Feeding Behavior, Gut Microbiota, and Transcriptome Analysis Reveal Individual Growth Differences in the Sea Urchin Strongylocentrotus intermedius
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Breeding and Experimental Design
2.2. DNA Extraction and High-Throughput Sequencing
2.3. Diversity Analysis and Functional Prediction
2.4. RNA Preparation, and cDNA Library Construction
2.5. Quality Control and Gene Annotation
2.6. Differential Gene Expression Analysis
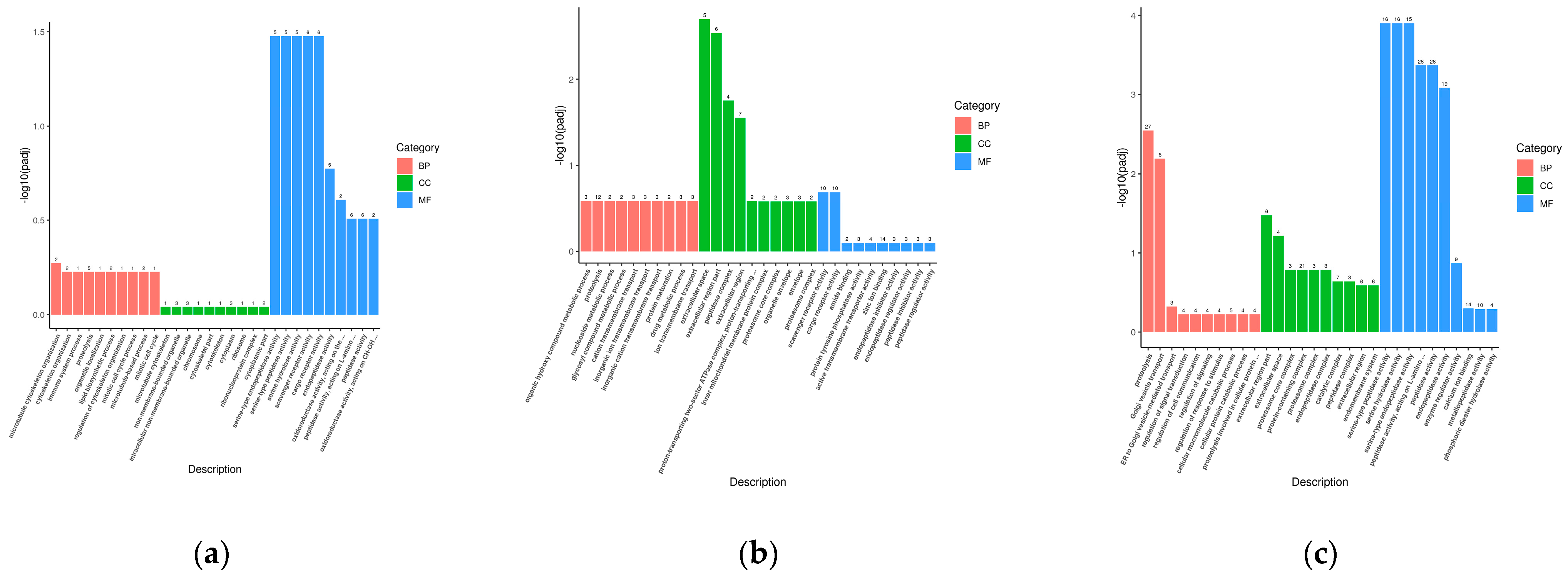
2.7. GO and KEGG Enrichment Analysis of Differentially Expressed Genes
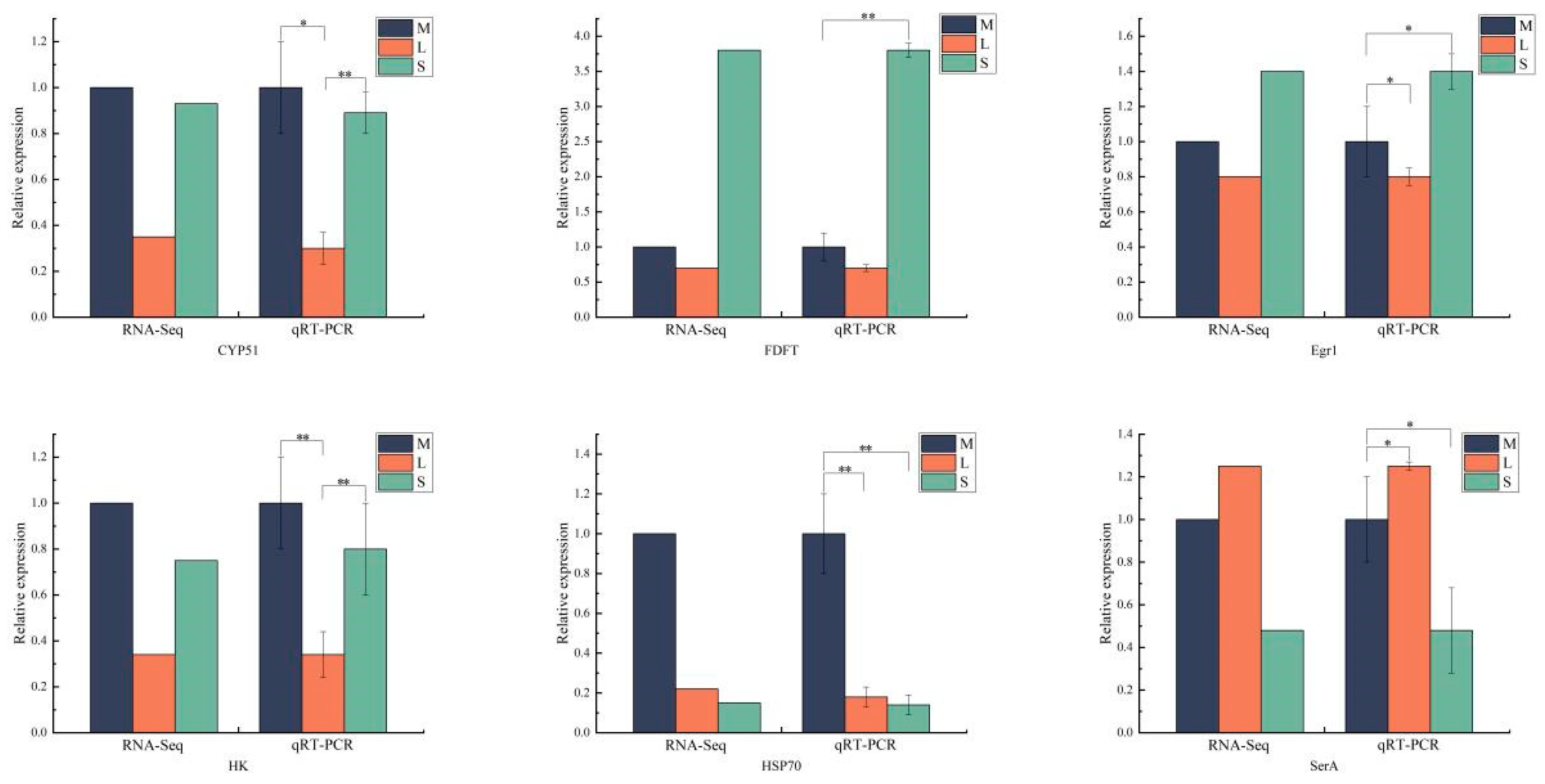
2.8. RT-qPCR Validation
3. Results
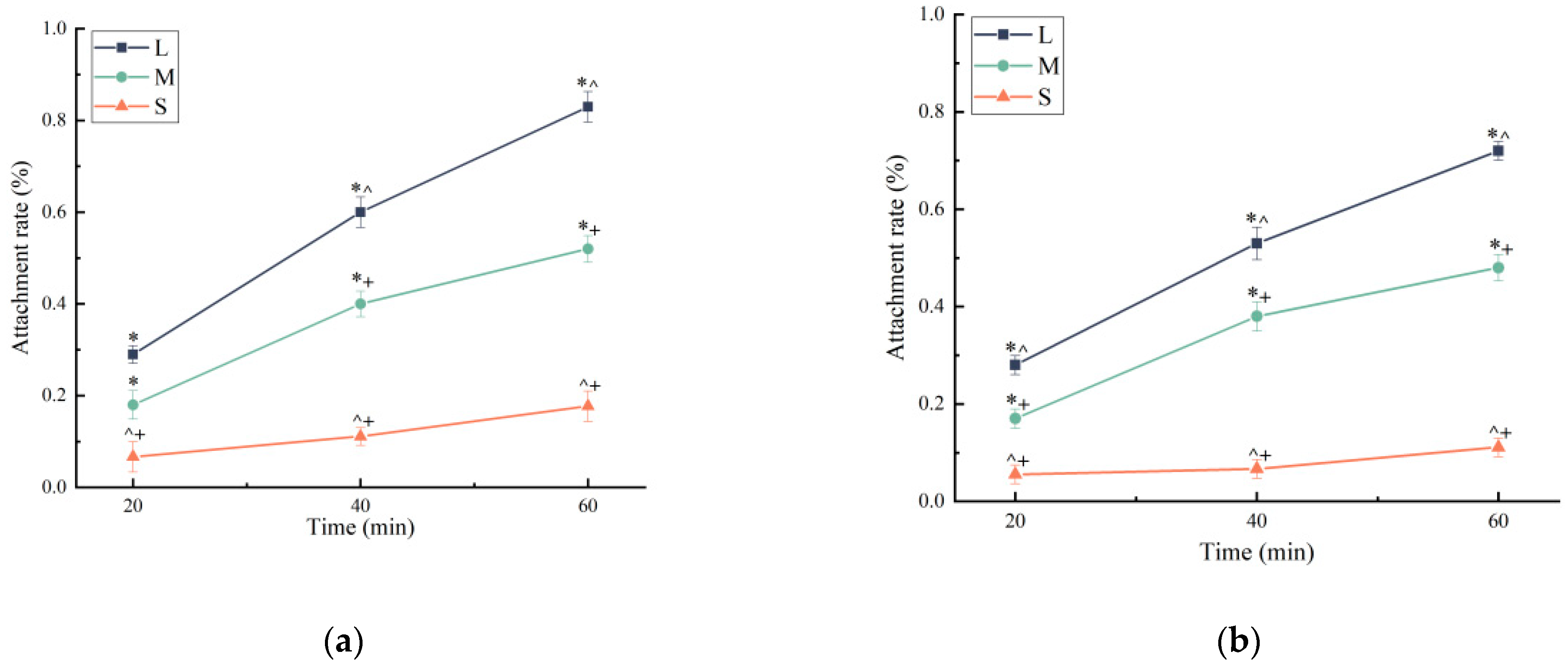
3.1. Sea Urchin Feeding Behavior at Different Sizes
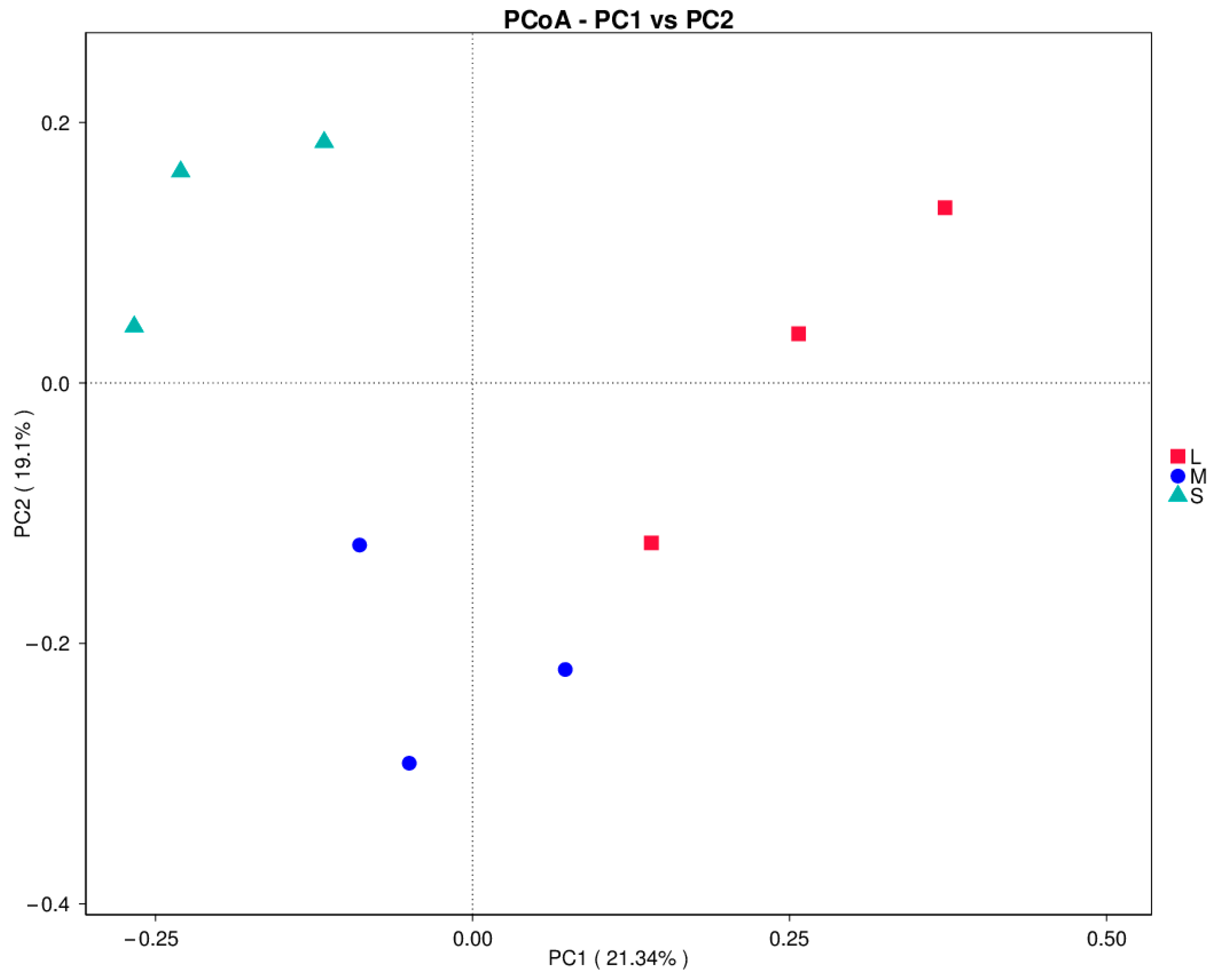
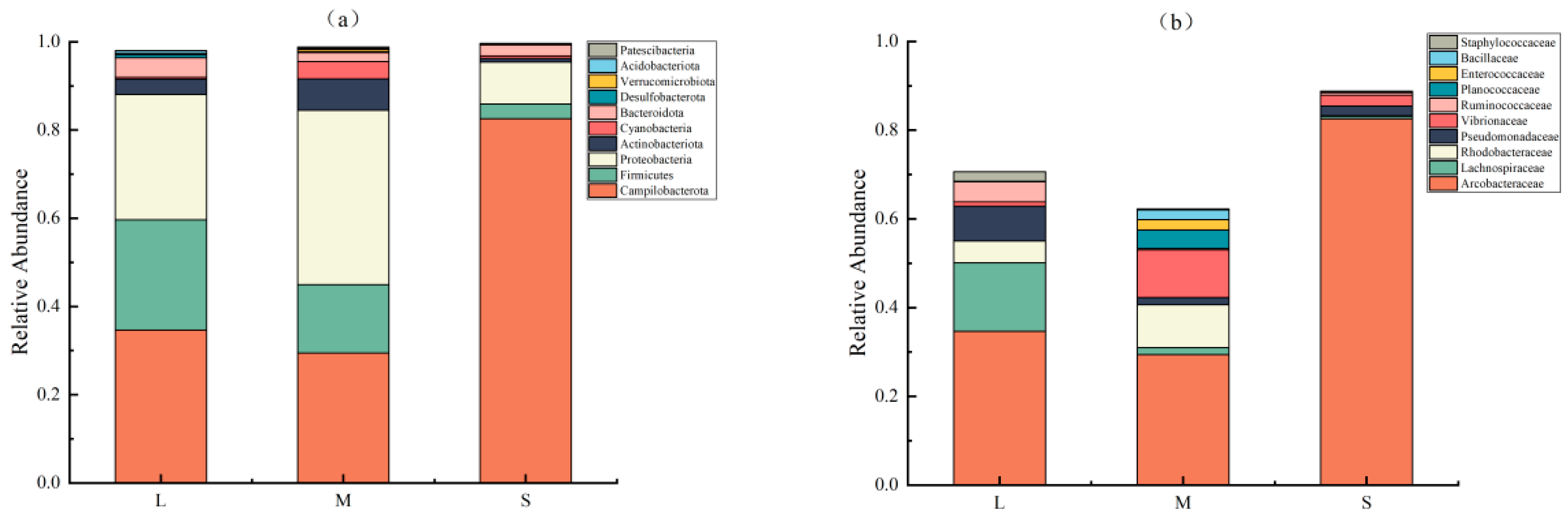
3.2. Composition of Intestinal Flora in the Different Size Groups
3.3. Quality of the Transcriptome Sequencing Data
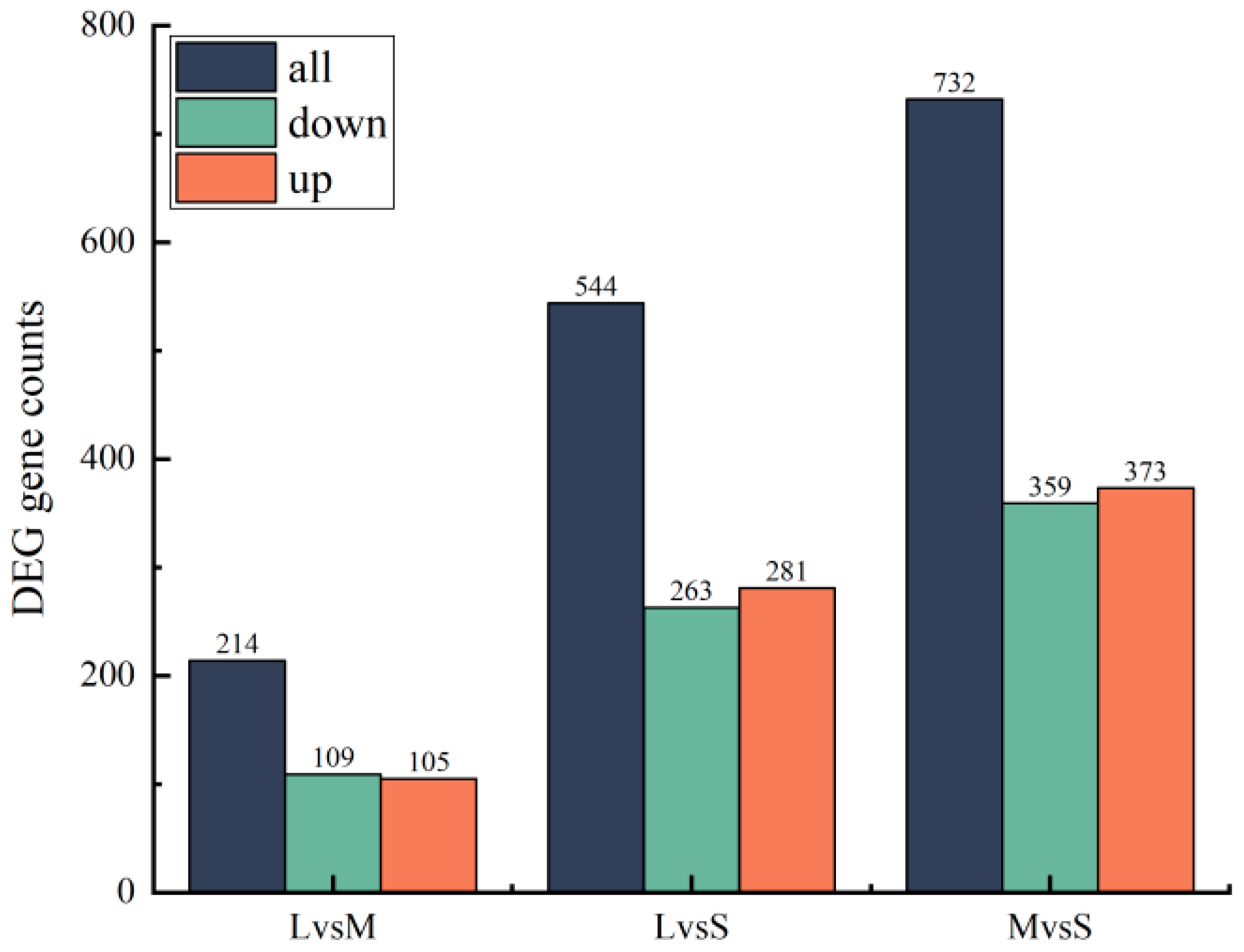
3.4. Annotation and Functional Characterization of Transcriptomes of Sea Urchins of Different Sizes
3.5. qRT-PCR Verification
4. Discussion
4.1. Growth Differences Caused by Different Sea Urchin Feeding Behaviors
4.2. Differences in Intestinal Flora Composition Affect the Growth of Sea Urchins
4.3. Preliminary Study on Transcriptome Differences in Sea Urchins of Different Sizes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, S.; Liang, M.; Gao, Q.; Wang, F.; Dong, Y.; Tian, X. Intra-specific effects of sea cucumber (Apostichopus japonicus) with reference to stocking density and body size. Aquac. Res. 2010, 41, 1170–1178. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chang, Y.Q.; Zhao, C.; Liu, P.J.; Song, J. Effects of phenotypic traits on gonad traits in sea urchins Strongylocentrotus intermedius. Prog. Fish. Sci. 2010, 31, 49–55. [Google Scholar] [CrossRef]
- Moutou, K.; McCarthy, I.; Houlihan, D. The effect of ration level and social rank on the development of fin damage in juvenile rainbow trout. J. Fish Biol. 1998, 52, 756–770. [Google Scholar] [CrossRef]
- Feng, Q.-M.; Ru, X.-S.; Zhang, L.-B.; Zhang, S.-Y.; Yang, H.-S. Differences in feeding behavior and intestinal microbiota may relate to different growth rates of sea cucumbers (Apostichopus japonicus). Aquaculture 2022, 559, 738368. [Google Scholar] [CrossRef]
- Moss, D.R.; Moss, S.M. Effects of gender and size on feed acquisition in the Pacific white shrimp Litopenaeus Vannamei. J. World Aquac. Soc. 2006, 37, 161–167. [Google Scholar] [CrossRef]
- Gregory, T.R.; Wood, C.M. Individual Variation and Interrelationships Between Swimming Performance, Growth Rate, and Feeding in Juvenile Rainbow Trout (Oncorhynchus mykiss). J. Can. Sci. Halieut. Aquat. 2007, 55, 1583–1590. [Google Scholar] [CrossRef]
- Gao, F.; Sun, H.L.; Xu, Q.; Tan, J.; YAN, J.P.; Wang, Q.Y. PCR-DGGE analysis of bacterial community composition in the gut contents of Apostichopus japonicus. J. Fish. Sci. China 2010, 17, 671–680. [Google Scholar]
