Study of Liposomes Containing Extract from the Leaves of Protium heptaphyllum (Aubl.) March in Animals Submitted to a Mutagenic Model Induced by Cyclophosphamide
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of Extract and Development of Liposomes
2.2. In Vivo Tests
2.2.1. Animals and Experimental Design
2.2.2. Analysis of Anthropometric Measurements
2.2.3. Micronucleus Test
2.2.4. Biochemical Analyses of Liver, Kidney, Brain, Heart, and Plasma
2.2.5. Histological Analysis
2.2.6. Immunological Analysis by ELISA
2.3. Data Analysis
3. Results
4. Discussion
5. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanno, T.Y.N.; Sensiate, L.A.; de Paula, N.A.; Salles, M.J.S. Toxic effects of different doses of cyclophosphamide on the reproductive parameters of male mice. Braz. J. Pharm. Sci. 2009, 45, 313–319. [Google Scholar] [CrossRef]
- Alrefaei, A.E.; Alzahrani, M.A.; Alsuhaim, S.A. Cyclophosphamide related toxicity; a systematic review. Int. J. Med. Dev. Ctries. 2022, 6, 740–747. [Google Scholar] [CrossRef]
- Mirkes, P.E. Cyclophosphamide teratogenesis: A review. Teratog. Carcinog. Mutagen. 1985, 5, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Nassef, M.; Abdel-Salam, S.; Alshorbagy, I.; Ata, A. Cytotoxic and anti-tumor effects of the chemotherapeutic drug cyclophosphamide in tumor bearing mice is time and dose dependent. Egypt. J. Zool. 2014, 62, 131–146. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Chaves, M.H.; Almeida, F.R.; Lima, R.C.; Silva, R.M.; Maia, J.L.; Brito, G.A.A.; Santos, F.A.; Rao, V.S. Protective effect of alpha- and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J. Ethnopharmacol. 2005, 98, 103–108. [Google Scholar] [CrossRef]
- Shen, Y.; Hao, X. Natural product sciences: An integrative approach to the innovations of plant natural products. Sci. China Life Sci. 2020, 63, 1634–1650. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K. Natural products: A lead for drug discovery and development. Phytother. Res. 2021, 35, 4660–4702. [Google Scholar] [CrossRef]
- Islam, R.; Kabir, M.F.; Alam, R.; Dahr, R.; Rana, M.N.; Islam, E.; Parvin, S.; Hossain, A. Sedative, membrane stability, cytotoxic and antioxidant properties of methanol extract of leaves of Protium serratum Wall. Asian Pac. J. Trop. Dis. 2014, 4, 928–933. [Google Scholar] [CrossRef]
- Nogueira, A.O.; Oliveira, Y.I.S.; Adjafre, B.L.; de Moraes, M.E.A.; Aragão, G.F. Pharmacological effects of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum: A literature review. Fundam. Clin. Pharm. 2019, 33, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Tafurt-García, G.; Muñoz-Acevedo, A. Volatile metabolites present in Protium heptaphyllum (Aubl.) March. collected in Tame (Arauca—Colombia). Lat. Am. Caribb. Bull. Med. Aromat. Plants 2012, 11, 223–232. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/lil-647661 (accessed on 2 June 2024).
- Bandeira, P.N.; Pessoa, O.D.L.; Trevisan, M.T.S.; Lemos, T.L.G. Secondary metabolites of Protium heptaphyllum March. Quim. Nova 2002, 25, 1078–1080. [Google Scholar] [CrossRef]
- Marques, D.D.; Sartori, R.A.; Lemos, T.L.G.; Machado, L.L.; Souza, J.S.N.; Monte, F.J.Q. Chemical composition of the essential oils from two subspecies of Protium heptaphyllum. Acta Amaz. 2010, 40, 227–230. [Google Scholar] [CrossRef]
- Maia, R.M.; Barbosa, P.R.; Cruz, F.G.; Roque, N.F.; Fascio, M. Triterpenes from the resin of Protium heptaphyllum March (Burseraceae): Characterization in binary mixtures. Quím. Nova 2000, 23, 623–626. [Google Scholar] [CrossRef]
