Near-Infrared Spectroscopic Determination of Pentacyclic Triterpenoid Concentrations in Additives for Animal Food
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Food Additive Samples and Chemicals
2.2. Extraction of Triterpenoids
2.3. Analysis and Determination of the Triterpenoid Content
2.4. Collection of NIR Spectra, NIRS Model, and Equation Development
- Standard normal variate (SNV) correction was used for the correction of variations in the baseline produced by the particle size and the dispersion. The spectra were transformed to log(1/R). This consisted of subtracting the mean of the spectrum from each original absorbance value and dividing this result by its standard deviation [17].
- Detrend (DT) correction. The application of this second-grade polynomic function to the absorbance values in log(1/R) and the lambda allows for removal of the linear or quadratic curve in the baseline of the spectra due to the different packing pressures [17].
- SNV and DT (SNVD) correction. This is a combination of both previous corrections, SNV and DT. It corrects the baseline and removes the differences between the spectra corresponding to samples of a similar chemical composition but with different particle sizes [17].
2.5. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rufino-Palomares, E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical role of polyphenols and triterpenes present in the extracts of fruits and leaves of Olea europaea as antioxidants, anti-infectives and anticancer agents on healthy growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef] [PubMed]
- Peragón, J. Time course of pentacyclic triterpenoids from fruits and leaves of olive tree (Olea europaea L.) cv. Picual and cv. Cornezuelo during ripening. J. Agric. Food Chem. 2013, 61, 6671–6678. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Herrera, R.; Pacheco-López, B.; Peragón, J. Water stress, irrrigation and concentrations of pentacyclic triterpenes and phenols in Olea europaea L. cv. Picual olive trees. Antioxidants 2019, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Yera, M.; Peragón, J. Time course profile of pentacyclic triterpenes from stem and root of cv. Picual olive tree (Olea europaea, L.) along ripening. J. Plant Sci. 2020, 4, 186–193. [Google Scholar]
