liu, H.; Yin, Z.; Duan, L. Analysis of climate change and circulation features of frost days in Hunan Province, China in recent 67 years. J. Geosci. Environ. Prot. 2019, 7, 124–137. [Google Scholar] [CrossRef][Green Version]
- Kang, M.J.; Kim, S.C.; Lee, H.R.; Lee, S.A.; Lee, J.W.; Kim, T.D.; Park, E.J. The complete chloroplast genome of Korean Gastrodia elata Blume. Mitochondrial DNA Part B Resour. 2020, 5, 1015–1016. [Google Scholar] [CrossRef]
- Schroers, H.J. A monograph of bionectria ascomycota, Hypocreales, Bionectriaceae and its Clonostachys anamorphs. Stud. Mycol. 2001, 46, 1–214. [Google Scholar]
- Li, X.P.; Xu, S.Y.; Li, J.J.; Zhang, Y.X.; Qi, Y.H.; Wang, X.M.; Jiang, J.J.; Fan, Y.X.; Li, M.Q. Clonostachys rosea, a pathogen of root rot in naked barley (Hordeum vulgare L. var. nudum Hook. f.) on the Qinghai-Tibet Plateau, China. Microbiol. China 2022, 49, 598–605. [Google Scholar] [CrossRef]
- Afshari, N.; Hemmati, R. First report of the occurrence and pathogenicity of Clonostachys rosea on faba bean. Australas. Plant Pathol. 2017, 46, 231–234. [Google Scholar] [CrossRef]
- Farhaoui, A.; Tahiri, A.; Radouane, N.; Khadiri, M.; Amiri, S.; El Alami, N.; Lahlali, R. First report of Clonostachys rosea causing sugar beet root rot in Morocco. New Dis. Rep. 2023, 48, e12235. [Google Scholar] [CrossRef]
- Coyotl-Pérez, W.A.; Romero-Arenas, O.; Mosso-González, C.; Pacheco-Hernández, Y.; Rivera-Tapia, J.A.; Villa-Ruano, N. First report of Clonostachys rosea associated with avocado fruit rot in Puebla, Mexico. Rev. Mex. Fitopatol. 2022, 40, 298–307. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Li, S.; Zhao, X.; Abiodun, O.; Han, Z. First Report of root rot caused by Clonostachys rosea on Xanthoceras sorbifolium in China. Plant Dis. 2024, 108, 798. [Google Scholar] [CrossRef]
- Díaz, R.; Chávez, E.C.; Delgado-Ortiz, J.C.; Guerrero, J.J.V.; Roque, A.; Ochoa, Y.M. First report of Clonostachys rosea causing root rot on garlic in Mexico. Plant Dis. 2022, 106, 3000. [Google Scholar] [CrossRef]
- Ma, H.; Duan, X.; Xu, W.; Ma, G.; Ma, W.; Qi, H. Root rot of Angelica sinensis caused by Clonostachys rosea and Fusarium acuminatum in China. Plant Dis. 2022, 106, 2264. [Google Scholar] [CrossRef]
- Ling, M.; Tang, X.; Zheng, C.; Liu, J.; Wei, L.; Pan, H.; Li, Z.; Ding, H. First Report of Leaf Spot Caused by Clonostachys rosea on Tea (Camellia sinensis) in China. Plant Dis. 2023, 107, 2537. [Google Scholar] [CrossRef]
- Wang, H.; He, W.; Chen, J.; Fang, Q.; Zhou, X.; Li, Q.; Wu, P.; Luo, B. First Report of Clonostachys rosea causing bulb rot on Fritillaria taipaiensis in China. Plant Dis. 2024, 108, 2568. [Google Scholar] [CrossRef]
- Qi, H.; Duan, X.; Wen, X.; Yuan, Z.; Hai, M.; Wei, M.; Gui, M. First Report disease of Clonostachys rosea causing root rot on Astragalus membranaceus in China. Plant Dis. 2022, 106, 1752. [Google Scholar] [CrossRef]
- Bienapfl, J.C.; Floyd, C.M.; Percich, J.A.; Malvick, D.K. First Report of Clonostachys rosea causing root rot of soybean in the United States. Plant Dis. 2012, 96, 1700. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).