- Patias, N.S.; Sinhorin, V.D.G.; de Moura, F.R.; da Cunha, A.P.F.; Lima, R.R.S.; Costa, R.J.; da Costa, T.B.; Cavalheiro, L.; Bicudo, R.C.; Sinhorin, A.P. Identification of flavonoids by lc-ms/ms in leaves extract from Protium heptaphyllum (Aubl.) March and antioxidant activity in mice. Nat. Prod. J. 2021, 11, 715–727. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, V.; Singh, S.K.; Dubey, A.K.; Kim, J.J. Flavonoids: Potential Candidates for the Treatment of Neurodegenerative Disorders. Biomedicines 2021, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Durairaj, V.; Hoda, M.; Shakya, G.; Babu, S.P.; Rajagopalan, R. Phytochemical screening and analysis of antioxidant properties of aqueous extract of wheatgrass. Asian Pac. J. Trop. Med. 2014, 7S1, S398–S404. [Google Scholar] [CrossRef]
- Bishnu, K.; Jyoti, S. A Review Study of Importance of Herbal Medicine. Int. J. Multidiscip. Res. 2023, 5. [Google Scholar] [CrossRef]
- Patias, N.S.; Queiroz, E.A.I.F.; Ferrarini, S.R.; Bomfim, G.F.; Aguiar, D.H.; Sinhorin, A.P.; Bello, A.A.; Silva, G.V.F.; Cavalheiro, L.; Sinhorin, V.D.G. Effect of liposomal extract of Protium heptaphyllum (Alb.) March in the treatment of obesity induced by a high-calorie diet. Biology 2024, 13, 535. [Google Scholar] [CrossRef]
- Anand, A.; Gautam, P.; Ojha, S. Application of Nanotechnology for Herbal Medicine Development: A Review. Lett. Drug Des. Discov. 2023, 21, 1325–1333. [Google Scholar] [CrossRef]
- Gad, S.; ElGogary, R.; Geneidi, A.; Hathout, R. Lipid nanocarriers encapsulating herbal drugs for brain diseases therapy. Arch. Pharm. Sci. Ain Shams Univ. 2023, 7, 60–86. [Google Scholar] [CrossRef]
- Halder, M.; Jha, S. Medicinal Plants and Bioactive Phytochemical Diversity: A Fountainhead of Potential Drugs against Human Diseases. In Medicinal Plants: Biodiversity, Biotechnology Conservation; Jha, S., Halder, M., Eds.; Springer: Singapore, 2023; Volume 33. [Google Scholar] [CrossRef]
- Hua, S.; WU, S.Y. The use of lipid-based nanocarriers for targeted pain therapies. Front. Pharmacol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.H. The pharmacological evaluation of natural products—General and specific approaches to screening ethnophar-maceuticals. J. Ethnopharmacol. 1983, 8, 127–147. [Google Scholar] [CrossRef]
- Bhat, N.; Kalthur, S.G.; Padmashali, S.; Monappa, V. Toxic Effects of Different Doses of Cyclophosphamide on Liver and Kidney Tissue in Swiss Albino Mice: A Histopathological Study. Ethiop. J. Health Sci. 2018, 28, 711–716. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, J.T.; Heddle, J.A.; Hite, M.; Margolin, B.H.; Ramel, C.; Salamone, M.F.; Tia, R.R.; Wild, D. Guidelines for the conduct of micronucleus assay in mammalian bone marrow erythrocytes. Mutat. Res. 1987, 189, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.P.; Kiesow, L.A. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H2O2 solutions in the UV). Anal. Biochem. 1972, 49, 474–478. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. Available online: https://pubmed.ncbi.nlm.nih.gov/4623845/ (accessed on 2 July 2024). [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. Available online: https://pubmed.ncbi.nlm.nih.gov/4436300/ (accessed on 16 July 2024). [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. Available online: https://pubmed.ncbi.nlm.nih.gov/6066618/ (accessed on 4 July 2024).
