Changes in Anxiety-Related Behaviors, Voiding Patterns, and Urinary Bladder Contractile Properties in Male Mice Exposed to Water Avoidance Stress for 1 Day and 28 Days
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Water Avoidance Stress (WAS) Protocol
2.3. Voiding Spot Analysis
2.4. Open Field Test (OFT)
2.5. Organ Bath Studies
2.6. Toluidine Blue Staining for Mast Cell Quantification
2.7. Statistical Analysis
3. Results
3.1. Changes in Locomotor Activity and Anxiety-Related Behavior
3.2. Body Weight and Bladder Weight
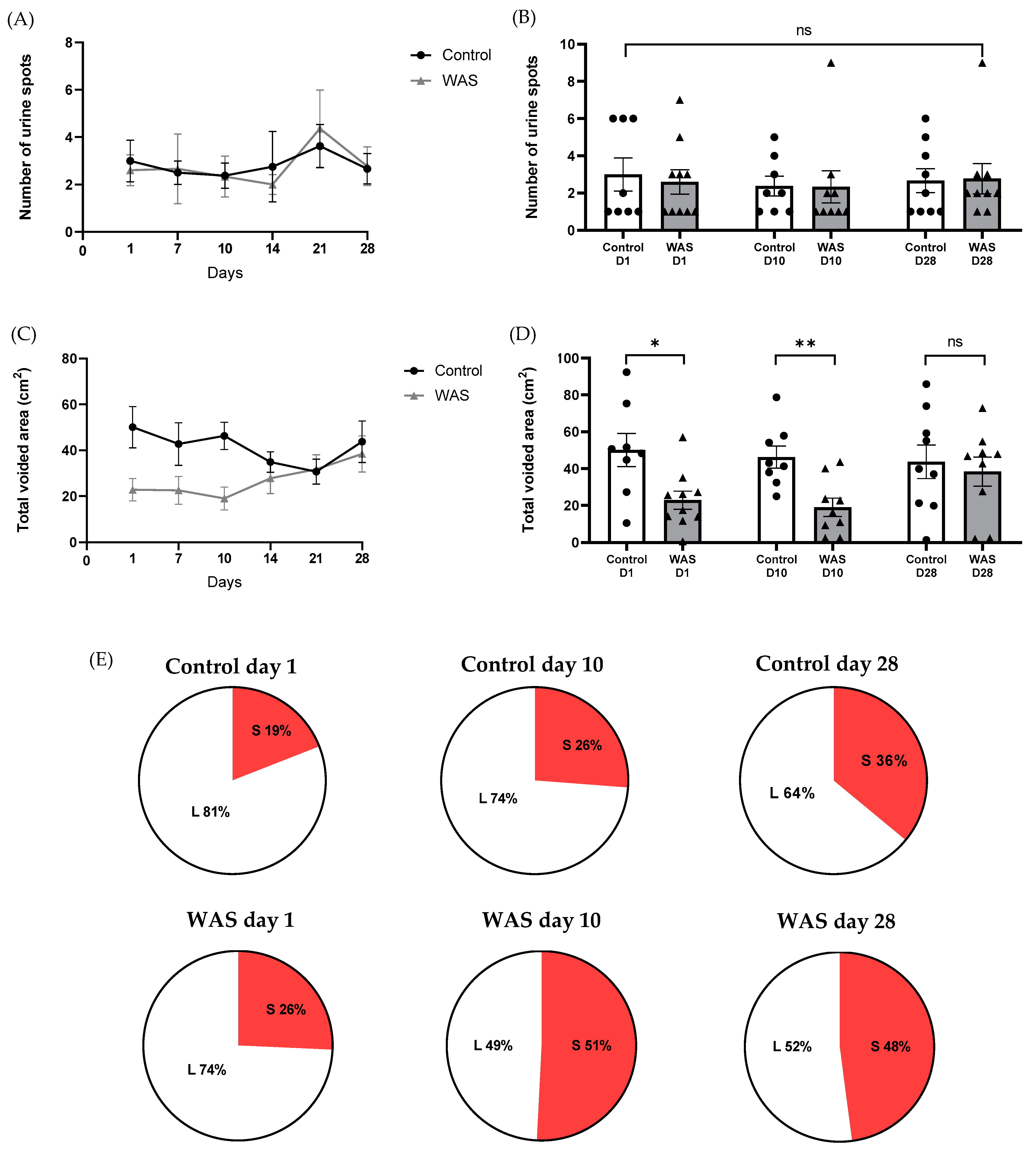
3.3. Urine Pattern Analysis
3.4. Mast Cell Number in Bladder Tissues
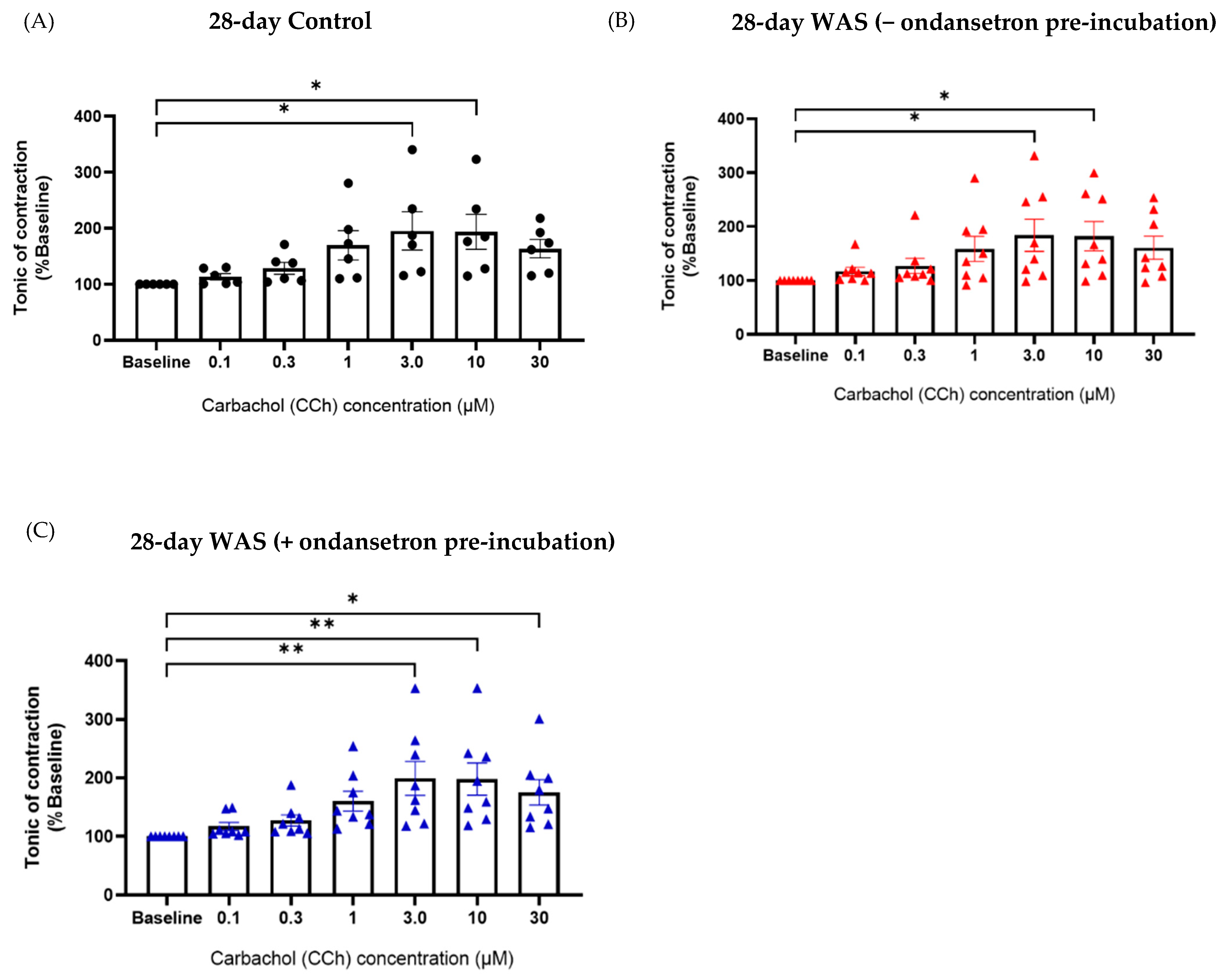
3.5. Tonic Contractile Properties of Bladder Tissues in Response to Carbachol (CCh) and Ondansetron Pre-Incubation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function. A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Rothrock, N.E.; Lutgendorf, S.K.; Kreder, K.J.; Ratliff, T.; Zimmerman, B. Stress and symptoms in patients with interstitial cystitis: A life stress model. Urology 2001, 57, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Koseki, J.; Oshima, T.; Hattori, T.; Kase, T.; Kondo, T.; Fukui, H.; Tomita, T.; Ohda, Y.; Watari, J. Impairment of gastric accommodation induced by water-avoidance stress is mediated by 5-HT2B receptors. Neurogastroenterol. Motil. 2016, 28, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Leron, E.; Weintraub, A.Y.; Mastrolia, S.A.; Schwarzman, P. Overactive bladder syndrome: Evaluation and management. Currenturology 2017, 11, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Bradesi, S.; Schwetz, I.; Ennes, H.S.; Lamy, C.M.; Ohning, G.; Fanselow, M.; Pothoulakis, C.; McRoberts, J.A.; Mayer, E.A. Repeated exposure to water avoidance stress in rats: A new model for sustained visceral hyperalgesia. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 289, G42–G53. [Google Scholar] [CrossRef]
- Söderholm, J.D.; Yates, D.A.; Gareau, M.G.; Yang, P.C.; MacQueen, G.; Perdue, M.H. Neonatal maternal separation pre-disposes adult rats to colonic barrier dysfunction in response to mild stress. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G1257–G1263. [Google Scholar] [CrossRef]