- Zhang, Y.T.; Huang, J.; Jin, W.; Ma, X.; Lv, G.; Ye, C.; Mu, J.; Wen, H.; Chen, S.X. Comparative analysis of gut microbiota and intestinal transcriptomic profile between fast-and slow-growing American eels (Anguilla rostrata). Aquac. Rep. 2024, 36, 102087. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, B.; David, M.A.; Gao, J.-Z.; Chen, Z.-Z. Comparative analysis of intestinal microbiota of discus fish (Symphysodon haraldi) with different growth rates. Aquaculture 2021, 540, 736740. [Google Scholar] [CrossRef]
- Wang, S.T. Identification of Intestinal Microbiotas in Grass Carp with Different Growth and Functional Research of Short-Chain Fatty Acid Receptor Gene; Tongfang CNKI (Beijing) Technology Co., Ltd.: Beijing, China, 2021. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, X.; Song, T.Y. Differences in intestinal flora of cultured large yellow croaker Pseudosciaena crocea with different growth rates. J. Dalian Ocean. Univ. 2017, 32, 509–513. [Google Scholar] [CrossRef]
- Chen, B.; Xiao, W.; Zou, Z.; Zhu, J. Comparing transcriptomes reveals key metabolic mechanisms in superior growth performance Nile tilapia (Oreochromis niloticus). Front. Genet. 2022, 13, 879570. [Google Scholar] [CrossRef]
- Nie, H.; Zheng, M.; Wang, Z.; Xu, Q.; Yin, Z.; Zhang, Y.; Yan, X. Transcriptomic analysis provides insights into candidate genes and molecular pathways involved in growth of Manila clam Ruditapes philippinarum. Funct. Integr. Genom. 2021, 21, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Liu, Z.W.; Dai, X.L. Transcriptome Analysis of Growth Retardation and Normal Macrobrachium rosenbergii. Genom. Appl. Biol. 2021, 40, 89–100. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Liang, X.F.; He, S.; Zhang, Q.; Li, L. Differences of gut microbiota and lipid metabolism in Chinese perch (Siniperca chuatsi) with different growth rates. Aquac. Res. 2022, 53, 1766–1781. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; Byrne, M. Impacts of ocean acidification on sea urchin growth across the juvenile to mature adult life-stage transition is mitigated by warming. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172684. [Google Scholar] [CrossRef]
- Shpigel, M.; Erez, J. Effect of diets and light regimes on calcification and somatic growth of the sea urchin Tripneustes gratilla elatensis. Aquaculture 2020, 529, 735547. [Google Scholar] [CrossRef]
- Qi, S.; Zhang, W.; Jing, C.; Wang, H.; Zhao, S.; Zhou, M.; Chang, Y. Long-term effects of stocking density on survival, growth performance and marketable production of the sea urchin Strongylocentrotus intermedius. Aquac. Int. 2016, 24, 1323–1339. [Google Scholar] [CrossRef]
- Li, M.; Zhang, F.; Ding, J.; Zuo, R.; Chang, Y. Effects of lipid sources on the growth performance, gonad development, fatty acid profile and transcription of related genes in juvenile sea urchin (Strongylocentrotus intermedius). Aquac. Nutr. 2021, 27, 28–38. [Google Scholar] [CrossRef]
- He, Q.Y.Q.; Di, W.X.; Gou, D.; Gong, P.K.; Tang, L.; Chang, Y.Q.; Ding, J.; Zuo, R.T. Effects of Different Feeding Programs on Growth and Gonad Development, Sensory Quality and Nutritional Quality of Sea Urchin Strongylocentrotus intermedius). Chin. J. Anim. Nutr. 2023, 35, 4507–4519. [Google Scholar] [CrossRef]