- Sedlack, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Roe, J.H. Chemical determination of ascorbic, dehydroascorbic, and diketogulonic acids. Methods Biochem. Anal. 1954, 1, 115–139. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Clerici, M.; Garavaglia, M.E.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1019, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.S.L.; Rissi, D.R. Down the rabbit hole: A quick guide for histopathology description. Pesq. Vet. Bras. 2021, 41, e06927. [Google Scholar] [CrossRef]
- Pereira, C.A.B.; Rabello, G.M.N. Statistical test to compare proportions in cytogenetic problems. Mutagenesis, carcinogenesis and teratogenesis: Methods and evaluation criteria. Ribeirão Preto. Braz. Soc. Genet. 1991, 113–121. [Google Scholar]
- Waters, M.D.; Brady, A.L.; Stack, H.F.; Brockman, H.E. Antimutagenicity profiles for some model compounds. Mutat. Res. 1990, 1, 57–85. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Al-Kubaisy, K.N.; Al-Essa, L.Y.; Shawagfeh, M.T. Stimulation of Hepatocytes Repair by Fruit Juice of Opuntia ficus indica in Anti Cancer Drug Cyclophosphamide (CP)-Induced Liver Toxicity in Mice. Annu. Res. Rev. Biol. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Roszkowski, S. Application of Polyphenols and Flavonoids in Oncological Therapy. Molecules 2023, 28, 4080. [Google Scholar] [CrossRef]
- Alhowail, A.; Sajid, S.; Almogbel, Y.; Rabbani, S.I.; Alsharidah, M. Effect of Cyclophosphamide and Its Combination with Metformin on the Survival Rate in Mice. J. Pharm. Res. Int. 2019, 30, 1–6. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Ojo, F.O.; Onaolapo, O.J. Biflavonoid quercetin protects against cyclophosphamide-induced organ toxicities via modulation of inflammatory cytokines, brain neurotransmitters, and astrocyte immunoreactivity. Food Chem. Toxicol. 2023, 178, 113879. [Google Scholar] [CrossRef] [PubMed]
- Zahraa, Z.A.; Haider, H.H.; Amer, A. Toxic effects of Cyclophosphamide on Hepatic and Kidney tissues in Albino Mice Model. Res. J. Pharm. Technol. 2022, 15, 4655–4659. [Google Scholar] [CrossRef]
- Ying, Y.; Hu, J.; Li, L.; Li, Y.; Wang, S.; Qu, H. The Influence of Cyclophosphamide on Growth Peripheral Blood Biochemidtry Index and Organ Coefficient of SD Rat. Chin. J. Cancer 2010, 37, 18–21. Available online: https://www.chvm.net/EN/Y2010/V37/I10/18 (accessed on 2 June 2024).
- Golan, E.D.; Tashjian, H.A.; Armstrong, J.E.; Armstrong, W.A. Princípios de Farmacologia: A Base Fisiopatológica da Farmacoterapia, 3rd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Luiz, T.C.; Cunha, A.P.S.; Aguiar, D.; Sugui, M.M.; Bicudo, R.C.; Sinhorin, A.P.; Sinhorin, V.D.G. Antioxidant potential of Carica papaya Linn (Caricaceae) leaf extract in mice with cyclophosphamide induced oxidative stress. Sci. Med. 2020, 30, e34702. [Google Scholar] [CrossRef]
- Bragante, W.; Sinhorin, V.D.G.; Sugui, M.M.; Cunha, A.P.S.; Santos, W.B.; Sinhorin, A.P. In vivo mutagenic effects and oxidative stress parameters evaluation of cypermethrin and benzoate of emamectin and their mixtures in female mice. J. Environ. Sci. Health Part B 2022, 57, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sinhorin, V.D.G.; Cordeiro Luiz, T.; Baldissera, L.; Simões, C.A.P.; Bressan, S.W.; Lopes, B.A.J.; Sugui, M.M.; Valadão, D.M.S. Prophylactic action of the isotonic drink made from the skins of the fruit of Oenocarpus bacaba Mart. (Arecaceae) against cyclophosphamide-induced oxidative stress in mice. Native 2024, 11, 251–258. [Google Scholar] [CrossRef]
- Ribeiro, L.R.; Salvadori, D.M.; Marques, E.K. Mutagênse Ambiental, 1st ed.; Editora Ulbra: Canoas, RS, Brazil, 2024; Available online: https://www.researchgate.net/publication/284581917_Mutagenese_Ambiental (accessed on 11 June 2024).
- Gomes-Carneiro, M.R.; Viana, M.E.; Felzenszwalb, I.; Paumgartten, F.J. Evaluation of beta-myrcene, alpha-terpinene and (+)- and (−)-alpha-pinene in the Salmonella/microsome assay. Food Chem. Toxicol. 2005, 43, 247–252. [Google Scholar] [CrossRef]
- Masfria, M.; Marianne, M.; Permata, Y.M.; Octavio, S.; Mulyani, S. Antimutagenic activity of nanoparticles of Rhaphidophora pinnata leaves in mice using micronucleus assay. J. Adv. Pharm. Technol. Res. 2021, 12, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, S. Evaluation of Mutagenic Effect (Antimutagenic) of Dalbergia Latifolia on Swiss Albino Mice. Int. J. Toxicol. Pharmacol. Res. 2015, 7, 80–84. Available online: https://www.researchgate.net/publication/379025686 (accessed on 20 July 2024).
- Vasantha, K.C.; Nagarathna, P.K.M.; Sudhir, C.K.; Sri Sainadh, N. Evaluation of Antimutagenic Effect of Flavonoid of Kigelia Africana on Swiss-Albino Mice. Int. J. Pharm. Sci. Rev. Res. 2013, 21, 105–108. Available online: https://www.researchgate.net/publication/287919880_Evaluation_of_antimutagenic_effect_of_flavonoid_of_Kigelia_Africana_on_Swiss-Albino_mice (accessed on 22 July 2024).
