The Impact of Diabetes on Male Silkworm Reproductive Health
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Experimental Model
2.3. Preparation of Silkworm Hemolymph
2.4. Preparation of Silkworm Fat Body Samples
2.5. Determination of T, LH, FSH, and TG
2.6. Histological Analysis of the Silkworm Testicles
2.7. Determination of SOD, MDA, and GSH-Px
2.8. Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. Type II Diabetes Silkworm Model
3.2. Diabetic Silkworm Reproductive Damage Model
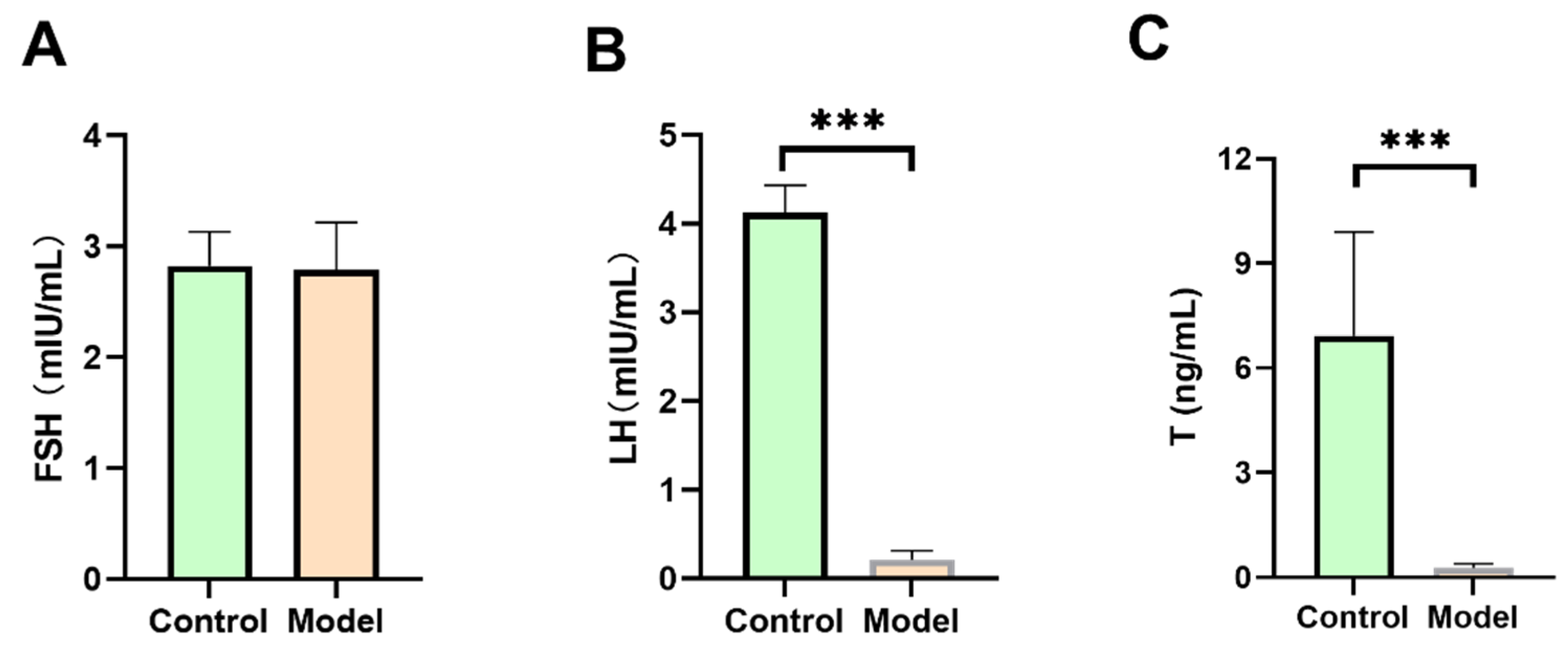
3.2.1. FSH, LH, and T Levels in Diabetic Silkworms
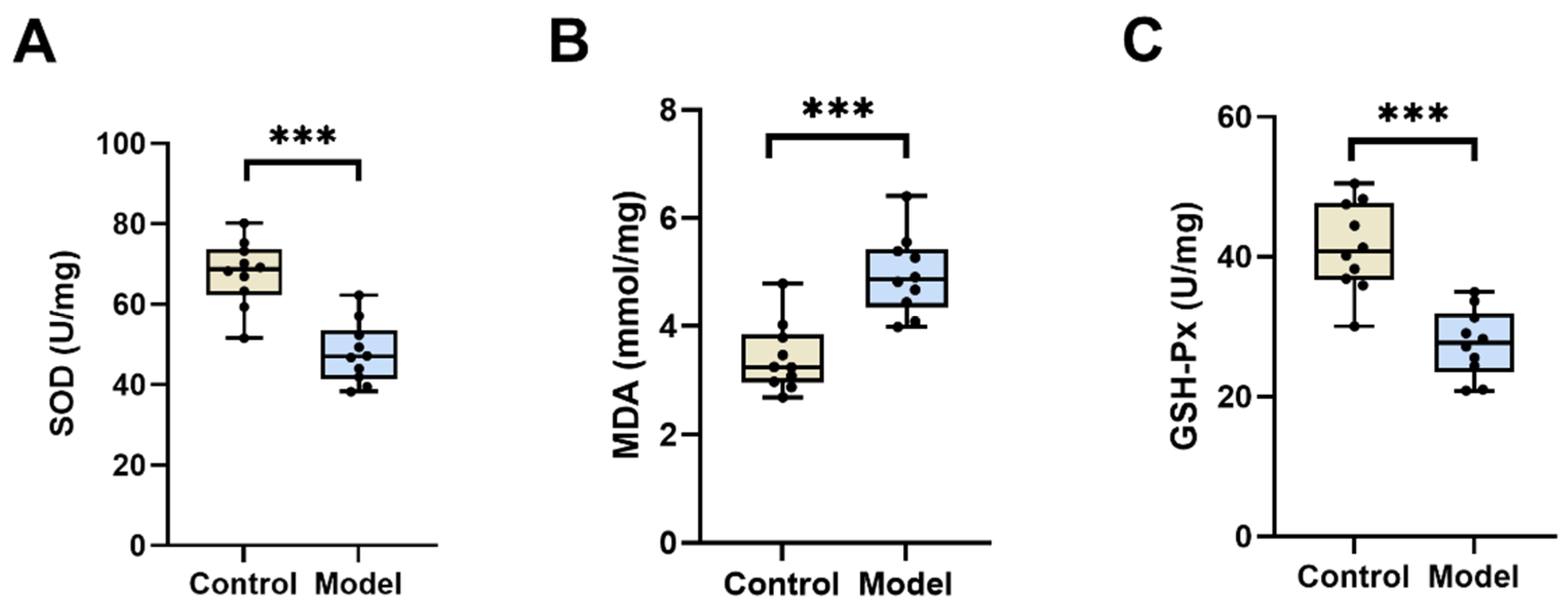
3.2.2. SOD, MDA, and GSH-Px Levels in the Testis of Diabetic Silkworms
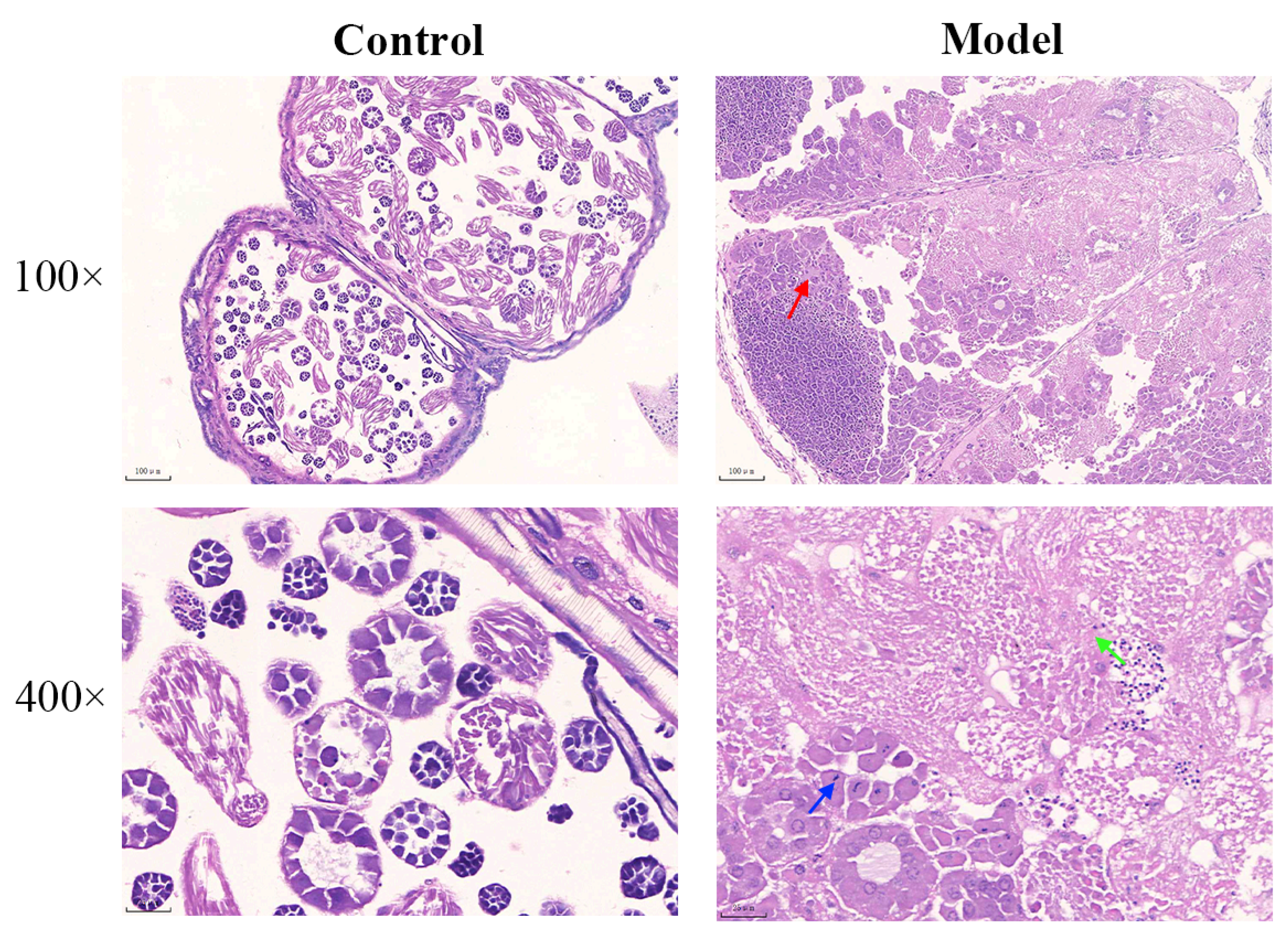
3.2.3. Diabetic Silkworm Testis Histopathological Changes
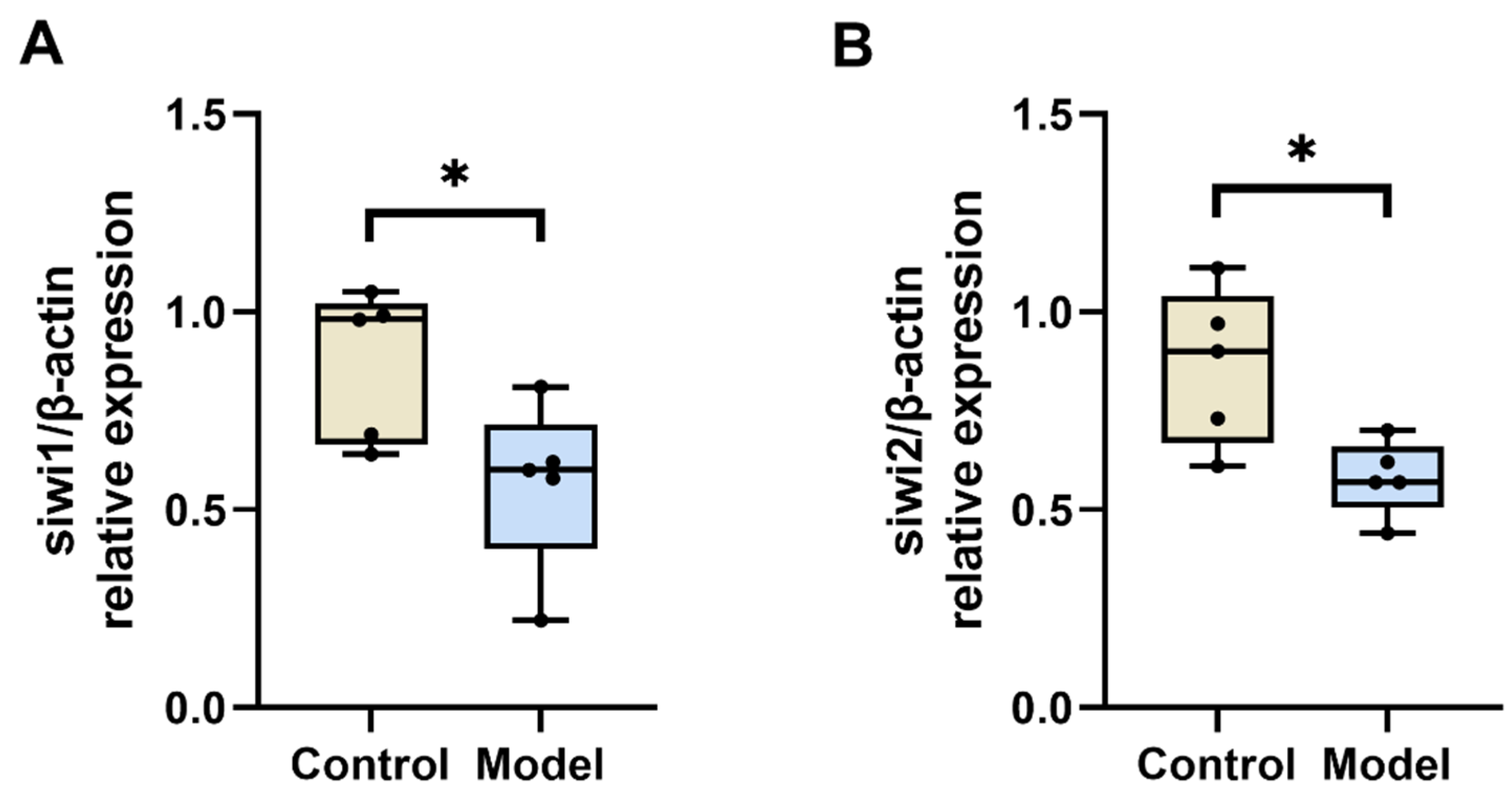
3.2.4. Diabetic Silkworm Testicular Tissue siwi1 and siwi2 mRNA Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giribabu, N.; Kumar, K.E.; Rekha, S.S.; Muniandy, S.; Salleh, N. Chlorophytum borivilianum root extract maintains near normal blood glucose, insulin and lipid profile levels and prevents oxidative stress in the pancreas of streptozotocin-induced adult male diabetic rats. Int. J. Med. Sci. 2014, 11, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Claude Mbanya, J.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119, Erratum in Diabetes Res. Clin. Pract. 2023, 204, 110945. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes Mellitus Causes Male Reproductive Dysfunction: A Review of the Evidence and Mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Ishii, M.; Hayashi, Y.; Miyazaki, S.; Sugita, T.; Sumiya, E.; Sekimizu, K. Diabetic silkworms for evaluation of therapeutically effective drugs against type II diabetes. Sci. Rep. 2015, 29, 10722. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef]
- Yuan, H.D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27–39. [Google Scholar] [CrossRef]
- Phong, N.V.; Gao, D.; Kim, J.A.; Yang, S.Y. Optimization of Ultrasonic-Assisted Extraction of α-Glucosidase Inhibitors from Dryopteris crassirhizoma Using Artificial Neural Network and Response Surface Methodology. Metabolites 2023, 13, 557. [Google Scholar] [CrossRef]
- Brito-Casillas, Y.; Melián, C.; Wägner, A.M. Study of the pathogenesis and treatment of diabetes mellitus through animal models. Endocrinol. Nutr. 2016, 63, 345–353. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Zhang, R.; Liu, Y.; Wang, C.; Song, G.; Yu, J.; Chen, Z. Phenotypic characterization of a novel type 2 diabetes animal model in a SHANXI MU colony of Chinese hamsters. Endocrine 2019, 65, 61–72. [Google Scholar] [CrossRef]
- Landi, M.; Everitt, J.; Berridge, B. Bioethical, Reproducibility, and Translational Challenges of Animal Models. ILAR J. 2021, 62, 60–65. [Google Scholar] [CrossRef]
- Philipson, T.J.; Sun, E.; Goldman, D.; Jena, A.B. A Reexamination of the Costs of Medical R&D Regulation. Forum Health Econ. Policy 2012, 15, 1–28. [Google Scholar] [CrossRef]