- Chi, X.; Hu, F.; Qin, C.; Huang, X.; Sun, J.; Cui, Z.; Ding, J.; Yang, M.; Chang, Y.; Zhao, C. Conspecific alarm cues are a potential effective barrier to regulate foraging behavior of the sea urchin Mesocentrotus nudus. Mar. Environ. Res. 2021, 171, 105476. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Price, M.; Dehal, P.; Arkin, A. FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Thomas, C.; Carter, C.; Crear, B. Feed availability and its relationship to survival, growth, dominance and the agonistic behaviour of the southern rock lobster, Jasus edwardsii in captivity. Aquaculture 2003, 215, 45–65. [Google Scholar] [CrossRef]
- Chen, X.L.; Lin, Q.W.; Li, S.J.; Wang, G.Z.; Ai, C.X.; LI, B.L.; Lin, T. Observations and Studies on the Cannibalism Among Post Larvae of Kuruma Prawn Penaeus japonicus. J. Xiamen Univ. (Nat. Sci.) 2003, 42, 358–362. [Google Scholar] [CrossRef]
- Li, Y.Q.; Sun, X. Agonistic behaviors of aquatic animals. Zool. Res. 2013, 34, 214–220. [Google Scholar] [CrossRef]
- Liang, M. Experimental Studies on Individual Variation in Growth Andeauses in Sea Cucumber, Apostichopus japonicus. 2011. Available online: https://scholar.google.com.hk/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E5%88%BA%E5%8F%82%EF%BC%88Apostichopus+japonicus%EF%BC%89%E4%B8%AA%E4%BD%93%E7%94%9F%E9%95%BF%E5%B7%AE%E5%BC%82%E7%9A%84%E5%AE%9E%E9%AA%8C%E7%A0%94%E7%A9%B6&btnG= (accessed on 1 August 2023).
- Qin, Y.J.; Li, X.; Wu, L.X.; Zhou, Y.B. Effects of starvation and refeeding on metabolism and growth in sea urchin, Strongylocentrotus intermedius. J. Dalian Ocean. Univ. 2011, 26, 521–525. [Google Scholar] [CrossRef]