- Smith, A.L.; Leung, J.; Kun, S.; Zhang, R.; Karagiannides, I.; Raz, S.; Lee, U.; Glovatscka, V.; Pothoulakis, C.; Bradesi, S.; et al. The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 2011, 78, 967.e1–967.e7. [Google Scholar] [CrossRef]
- Sattayachiti, S.; Waemong, A.; Cheaha, D.; Konthapakdee, N. 5-HT3 receptors modulate changes in voiding pattern and bladder contractility in water avoidance stress-induced bladder overactivity in male mice. Auton. Neurosci. Basic Clin. 2022, 243, 103040. [Google Scholar] [CrossRef]
- West, E.G.; McDermott, C.; Chess-Williams, R.; Sellers, D.J. Partial recovery of voiding function in female mice following repeated psychological stress exposure. PLoS ONE 2022, 17, e0266458. [Google Scholar] [CrossRef]
- West, E.G.; Sellers, D.J.; Chess-Williams, R.; McDermott, C. Bladder overactivity induced by psychological stress in female mice is associated with enhanced bladder contractility. Life Sci. 2021, 265, 118735. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, H.H.; Gao, Y.; Zhang, R.; Guo, Y.; Holschneider, D.P.; Rodriguez, L.V. Effects of water avoidance stress on peripheral and central responses during bladder filling in the rat: A multidisciplinary approach to the study of urologic chronic pelvic pain syndrome (MAPP) research network study. PLoS ONE 2017, 12, e0182976. [Google Scholar] [CrossRef] [PubMed]
- Matos, R.; Serrão, P.; Rodrigues, L.; Birder, L.A.; Cruz, F.; Charrua, A. The water avoidance stress induces bladder pain due to a prolonged adrenergic (alpha1A) stimulation of the bladder. Eur. Urol. Suppl. 2017, 16, e350–e351. [Google Scholar] [CrossRef]
- Kim, J.K.; Hong, S.; Park, J.; Kim, S. Metabolic and transcriptomic changes in the mouse brain in response to short-term high-fat metabolic stress. Metabolites 2023, 13, 407. [Google Scholar] [CrossRef]
- McGonagle, E.; Smith, A.; Butler, S.; Sliwoski, J.; Valentino, R.; Canning, D.; Zderic, S.A. Water avoidance stress results in an altered voiding phenotype in male mice. Neurourol. Urodyn. 2012, 31, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Waemong, A.; Sattayachiti, S.; Cheaha, D.; Konthapakdee, N. Effects of oral administration of ondansetron, a 5-HT3 receptor antagonist, on anxiety-related behaviors and colonic hypercontractility in repeated stress-induced mice. Auton. Neurosci. Basic Clin. 2024, 253, 103178. [Google Scholar] [CrossRef]