- Lima, E.M.; Cazelli, D.S.; Pinto, F.E.; Mazuco, R.A.; Kalil, I.C.; Lenz, D.; Scherer, R.; de Andrade, T.U.; Endringer, D.C. Essential Oil from the Resin of Protium heptaphyllum: Chemical Composition, Cytotoxicity, Antimicrobial Activity, and Antimutagenicity. Pharmacogn. Mag. 2016, 12, S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Araujo, D.A.; Takayama, C.; Faria-de, F.M.; Socca, E.A.; Dunder, R.J.; Manzo, L.P.; Luiz-Ferreira, A.; Souza-Brito, A.R.M. Gastroprotective effects of essential oil from Protium heptaphyllum on experimental gastric ulcer models in rats. Braz. J. Pharmacogn. 2011, 21, 721–729. [Google Scholar] [CrossRef]
- Bandeira, P.N.; Fonseca, A.M.; Costa, S.M.; Lins, M.U.; Pessoa, O.D.; Monte, F.J.; Nogueira, N.; Lemos, T.L.G. Anti-bacterial and anti-oxidant activities of the essential oil of resin of Protium heptaphyllum. Nat. Prod. Commun. 2006, 1, 117–120. [Google Scholar] [CrossRef]
- Soto-Reyes, E.; Del Razo, L.M.; Valverde, M.; Rojas, E. Role of the alkali labile sites, reactive oxygen species and antioxidants in DNA damage induced by methylated trivalent metabolites of inorganic arsenic. Biometals 2005, 18, 493–506. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Habibullah-Al-Mamun, M.; Hossain, M.A.; Arif, M.; Parvin, E.; Akter, M.S.; Khan, M.S.; Islam, M.M. Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere 2011, 84, 143–149. [Google Scholar] [CrossRef]
- Shao, B.; Zhu, L.; Dong, M.; Wang, J.; Wang, J.; Xie, H.; Zhang, Q.; Du, Z.; Zhu, S. DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio). Ecotoxicology 2012, 21, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.; Batool, U.; Abdul, S.M.; Sana, S. Estresse oxidativo: Uma espada de dois gumes. BioSight 2024, 2, 4–12. [Google Scholar] [CrossRef]
- Sadasivam, N.; Kim, Y.J.; Radhakrishnan, K.; Kim, D.K. Estresse oxidativo, integridade genômica e doenças hepáticas. Moléculas 2022, 27, 3159. [Google Scholar] [CrossRef]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFjB/MAPK pathway. Chem. Biol. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef]