- Cox, L.M.; Blaser, M.J. Antibiotics in early life and obesity. Nat. Rev. Endocrinol. 2015, 11, 182–190. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, L.; Han, Y.; Wu, L.; Wang, B. The development of early life microbiota in human health and disease. Engineering 2022, 12, 101–114. [Google Scholar] [CrossRef]
- Xun, P.; Lin, H.; Wang, R.; Huang, Z.; Zhou, C.; Yu, W.; Huang, Q.; Tan, L.; Wang, Y.; Wang, J. Effects of dietary vitamin B1 on growth performance, intestinal digestion and absorption, intestinal microflora and immune response of juvenile golden pompano (Trachinotus ovatus). Aquaculture 2019, 506, 75–83. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Ma, M.X. Study on the Diversity of the Intestinal Flora of Sebastodes schlegeli under Different Culture Densities and Patterns. 2023. Available online: https://link.cnki.net/doi/10.27821/d.cnki.gdlhy.2023.000348 (accessed on 15 August 2023).
- Liu, Y.Y. Changes of Gut Microbial of Atlantic Salmon Infected with Aeromonas salmonidis and Its Intestinal Mechanism. 2023. Available online: https://link.cnki.net/doi/10.27821/d.cnki.gdlhy.2023.000336 (accessed on 7 May 2023).
- Li, P.F. Effect of Clostridium Butyricum on Intestinal Microbiota and Resistance to Vibrio Alginolyticus of Penaeus Vannamei. 2023. Available online: https://kns.cnki.net/kcms2/article/abstract?v=PAev8JwjQisccbTAct8gW-5OZkYAOFwea7xzAMbsA989l8x24e9KoI87N7IQJvWBju_y4KAGM_HwBtrGDqroUndPJ_REYk3VfNkgOjd6oEnh7MOAPh6QPlfnHE6XTq_rf-POZaSjKI28yav5zUDPjiUxh1ZBRG9SYorXoQ18ZJYyowTqVKKHbL5HF6X7alVfzARA1hxgupA0s6V1BOmbvL_vPun-zvZqXxXttFIWY7RDtQ-Be1e6BRjCZ2RRWN3Dbk8v4wRpbwBQuQsuB_vsaqsSuGXhtDVKU1MH8oBh2hKJ3syeVpWZDuUw8JtXzg5s-e2doAHorYs=&uniplatform=NZKPT&language=CHS (accessed on 22 June 2023).