- Whimbey, A.E.; Denenberg, V.H. Two independent behavioral dimensions in open-field performance. J. Comp. Physiol. Psychol. 1967, 63, 500–504. [Google Scholar] [CrossRef]
- Zelena, D.; Filaretova, L. Stress adaptation with special emphasis on gastric erosion: Is stress a bad guy or a good guy? In Chronic Stress and Health; Nova Science Publisher: Hauppauge, NY, USA, 2017; Volume 4, pp. 65–88. [Google Scholar]
- Chaves, T.; Fazekas, C.L.; Horváth, K.; Correia, P.; Szabó, A.; Török, B.; Bánrévi, K.; Zelena, D. Stress adaptation and the brainstem with focus on corticotropin-releasing hormone. Int. J. Mol. Sci. 2021, 22, 9090. [Google Scholar] [CrossRef]
- Charrua, A.; Pinto, R.; Birder, L.A.; Cruz, F. Sympathetic nervous system and chronic bladder pain: A new tune for an old song. Transl. Androl. Urol. 2015, 4, 534–542. [Google Scholar] [CrossRef]
- Gao, Y.; Rodríguez, L.V. The effect of chronic psychological stress on lower urinary tract function: An animal model perspective. Front. Physiol. 2022, 13, 818993. [Google Scholar] [CrossRef]
- Lin, G.G.; Scott, J.G. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Bradley, C.S.; Nygaard, I.E.; Hillis, S.L.; Torner, J.C.; Sadler, A.G. Longitudinal associations between mental health conditions and overactive bladder in women veterans. Am. J. Obstet. Gynecol. 2017, 217, e1–e430. [Google Scholar] [CrossRef] [PubMed]
- Davila, G.W.; Bernier, F.; Franco, J.; Kopka, S.L. Bladder dysfunction in sexual abuse survivors. J. Urol. 2003, 170, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.H.; Morgan, C.D.; Vetter, J.; Andriole, G.L. Impact of childhood and recent traumatic events on the clinical presentation of overactive bladder. Neurourol. Urodyn. 2016, 35, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Karagkouni, A.; Alevizos, M.; Theoharides, T.C. Effect of stress on brain inflammation and multiple sclerosis. Autoimmun. Rev. 2013, 12, 947–953. [Google Scholar] [CrossRef]
- Jennings, S.V.; Slee, V.M.; Zack, R.M.; Verstovsek, S.; George, T.I.; Shi, H.; Lee, P.; Castells, M.C. Patient perceptions in mast cell disorders. Immunol. Allergy Clin. N. Am. 2018, 38, 505–525. [Google Scholar] [CrossRef]