- Fernández-Navarro, M.; Peragón, J.; Esteban, F.J.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid as a feed additive to stimulate growth and hepatic protein-turnover rates in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2006, 144, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Navarro, M.; Peragón, J.; Amores, V.; de la Higuera, M.; Lupiáñez, J.A. Maslinic acid added to the diet increases growth and protein-turnover rates in the white muscle of rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2008, 147, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Palomares, E.; Reyes-Zurita, F.; García-Salguero, L.; Peragón, J.; De La Higuera, M.; Lupiáñez, J.A. Maslinic acid, a natural triterpene, added to diet stimulates growth and protein turnover of white muscle in gilthead sea bream (Sparus aurata) cultured in farmed conditions. Aquac. Nutr. 2012, 18, 568–580. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.; Reyes-Zurita, F.; Fuentes-Almagro, C.; De La Higuera, M.; Lupiáñez, J.A.; Peragón, J. Proteomics in the liver of gilthead sea bream (Sparus aurata) to elucidate the cellular response induced by the intake of maslinic acid. Proteomics 2012, 11, 3312–3325. [Google Scholar] [CrossRef]
- Torrecilla, J.S.; Cancilla, J.C.; Perez-Calabuig, A.; Pradana-Lopez, S. Spectroscopy to evaluate the quality control of extra-virgin olive oils. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: London, UK, 2021; Volume 8, pp. 91–97. [Google Scholar]
- Mustorgi, E.; Malegori, C.; Oliveri, P.; Hooshyary, M.; Bounneche, H.; Mondello, L.; Oteri, M.; Casale, M. A chemometric strategy to evaluate the comparability of PLS models obtained from quartz cuvettes and disposable glass vials in the determination of extra virgin olive oil quality parameters by NIR spectroscopy. Chemom. Intell. Lab. Syst. 2020, 199, 103974. [Google Scholar] [CrossRef]
- Casson, A.; Beghi, R.; Giovenzana, V.; Riorind, I.; Tugnolo, A.; Guidetti, R. Environmental advantages of visible and near infrared spectroscopy for the prediction of intact olive ripeness. Biosyst. Eng. 2020, 180, 1–10. [Google Scholar] [CrossRef]
- Altieri, G.; Matera, A.; Genovese, F.; Di Renzo, G.C. Models for the rapid assessment of water and oil content in olive pomace by near-infrared spectrometry. J. Sci. Food Agric. 2020, 100, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Peragón, J.; Rufino-Palomares, E.E.; Muñoz-Espada, I.; Reyes-Zurita, F.J.; Lupiáñez, J.A. A new HPLC method for measuring maslinic acid and oleanolic acid in HT29 and HepG2 human cancer cells. Int. J. Mol. Sci. 2015, 16, 21681–21694. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Chen, G.; Zhang, G.; Wang, D.; Tilley, M.; Li, Y. Rapid determination of total phenolic content of whole wheat flour using near-infrared spectroscopy and chemometrics. Food Chem. 2021, 344, 128633. [Google Scholar] [CrossRef]