- Zeng, F.; Wang, L.; Zhen, H.; Guo, C.; Liu, A.; Xia, X.; Pei, H.; Dong, C.; Ding, J. Nanoplastics affect the growth of sea urchins (Strongylocentrotus intermedius) and damage gut health. Sci. Total Environ. 2023, 869, 161576. [Google Scholar] [CrossRef]
- Li, Y.-F.; Yang, N.; Liang, X.; Yoshida, A.; Power, D.; Batista, F.M.; Yang, J.-L. Elevated seawater temperatures decrease microbial diversity in the gut of Mytilus coruscus. Front. Physiol. 2018, 9, 380760. [Google Scholar] [CrossRef]
- Yang, M.-J.; Song, H.; Sun, L.-N.; Yu, Z.-L.; Hu, Z.; Wang, X.-L.; Zhu, J.-Y.; Zhang, T. Effect of temperature on the microflora community composition in the digestive tract of the veined rapa whelk (Rapana venosa) revealed by 16S rRNA gene sequencing. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 145–153. [Google Scholar] [CrossRef]
- Yu, G.; Ou, W.; Liao, Z.; Xu, H.; Liang, M.; Zhang, Y.; Mai, K. Intestinal homeostasis of juvenile tiger puffer Takifugu rubripes was sensitive to dietary arachidonic acid in terms of mucosal barrier and microbiota. Aquaculture 2019, 502, 97–106. [Google Scholar] [CrossRef]
- Bozcal, E.; Dagdeviren, M. Bacterial metagenome analysis of Mytilus galloprovincialis collected from Istanbul and Izmir coastal stations of Turkey. Environ. Monit. Assess. 2020, 192, 186. [Google Scholar] [CrossRef]
- Venâncio, I.; Luís, Â.; Domingues, F.; Oleastro, M.; Pereira, L.; Ferreira, S. The prevalence of Arcobacteraceae in aquatic environments: A systematic review and meta-analysis. Pathogens 2022, 11, 244. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Meirelles, P.M.; Mino, S.; Suda, W.; Oshima, K.; Hattori, M.; Thompson, F.L.; Sakai, Y.; Sawabe, T.; Sawabe, T. Individual Apostichopus japonicus fecal microbiome reveals a link with polyhydroxybutyrate producers in host growth gaps. Sci. Rep. 2016, 6, 21631. [Google Scholar] [CrossRef]
- Dong, P.; Guo, H.; Wang, Y.; Wang, R.; Chen, H.; Zhao, Y.; Wang, K.; Zhang, D. Gastrointestinal microbiota imbalance is triggered by the enrichment of Vibrio in subadult Litopenaeus vannamei with acute hepatopancreatic necrosis disease. Aquaculture 2021, 533, 736199. [Google Scholar] [CrossRef]
- Pei, P.; Liu, X.; Chen, Y.; Wu, J.; Zhong, M.; Lin, Q.; Du, H. Effects of biological water purification grid on microbial community of culture environment and intestine of the shrimp Litopenaeus vannamei. Aquac. Res. 2019, 50, 1300–1312. [Google Scholar] [CrossRef]
- Carasso, S.; Fishman, B.; Lask, L.S.; Shochat, T.; Geva-Zatorsky, N.; Tauber, E. Metagenomic analysis reveals the signature of gut microbiota associated with human chronotypes. bioRxiv 2021. [Google Scholar] [CrossRef]
- Xu, Z.; Gan, L.; Li, T.; Xu, C.; Chen, K.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Transcriptome profiling and molecular pathway analysis of genes in association with salinity adaptation in Nile tilapia Oreochromis niloticus. PLoS ONE 2015, 10, e0136506. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, S.; Wang, B.; Wu, H.; Tang, N.; Zhao, L.; Yang, S.; Liu, Q.; Zhou, B. Transcriptome reveals the effects of early weaning on lipid metabolism and liver health of Yangtze Sturgeon (Acipenser dabryanus). Int. J. Mol. Sci. 2022, 23, 10866. [Google Scholar] [CrossRef]
- Roh, H.; Kim, D.-H. Identification, classification and functional characterization of HSP70s in rainbow trout (Oncorhynchus mykiss) through multi-omics approaches. Fish Shellfish Immunol. 2022, 121, 205–214. [Google Scholar] [CrossRef]
- Vadas, R.; Elner, R.; Garwood, P.; Babb, I. Experimental evaluation of aggregation behavior in the sea urchin Strongylocentrotus droebachiensis: A reinterpretation. Mar. Biol. 1986, 90, 433–448. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Z.; Zhao, C.; Yu, Y.; Ding, P.; Ding, J.; Yang, M.; Chi, X.; Hu, F.; Chang, Y. Interaction among sea urchins in response to food cues. Sci. Rep. 2021, 11, 9985. [Google Scholar] [CrossRef]
- Webster, M.S.; Hixon, M.A. Mechanisms and individual consequences of intraspecific competition in a coral-reef fish. Mar. Ecol. Prog. Ser. 2000, 196, 187–194. [Google Scholar] [CrossRef]