- Blin, J.; Gautier, C.; Aubert, P.; Durand, T.; Oullier, T.; Aymeric, L.; Naveilhan, P.; Masson, D.; Neunlist, M.; Bach-Ngohou, K. Psychological stress induces an increase in cholinergic enteric neuromuscular pathways mediated by glucocorticoid receptors. Front. Neurosci. 2023, 17, 1100473. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D.; Marchand, J.; Vasiadi, M.; Katsarou-Katsari, A.; Makris, M.; Kalogeromitros, D. Urticaria pigmentosa associated with acute stress and lesional skin mast-cell expression of CRF-R1. Clin. Exp. Dermatol. 2008, 34, e163–e166. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Petra, A.I.; Stewart, J.M.; Tsilioni, I.; Panagiotidou, S.; Akin, C. High serum corticotropin-releasing hormone (CRH) and bone marrow mast cell CRH receptor expression in a mastocytosis patient. J. Allergy Clin. Immunol. 2014, 134, 1197–1199. [Google Scholar] [CrossRef]
- Imamura, T.; Ishizuka, O.; Ogawa, T.; Yamagishi, T.; Yokoyama, H.; Minagawa, T.; Gautam, S.S.; Nishizawa, O. Muscarinic receptors mediate cold stress-induced detrusor overactivity in type 2 diabetes mellitus rats. Int. J. Urol. 2014, 21, 1051–1058. [Google Scholar] [CrossRef]
- West, E.G.; McDermott, C.; Chess-Williams, R.; Sellers, D.J. Mirabegron and solifenacin are effective for the management of the increased urinary frequency induced by psychological stress in female mice. Sci. Rep. 2022, 12, 12365. [Google Scholar] [CrossRef] [PubMed]
- Messori, E.; Rizzi, C.A.; Candura, S.M.; Lucchelli, A.; Balestra, B.; Tonini, M. 5-hydroxytryptamine receptors that facilitate excitatory neuromuscular transmission in the Guinea-pig isolated detrusor muscle. Br. J. Pharmacol. 1995, 115, 677–683. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Acute Period (1 Day) | Chronic Period (10 Days) | Prolonged Period (28 Days) | |||
|---|---|---|---|---|---|---|
| 1-Day Control (N = 5) | 1-Day WAS (N = 5) | 10-Day Control (N = 6) | 10-Day WAS (N = 7) | 28-Day Control (N = 4) | 28-Day WAS (N = 4) | |
| Averaged speed (cm/s) | 4.112 ± 0.93 | 3.931 ± 0.64 | 3.858 ± 1.11 | 4.373 ± 1.01 | 3.490 ± 0.84 | 4.661 ± 1.02 |