- Xia, Q.; Zhou, Z.; Lu, C.; Cheng, D.; Dai, F.; Li, B.; Zhao, P.; Zha, X.; Cheng, T.; Chai, C.; et al. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 2004, 306, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; O’Brochta, D.A. Advanced technologies for genetically manipulating the silkworm Bombyx mori, a model Lepidopteran insect. Proc. Biol. Sci. 2015, 282, 1810. [Google Scholar] [CrossRef]
- Zhang, Z.; Teng, X.; Chen, M.; Li, F. Orthologs of human disease associated genes and RNAi analysis of silencing insulin receptor gene in Bombyx mori. Int. J. Mol. Sci. 2014, 15, 18102–18116. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Wang, Y.F.; Wang, Y.L.; Li, Q.Y.; Ma, H.Y.; Wang, L.; Sima, Y.H.; Xu, S.Q. Induced Hyperproteinemia and Its Effects on the Remodeling of Fat Bodies in Silkworm, Bombyx mori. Front. Physiol. 2018, 9, 302. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.D.; Monteagudo Santesteban, B.; Cenis, J.L. Products of Sericulture and Their Hypoglycemic Action Evaluated by Using the Silkworm, Bombyx mori (Lepidoptera: Bombycidae), as a Model. Insects 2021, 12, 1059. [Google Scholar] [CrossRef]
- Ishii, M.; Matsumoto, Y.; Sekimizu, K. Inhibitory effects of alpha-cyclodextrin and its derivative against sucrose-induced hyperglycemia in an in vivo evaluation system. Drug Discov. Ther. 2018, 12, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, M.; Ogihara, M.H.; Kataoka, H. Sterol Characteristics in Silkworm Brain and Various Tissues Characterized by Precise Sterol Profiling Using LC-MS/MS. Int. J. Mol. Sci. 2019, 20, 4840. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.L.; Pfotenhauer, K.M. Classification and Diagnosis of Diabetes. Prim. Care 2022, 49, 191–200. [Google Scholar] [CrossRef]
- Iranmanesh, A.; Lawson, D.; Veldhuis, J.D. Glucose ingestion acutely lowers pulsatile LH and basal testosterone secretion in men. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E724–E730. [Google Scholar] [CrossRef]
- Redl, S.; de Jesus Domingues, A.M.; Caspani, E.; Möckel, S.; Salvenmoser, W.; Mendez-Lago, M.; Ketting, R.F. Extensive nuclear gyration and pervasive non-genic transcription during primordial germ cell development in zebrafish. Development 2021, 148, 193060. [Google Scholar] [CrossRef]
- Doke, S.K.; Dhawale, S.C. Alternatives to animal testing: A review. Saudi Pharm. J. 2015, 23, 223–229. [Google Scholar] [CrossRef]
- Kaito, C.; Murakami, K.; Imai, L.; Furuta, K. Animal infection models using non-mammals. Microbiol. Immunol. 2020, 64, 585–592. [Google Scholar] [CrossRef]
- Hou, J.; Tan, C.; Chen, N.; Zhou, Y.; Huang, S.; Chen, H.; Qian, L. Establishment of diabetes mellitus model using Bombyx mori silkworms in a low-temperature environment. Arch. Insect Biochem. Physiol. 2024, 115, e22083. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Sekimizu, K. Evaluation of anti-diabetic drugs by using silkworm, Bombyx mori. Drug Discov. Ther. 2016, 10, 19–23. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Ji, H.; Ji, Y.; Yang, J.; Huang, J.; Sun, D. Involvement of hypoxia-inducible factor-1α in the oxidative stress induced by advanced glycation end products in murine Leydig cells. Toxicol. Vitr. 2016, 32, 146–153. [Google Scholar] [CrossRef]
- Fui, M.N.; Dupuis, P.; Grossmann, M. Lowered testosterone in male obesity: Mechanisms, morbidity and management. Asian J. Androl. 2014, 16, 223–231. [Google Scholar] [CrossRef]
- Keber, R.; Rozman, D.; Horvat, S. Sterols in spermatogenesis and sperm maturation. J. Lipid Res. 2013, 54, 20–33. [Google Scholar] [CrossRef]
- Kim, C.Y.; Kim, K.H. Curcumin prevents leptin-induced tight junction dysfunction in intestinal Caco-2 BBe cells. J. Nutr. Biochem. 2014, 25, 26–35. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Xin, C.; Liu, J.; Wang, Q.; He, Y.; Liu, M.; Fu, M.; Yang, Y.; Cao, X. The Identification of Anthocyanins from Padus racemosa and Its Protective Effects on H(2) O(2) -Induced INS-1 Cells Damage and STZ-Induced Diabetes Mice. Chem. Biodivers. 2020, 17, e2000382. [Google Scholar] [CrossRef]
- Shalaby, M.A.; El-Zorba, H.Y.; Kamel, G.M. Effect of alpha-tocopherol and simvastatin on male fertility in hypercholesterolemic rats. Pharmacol. Res. 2004, 50, 137–142. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- El-Missiry, M.A. Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 124, 233–237. [Google Scholar] [CrossRef]