- Golubkova, A.; Leiva, T.; Snyder, K.; Schlegel, C.; Bonvicino, S.M.; Agbaga, M.-P.; Brush, R.S.; Hansen, J.M.; Vitiello, P.F.; Hunter, C.J. Response of the Glutathione (GSH) Antioxidant Defense System to Oxidative Injury in Necrotizing Enterocolitis. Antioxidants 2023, 12, 1385. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Kaur, J. Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin. Chim. Acta 2003, 333, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Angie, M.B.; Paka, D.; Ntentie, G.; Ngondi, D.; Enyong, O. Protective effect of hydroethanolic extracts of Solanum scabrum and Cola verticillata against cyclophosphamide induced toxicity in female rats. J. Food Res. 2014, 3, 18–30. [Google Scholar] [CrossRef]
- Akomolafe, S.F.; Olasehinde, T.A.; Oyeleye, S.I.; Aluko, T.B.; Adewale, O.O.; Ijomone, O.M. Curcumin Administration Mitigates Cyclophosphamide-Induced Oxidative Damage and Restores Alteration of Enzymes Associated with Cognitive Function in Rats’ Brain. Neurotox. Res. 2020, 38, 199–210. [Google Scholar] [CrossRef] [PubMed]
- AbdElrazek, D.A.; Ibrahim, M.A.; Hassan, N.H.; Hassanen, E.I.; Farroh, K.Y.; Abass, H.I. Neuroprotective effect of quercetin and nano-quercetin against cyclophosphamide-induced oxidative stress in the rat brain: Role of Nrf2/ HO-1/Keap-1 signaling pathway. Neurotoxicology 2023, 98, 16–28. [Google Scholar] [CrossRef]
- Tan, Q.; Yan, X.; Chen, L.; Jiang, K.; Guo, Z. Dietary flavonoids protect human brain microvascular endothelial cell from oxidative stress-induced dysfunction. Mol. Cell. Toxicol. 2024, 1–11. [Google Scholar] [CrossRef]
- Ye, B.; Ling, W.; Wang, Y.; Jaisi, A.; Olatunji, O.J. Protective Effects of Chrysin against Cyclophosphamide-Induced Cardiotoxicity in Rats: A Biochemical and Histopathological Approach. Chem. Biodivers. 2022, 19, e202100886. [Google Scholar] [CrossRef]
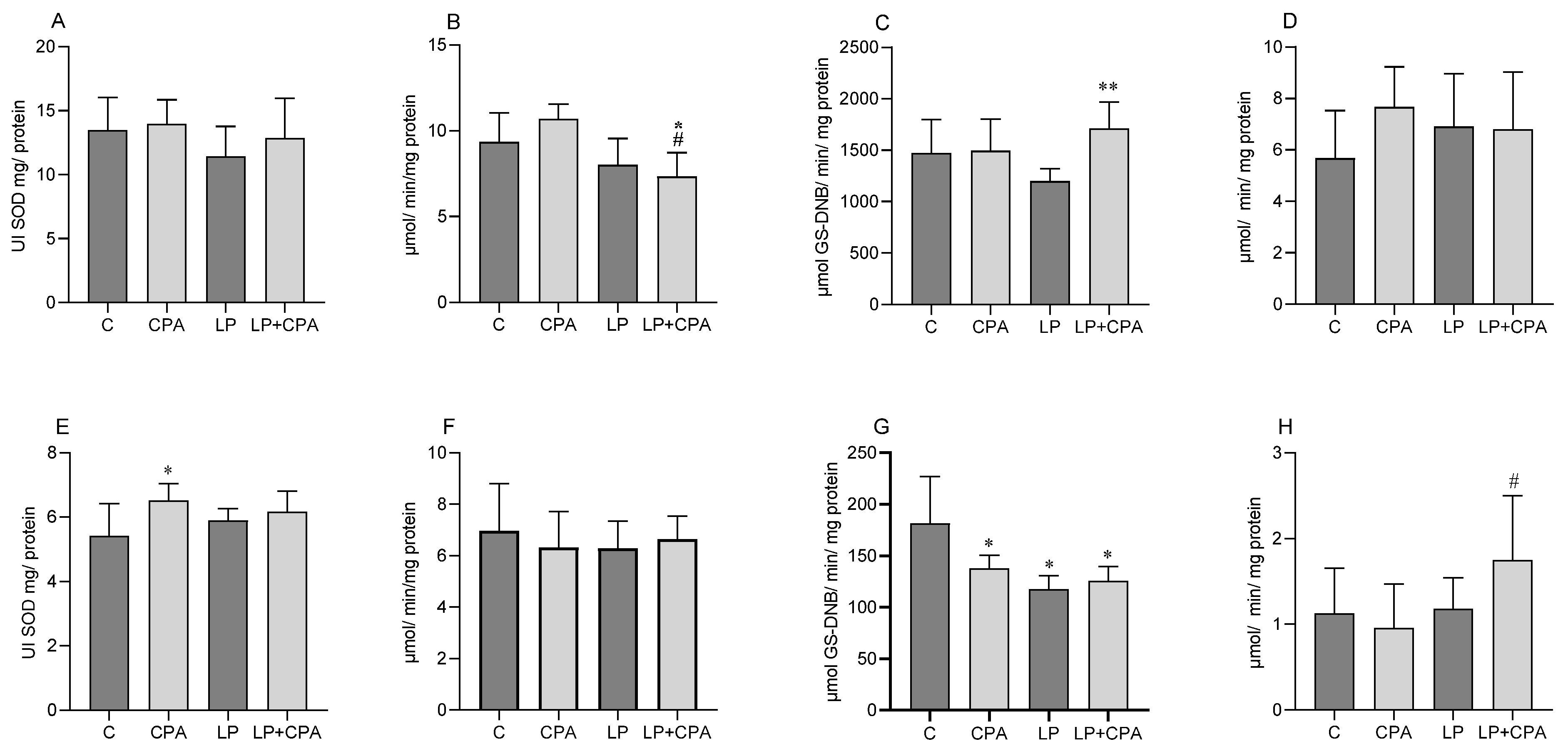
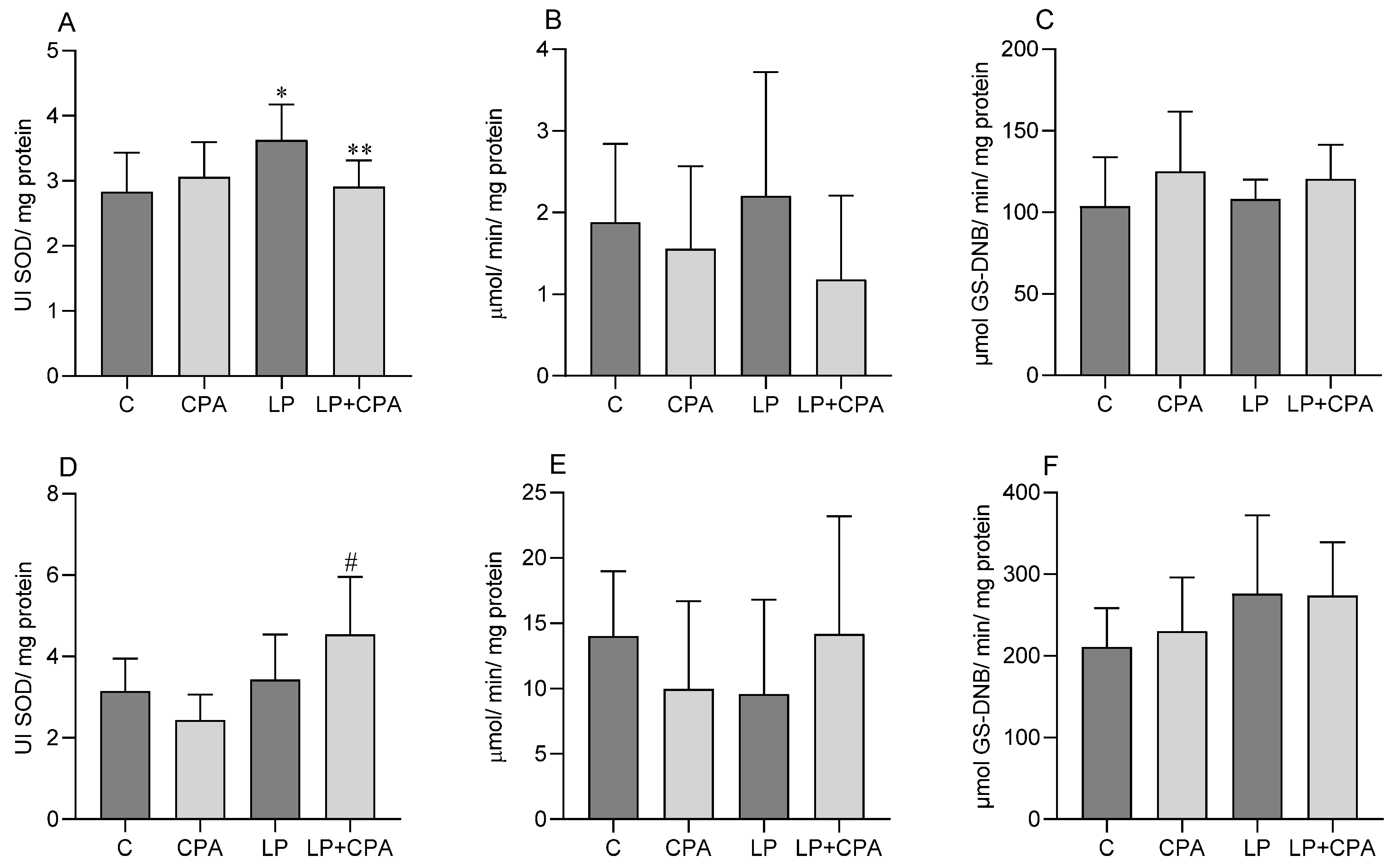