| Total distance traveled (cm) | 2465 ± 554.70 | 2335 ± 349.50 | 2313 ± 661.90 | 2623 ± 632.10 | 2092 ± 504.30 | 2795 ± 609.20 |
| Time spent in the center zone (sec) | 142.3 ± 62.89 | 166.7 ± 111.60 | 186.4 ± 138.60 | 108.7 ± 31.27 | 182.0 ± 140.60 | 164.8 ± 121.10 |
| Parameters | Acute Period (1 Day) | Chronic Period (10 Days) | Prolonged Period (28 Days) | |||
|---|---|---|---|---|---|---|
| 1-Day Control (N = 9) | 1-Day WAS (N = 11) | 10-Day Control (N = 10) | 10-Day WAS (N = 11) | 28-Day Control (N = 11) | 28-Day WAS (N = 10) | |
| Body weight (g) | 27.30 ± 2.854 | 25.90 ± 3.335 | 26.73 ± 1.890 | 26.89 ± 3.165 | 26.49 ± 1.342 | 26.23 ± 2.031 |
| Bladder weight (g) | 0.034 ± 0.004 | 0.036 ± 0.010 | 0.034 ± 0.005 | 0.036 ± 0.008 | 0.034 ± 0.009 | 0.037 ± 0.006 |
| % Bladder weight/body weight | 0.127 ± 0.018 | 0.140 ± 0.028 | 0.128 ± 0.020 | 0.133 ± 0.028 | 0.130 ± 0.034 | 0.139 ± 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattayachiti, S.; Chumpong, P.; Niyomdecha, S.; Cheaha, D.; Konthapakdee, N. Changes in Anxiety-Related Behaviors, Voiding Patterns, and Urinary Bladder Contractile Properties in Male Mice Exposed to Water Avoidance Stress for 1 Day and 28 Days. Biology 2024, 13, 707. https://doi.org/10.3390/biology13090707
Sattayachiti S, Chumpong P, Niyomdecha S, Cheaha D, Konthapakdee N. Changes in Anxiety-Related Behaviors, Voiding Patterns, and Urinary Bladder Contractile Properties in Male Mice Exposed to Water Avoidance Stress for 1 Day and 28 Days. Biology. 2024; 13(9):707. https://doi.org/10.3390/biology13090707
Chicago/Turabian StyleSattayachiti, Sarunnuch, Panida Chumpong, Seree Niyomdecha, Dania Cheaha, and Nipaporn Konthapakdee. 2024. "Changes in Anxiety-Related Behaviors, Voiding Patterns, and Urinary Bladder Contractile Properties in Male Mice Exposed to Water Avoidance Stress for 1 Day and 28 Days" Biology 13, no. 9: 707. https://doi.org/10.3390/biology13090707
APA StyleSattayachiti, S., Chumpong, P., Niyomdecha, S., Cheaha, D., & Konthapakdee, N. (2024). Changes in Anxiety-Related Behaviors, Voiding Patterns, and Urinary Bladder Contractile Properties in Male Mice Exposed to Water Avoidance Stress for 1 Day and 28 Days. Biology, 13(9), 707. https://doi.org/10.3390/biology13090707