- Shenk, J.S.; Waterhaus, M.O. Population Definition, Sample Selection and Calibration Procedures for Near Infrared Reflectance Spectroscopy. Crop Sci. 1991, 31, 469–474. [Google Scholar] [CrossRef]
- Mahalanobis, P.C. On the generalised distance in statistics. Proc. Natl. Inst. Sci. India 1936, 2, 49–55. [Google Scholar]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Mu, T.H.; Sun, H.N. Sweet potato leaf polyphenols: Preparation, individual phenolic compound composition and antioxidant activity. In Polyphenols in Plants, 2nd ed.; Academic Press: London, UK; Elsevier: London, UK, 2019; pp. 365–380. [Google Scholar]
- Williams, P. Near—Infrared Technology—Getting the Best Out of Light; PDK Projects, Inc.: Nanaimo, BC, Canada; Winnipeg, MB, Canada, 2007. [Google Scholar]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Posthavest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Mouazen, A.; Saeys, W.; Xing, J.; De Baerdemaeker, J.; Ramon, H. Near infrared spectroscopy for agricultural materials: An instrument. J. Near Infrared Spectrosc. 2005, 13, 87–98. [Google Scholar] [CrossRef]
- Salguero-Chaparro, L.; Baeten, V.; Fernández-Pierna, J.A.; Peña-Rodríguez, F. Near infrared spectroscopy (NIRS) for on-line determination of quality parameters in intact olives. Food Chem. 2013, 139, 1121–1126. [Google Scholar] [CrossRef]
- Salguero-Chaparro, L.; Gaitán-Jurado, A.J.; Ortiz-Somovilla, V.; Peña-Rodriguez, F. Feasibility of using NIR spectroscopy to detect herbicide residues in intact olives. Food Control 2013, 30, 504–509. [Google Scholar] [CrossRef]
- Valinger, D.; Kusen, M.; Tusek, A.J.; Panic, M.; Jurina, T.; Benkovic, M.; Redovnikovic, I.R.; Kljusuric, J.G. Development of near infrared spectroscopy models for quantitative prediction of the content of bioactive compounds in olive leaves. Chem. Biochem. Eng. Q. 2018, 32, 535–543. [Google Scholar] [CrossRef]
- Torres, I.; Sánchez, M.T.; Belloch-Gozález, M.; Pérez-Marín, D. Irrigation decision support base don leaf relative water content determination in olive grove using near infrared spectroscopy. Biosyst. Eng. 2019, 180, 50–58. [Google Scholar] [CrossRef]
- Grassi, S.; Jolayemi, O.S.; Giovenzana, V.; Tugnolo, A.; Squeo, G.; Conte, P.; De Bruno, A.; Flamminii, F.; Casiraghi, E.; Alamprese, C. Near infrared spectroscopy as a green technology for the quality prediction of intact olives. Foods 2021, 10, 1042. [Google Scholar] [CrossRef]