| Parameters | Control (C) | CPA | LP | LP + CPA |
|---|---|---|---|---|
| Initial body weight (g) | 41.75 ± 5.23 | 41.12 ± 2.74 | 41.37 ± 2.92 | 39.75 ± 3.73 |
| Final body weight (g) | 44.12 ± 5.54 | 42.50 ± 2.13 | 41.12 ± 2.90 | 42.37 ± 3.70 |
| Feed consumption (g/day/mouse) | 44.33 ± 9.64 | 47.00 ± 14.53 | 45.00 ± 10.23 | 47.83 ± 13.12 |
| Water intake (mL/day/mouse) | 28.73 ± 4.21 | 41.03 ± 13.04 | 31.20 ± 6.83 | 29.46 ± 6.89 |
| Liver (g) | 1.72 ± 0.13 | 2.06 ± 0.11 * | 1.81 ± 0.12 | 1.63 ± 0.11 **,# |
| Kidney (g) | 0.51 ± 0.05 | 0.56 ± 0.05 | 0.56 ± 0.05 | 0.51 ± 0.03 |
| Heart (g) | 0.19 ± 0.008 | 0.22 ± 0.01 * | 0.21 ± 0.02 | 0.19 ± 0.02 # |
| Brain (g) | 0.43 ± 0.05 | 0.42 ± 0.04 | 0.43 ± 0.06 | 0.43 ± 0.08 |
| Parameters | Control (C) | CPA | LP | LP + CPA |
|---|---|---|---|---|
| Glucose (mg/dL) | 229.25 ± 57.17 | 224.62 ± 44.51 | 185.12 ± 43.79 | 251.75 ± 53.54 |
| AST (U/L) | 154.87 ± 42.00 | 185.50 ± 150.00 | 182.25 ± 97.00 | 186.12 ± 181.00 |
| ALP (U/L) | 112.62 ± 25.39 | 100.12 ± 19.65 | 96.62 ± 18.57 | 76.87 ± 7.84 * |
| Creatinine (mg/dL) | 3.18 ± 0.66 | 2.13 ± 1.03 * | 1.31 ± 0.46 * | 1.45 ± 0.50 * |
| Cholesterol (mg/dL) | 85.87 ± 14.24 | 79.80 ± 17.72 | 102.88 ± 32.83 | 95.30 ± 25.04 |
| Triglycerides (mg/dL) | 145.25 ± 16.62 | 126.00 ± 3.84 * | 128.12 ± 16.27 | 136.12 ± 5.41 |
| PCEMNs | ||||
|---|---|---|---|---|
| Treatment | Number of PCEs Analyzed | MN | % | % Reduction |
| Control (Water + NaCl 0.9%) | 8000 | 290 | 3.62 | |
| CPA (Water + CPA) | 8000 | 535 | 6.69 | |
| LP (LP + NaCl 0.9%) | 8000 | 264 | 3.30 | |
| LP + CPA (LP + CPA) | 8000 | 470 * | 5.81 | 26% |
| Parameters | Control (C) | CPA | LP | LP + CPA | |
|---|---|---|---|---|---|
| Liver | TNF-α (pg/mL) | 0.72 ± 0.26 | 0.76 ± 0.24 | 0.83 ± 0.25 | 0.97 ± 0.15 |
| IFN-γ (pg/mL) | 1.17 ± 0.19 | 1.20 ± 0.12 | 1.24 ± 0.11 | 1.27 ± 0.07 | |
| IL-6 (pg/mL) | 1.05 ± 0.18 | 1.12 ± 0.14 | 1.13 ± 0.10 | 1.15 ± 0.04 | |
| IL-10 (pg/mL) | 1.15 ± 0.26 | 1.27 ± 0.17 | 1.28 ± 0.10 | 1.37 ± 0.08 | |