- Robey, R.A.; Hay, N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 2006, 25, 4683–4696. [Google Scholar] [CrossRef]
- Ru, X.; Zhang, L.; Liu, S.; Jiang, Y.; Li, L. Physiological traits of income breeding strategy in the sea cucumber Apostichopus japonicus. Aquaculture 2021, 539, 736646. [Google Scholar] [CrossRef]
- Satiro, T.M.; Carli, G.C.; de Arruda Amorim, J.P.; Koch, J.F.A.; Zanuzzo, F.S.; Takahashi, L.S. Dietary β-glucan ameliorates metabolic stress caused by a high dietary carbohydrate level in Nile tilapia. Aquaculture 2024, 579, 740186. [Google Scholar] [CrossRef]
- Dobly, A.; Martin, S.A.M.; Blaney, S.C.; Houlihan, D. Protein growth rate in rainbow trout (Oncorhynchus mykiss) is negatively correlated to liver 20S proteasome activity. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 137, 75–85. [Google Scholar] [CrossRef]
- Marjara, I.S.; Bain, N.; Evensen, Ø. Naïve Atlantic salmon (Salmo Salar L.) surviving a lethal challenge with infectious pancreatic necrosis virus (IPNV) shows upregulation of antiviral genes in head-kidney, including Vig-2. Aquaculture 2011, 318, 300–308. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Lu, Z.; Huang, R.; Tran, N.T.; Wu, J.; Yang, F.; Ge, H.; Zhong, C.; Sun, Q. Transcriptome and metabolome analyses of sea cucumbers Apostichopus japonicus in Southern China during the summer aestivation period. J. Ocean. Univ. China 2021, 20, 198–212. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Chen, L.; Li, B. The lysosome-phagosome pathway mediates immune regulatory mechanisms in Mesocentrotus nudus against Vibrio coralliilyticus infection. Fish Shellfish Immunol. 2023, 139, 108864. [Google Scholar] [CrossRef]
- Zhang, W.; Lv, Z.; Li, C.; Sun, Y.; Jiang, H.; Zhao, M.; Zhao, X.; Shao, Y.; Chang, Y. Transcriptome profiling reveals key roles of phagosome and NOD-like receptor pathway in spotting diseased Strongylocentrotus intermedius. Fish Shellfish Immunol. 2019, 84, 521–531. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Annealing Temperature (°C) | Sequence (5′ to 3′) |
|---|---|---|---|
| cyp51-F | XP_030851345.1 | 58 | GAGAGATGACATTGGCAGCA |
| cyp51-R | XP_030851345.1 | 58 | AGTGTGGAGGGAGTTTGACG |
| HK-F | XP_786955.2 | 60 | ACTCCATCGTCTCCGAATGC |
| HK-R | XP_786955.2 | 60 | CAACGCCTGCTACATGGAAG |
| Hsp70-F | XP_030845497.1 | 56 | ACACTCATCTCGGAGGAG |
| Hsp70-R | XP_030845497.1 | 56 | CTTTCTTATGCTTTCGCTTGA |
| Egr1-F | XP_030852777.1 | 59 | TCCTGCGAGACGGCTTGTTTC |
| Egr1-R | XP_030852777.1 | 59 | AGGTTGCTGGATGCGTATAGGC |
| FDFT-F | XP_030839983.1 | 59 | GCAGCAGTCATTCAGGCATTGG |
| FDFT-R | XP_030839983.1 | 59 | CAGTGTCCAGGGCTCTCAGAAC |
| SerA-F | XP_030841752.1 | 57 | AGCACCGTTGAAGCACAGGAG |
| SerA-R | XP_030841752.1 | 57 | GCGTGAGGGCGTTGGACAG |
| 18S-F | XP_786791.2 | 56 | GTTCGAAGGCGATCAGATAC |
| 18S-R | XP_786791.2 | 56 | CTGTCAATCCTCACTGTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Gao, C.; Xiao, H.; Ruan, S.; Wang, Y.; Li, X.; Chang, Y.; Zhao, C.; Wang, H.; Han, B.; et al. Feeding Behavior, Gut Microbiota, and Transcriptome Analysis Reveal Individual Growth Differences in the Sea Urchin Strongylocentrotus intermedius. Biology 2024, 13, 705. https://doi.org/10.3390/biology13090705
Ye Q, Gao C, Xiao H, Ruan S, Wang Y, Li X, Chang Y, Zhao C, Wang H, Han B, et al. Feeding Behavior, Gut Microbiota, and Transcriptome Analysis Reveal Individual Growth Differences in the Sea Urchin Strongylocentrotus intermedius. Biology. 2024; 13(9):705. https://doi.org/10.3390/biology13090705
Chicago/Turabian StyleYe, Qi, Chuang Gao, Haoran Xiao, Shuchao Ruan, Yongjie Wang, Xiaonan Li, Yaqing Chang, Chong Zhao, Heng Wang, Bing Han, and et al. 2024. "Feeding Behavior, Gut Microbiota, and Transcriptome Analysis Reveal Individual Growth Differences in the Sea Urchin Strongylocentrotus intermedius" Biology 13, no. 9: 705. https://doi.org/10.3390/biology13090705
APA StyleYe, Q., Gao, C., Xiao, H., Ruan, S., Wang, Y., Li, X., Chang, Y., Zhao, C., Wang, H., Han, B., & Ding, J. (2024). Feeding Behavior, Gut Microbiota, and Transcriptome Analysis Reveal Individual Growth Differences in the Sea Urchin Strongylocentrotus intermedius. Biology, 13(9), 705. https://doi.org/10.3390/biology13090705