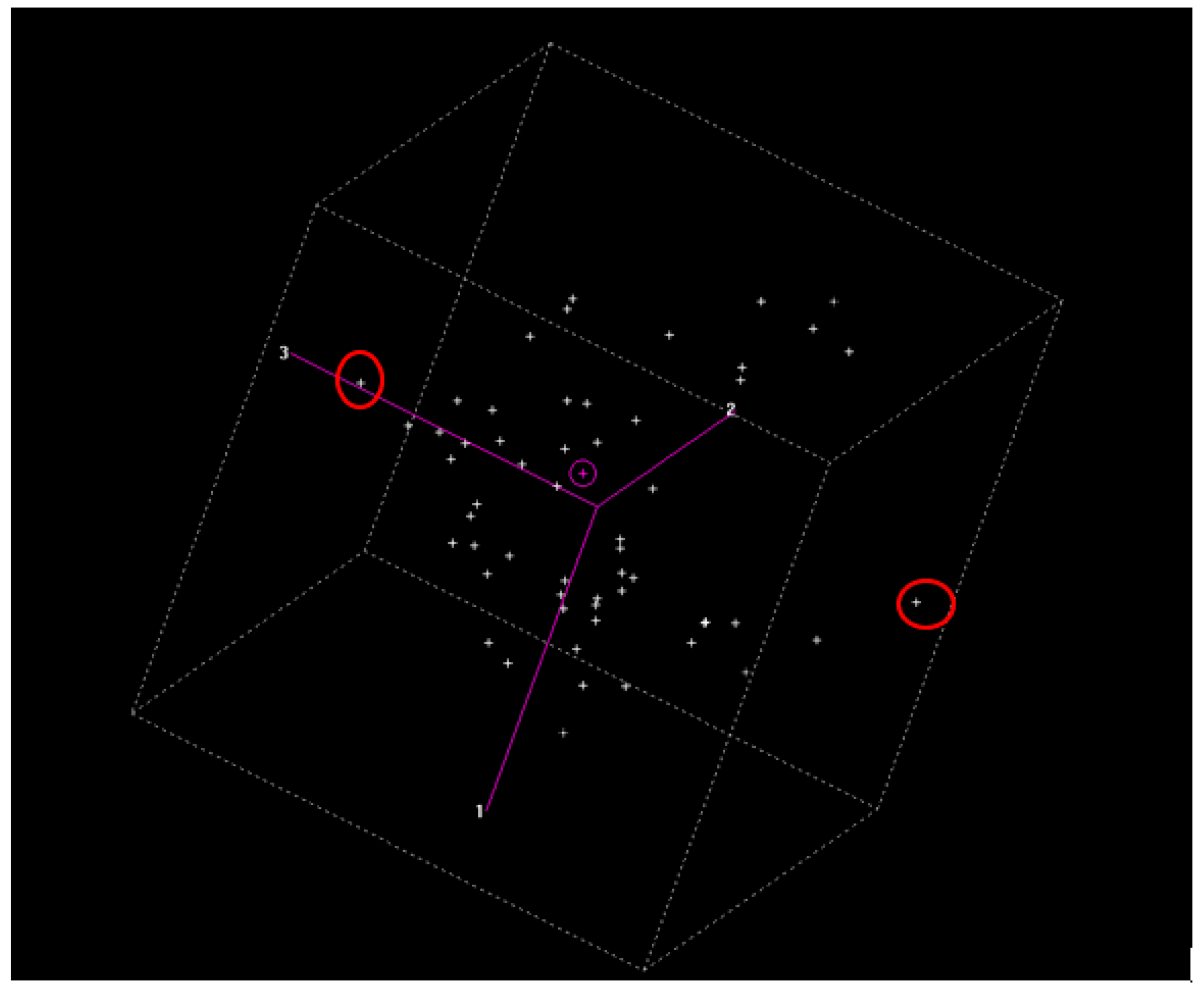
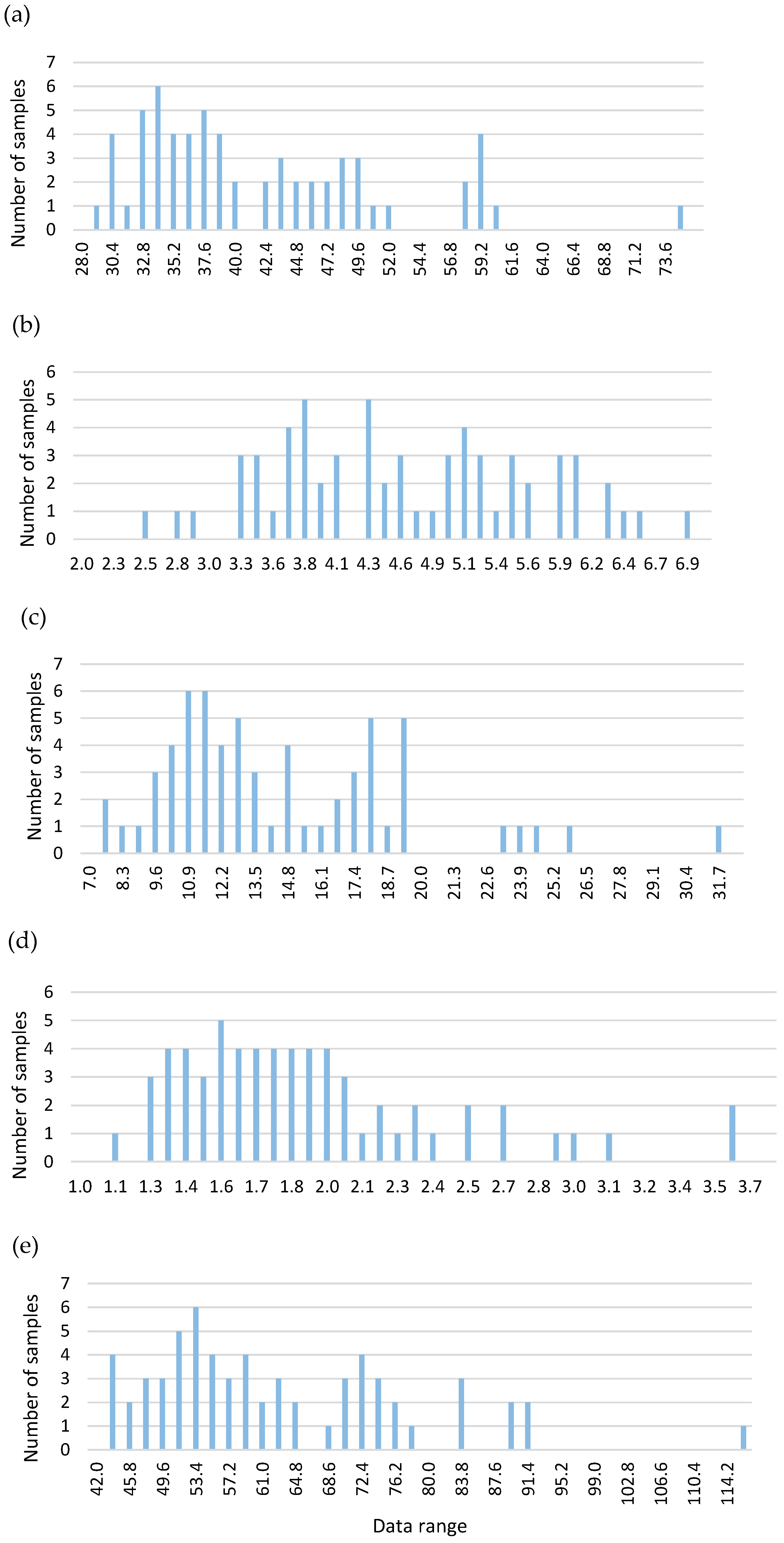
| MA | iBA | OA | UO | Total | |
|---|---|---|---|---|---|
| Minimum | 28.89 | 2.40 | 10.08 | 1.10 | 42.47 |
| Maximum | 74.08 | 6.83 | 29.75 | 3.60 | 114.26 |
| Mean | 41.27 | 4.57 | 14.19 | 1.87 | 61.90 |
| SD | 9.59 | 1.03 | 2.13 | 0.54 | 14.68 |
| Total Samples | 63 | 63 | 63 | 63 | 63 |
| Mean | Minimum | Maximum | R2 | SECV | |
|---|---|---|---|---|---|
| MA | 39.85 | 16.28 | 63.43 | 0.75 | 4.97 |
| iBA | 4.59 | 1.48 | 7.69 | 0.77 | 0.78 |
| OA | 13.76 | 0.90 | 26.62 | 0.76 | 2.49 |
| UO | 1.78 | 0.66 | 2.89 | 0.75 | 0.28 |
| Total | 60.35 | 21.83 | 98.87 | 0.77 | 7.40 |
| MA | iBA | OA | UO | Total | |
|---|---|---|---|---|---|
| N | 6 | 6 | 6 | 6 | 6 |
| Sep | 4.61 | 0.70 | 1.32 | 0.44 | 2.91 |
| R2 | 0.90 | 0.76 | 0.99 | 0.54 | 0.97 |
| BIAS | 1.75 | 0.10 | -0.66 | 0.12 | 0.73 |
| mg trolox equivalents/g of wet sample | 9877.62 ± 466.5 (9) |
| mmol trolox equivalents/100 g of wet sample | 3944.90 ± 186.3 (9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugráñez-Pérez, C.; Sugráñez-Serrano, R.; López-González, M.; Martínez-Vaquero, S.; Moral-Martos, D.; Cortés-Jiménez, S.; Peragón-Sánchez, J. Near-Infrared Spectroscopic Determination of Pentacyclic Triterpenoid Concentrations in Additives for Animal Food. Biology 2024, 13, 578. https://doi.org/10.3390/biology13080578
Sugráñez-Pérez C, Sugráñez-Serrano R, López-González M, Martínez-Vaquero S, Moral-Martos D, Cortés-Jiménez S, Peragón-Sánchez J. Near-Infrared Spectroscopic Determination of Pentacyclic Triterpenoid Concentrations in Additives for Animal Food. Biology. 2024; 13(8):578. https://doi.org/10.3390/biology13080578
Chicago/Turabian StyleSugráñez-Pérez, Carmen, Rafael Sugráñez-Serrano, Marta López-González, Sara Martínez-Vaquero, Daniel Moral-Martos, Sofía Cortés-Jiménez, and Juan Peragón-Sánchez. 2024. "Near-Infrared Spectroscopic Determination of Pentacyclic Triterpenoid Concentrations in Additives for Animal Food" Biology 13, no. 8: 578. https://doi.org/10.3390/biology13080578
APA StyleSugráñez-Pérez, C., Sugráñez-Serrano, R., López-González, M., Martínez-Vaquero, S., Moral-Martos, D., Cortés-Jiménez, S., & Peragón-Sánchez, J. (2024). Near-Infrared Spectroscopic Determination of Pentacyclic Triterpenoid Concentrations in Additives for Animal Food. Biology, 13(8), 578. https://doi.org/10.3390/biology13080578