| IL-17 (pg/mL) | 0.97 ± 0.22 | 1.11 ± 0.24 | 1.13 ± 0.17 | 1.18 ± 0.11 | |
| IL-β (pg/mL) | 0.77 ± 0.22 | 0.89 ± 0.11 | 0.97 ± 0.17 | 1.01 ± 0.13 | |
| Kidney | TNF-α (pg/mL) | 0.96 ± 0.21 | 1.15 ± 0.32 | 0.93 ± 0.30 | 0.94 ± 0.21 |
| IFN-γ (pg/mL) | 1.30 ± 0.14 | 1.46 ± 0.09 | 1.34 ± 0.20 | 1.42 ± 0.19 | |
| IL-6 (pg/mL) | 1.21 ± 0.18 | 1.38 ± 0.20 | 1.28 ± 0.25 | 1.24 ± 0.23 | |
| IL-10 (pg/mL) | 1.24 ± 0.18 | 1.39 ± 0.11 | 1.31 ± 0.27 | 1.28 ± 0.27 | |
| IL-17 (pg/mL) | 1.25 ± 0.13 | 1.16 ± 0.17 | 1.09 ± 0.23 | 1.17 ± 0.13 | |
| IL-β (pg/mL) | 1.00 ± 0.23 | 1.16 ± 0.36 | 1.23 ± 0.28 | 1.12 ± 0.26 |
| Parameters | Control (C) | CPA | LP | LP + CPA | |
|---|---|---|---|---|---|
| Liver | GSH (µmol de GSH/mg protein) | 3.65 ± 1.15 | 1.96 ± 0.49 * | 2.60 ± 0.53 * | 2.36 ± 0.64 * |
| ASA (μmol ASA/g tissue) | 3.05 ± 0.48 | 3.33 ± 1.04 | 2.73 ± 0.49 | 3.28 ± 0.97 | |
| TBARS (nmol MDA/mg protein) | 0.71 ± 0.20 | 0.89 ± 0.19 | 0.56 ± 0.18 | 0.66 ± 0.42 | |
| Carbonyl (nmol carbonyl/mg protein) | 13.60 ± 2.98 | 19.06 ± 3.76 * | 15.30 ± 4.73 | 17.16 ± 3.55 | |
| Kidney | GSH (µmol de GSH/mg protein) | 2.82 ± 3.65 | 3.53 ± 5.54 | 1.68 ± 4.08 | 2.35 ± 5.68 |
| ASA (μmol ASA/g tissue) | 1.40 ± 0.22 | 1.68 ± 0.21 | 1.38 ± 0.35 | 1.42 ± 0.33 | |
| TBARS (nmol MDA/mg protein) | 0.10 ± 0.15 | 0.09 ± 0.05 | 0.07 ± 0.05 * | 0.08 ± 0.07 | |
| Carbonyl (nmol carbonyl/mg protein) | 5.35 ± 2.25 | 5.99 ± 1.41 | 6.08 ± 2.02 | 4.89 ± 2.27 |
| Parameters | Control (C) | CPA | LP | LP + CPA | |
|---|---|---|---|---|---|
| Brain | GSH (µmol de GSH/mg protein) | 2.60 ± 3.42 | 3.67 ± 3.35 | 1.65 ± 1.30 | 1.93 ± 1.90 # |
| ASA (μmol ASA/g tissue) | 3.46 ± 0.34 | 3.31 ± 0.32 | 3.54 ± 0.40 | 3.13 ± 0.26 | |
| TBARS (nmol MDA/mg protein) | 4.57 ± 1.40 | 5.15 ± 0.72 | 4.41 ± 0.66 | 5.00 ± 0.87 | |
| Carbonyl (nmol carbonyl mg protein) | 18.45 ± 4.25 | 17.37 ± 3.40 | 24.30 ± 6.87 | 23.50 ± 3.08 | |
| Heart | GSH (µmol de GSH/mg protein) | 1.05 ± 0.34 | 0.91 ± 0.32 | 1.81 ± 0.65 | 1.38 ± 0.79 |
| ASA (μmol ASA/g tissue) | 1.77 ± 0.39 | 1.30 ± 0.31 * | 1.52 ± 0.35 | 1.46 ± 0.28 | |
| TBARS (nmol MDA/mg protein) | 0.67 ± 0.21 | 0.63 ± 0.10 | 0.55 ± 0.18 | 0.78 ± 0.13 | |
| Carbonyl (nmol carbonyl/mg protein) | 23.56 ± 11.48 | 21.79 ± 18.93 | 25.81 ± 16.33 | 36.15 ± 31.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patias, N.S.; Sinhorin, V.D.G.; Ferneda, A.J.L.B.; Ferneda, J.M.A.; Sugui, M.M.; Ferrarini, S.R.; Bomfim, G.F.; Lopes, J.W.; Antoniassi, N.A.B.; Cavalheiro, L.; et al. Study of Liposomes Containing Extract from the Leaves of Protium heptaphyllum (Aubl.) March in Animals Submitted to a Mutagenic Model Induced by Cyclophosphamide. Biology 2024, 13, 706. https://doi.org/10.3390/biology13090706
Patias NS, Sinhorin VDG, Ferneda AJLB, Ferneda JMA, Sugui MM, Ferrarini SR, Bomfim GF, Lopes JW, Antoniassi NAB, Cavalheiro L, et al. Study of Liposomes Containing Extract from the Leaves of Protium heptaphyllum (Aubl.) March in Animals Submitted to a Mutagenic Model Induced by Cyclophosphamide. Biology. 2024; 13(9):706. https://doi.org/10.3390/biology13090706
Chicago/Turabian StylePatias, Naiéle Sartori, Valéria Dornelles Gindri Sinhorin, Ana Júlia Lopes Braga Ferneda, João Maurício Andrade Ferneda, Marina Mariko Sugui, Stela Regina Ferrarini, Gisele Facholi Bomfim, Joaz Wellington Lopes, Nadia Aline Bobbi Antoniassi, Larissa Cavalheiro, and et al. 2024. "Study of Liposomes Containing Extract from the Leaves of Protium heptaphyllum (Aubl.) March in Animals Submitted to a Mutagenic Model Induced by Cyclophosphamide" Biology 13, no. 9: 706. https://doi.org/10.3390/biology13090706
APA StylePatias, N. S., Sinhorin, V. D. G., Ferneda, A. J. L. B., Ferneda, J. M. A., Sugui, M. M., Ferrarini, S. R., Bomfim, G. F., Lopes, J. W., Antoniassi, N. A. B., Cavalheiro, L., Domingues, N. L. d. C., & Sinhorin, A. P. (2024). Study of Liposomes Containing Extract from the Leaves of Protium heptaphyllum (Aubl.) March in Animals Submitted to a Mutagenic Model Induced by Cyclophosphamide. Biology, 13(9), 706. https://doi.org/10.3390/biology13090706