- Kong, Z.L.; Sudirman, S.; Hsu, Y.C.; Su, C.Y.; Kuo, H.P. Fucoxanthin-Rich Brown Algae Extract Improves Male Reproductive Function on Streptozotocin-Nicotinamide-Induced Diabetic Rat Model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef]
- Chen, Y.; Jiao, N.; Jiang, M.; Liu, L.; Zhu, Y.; Wu, H.; Chen, J.; Fu, Y.; Du, Q.; Xu, H.; et al. Loganin alleviates testicular damage and germ cell apoptosis induced by AGEs upon diabetes mellitus by suppressing the RAGE/p38MAPK/NF-κB pathway. J. Cell Mol. Med. 2020, 24, 6083–6095. [Google Scholar] [CrossRef]
- Luo, L.F.; Hou, C.C.; Yang, W.X. Small non-coding RNAs and their associated proteins in spermatogenesis. Gene 2016, 578, 141–157. [Google Scholar] [CrossRef]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef]
- Grivna, S.T.; Beyret, E.; Wang, Z.; Lin, H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006, 20, 1709–1714. [Google Scholar] [CrossRef]
- Watanabe, T.; Takeda, A.; Tsukiyama, T.; Mise, K.; Okuno, T.; Sasaki, H.; Minami, N.; Imai, H. Identification and characterization of two novel classes of small RNAs in the mouse germline: Retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev. 2006, 20, 1732–1743. [Google Scholar] [CrossRef]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006, 442, 203–207. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, T.; Tong, J.; Caidan, R.; Wang, K.; Kai, G.; Zhang, W.; Ru, L.; Pengcuo, J.; Tong, L. Screening active components from Rubus amabilis for pancreatic β-cells protection. Pharm. Biol. 2020, 58, 674–685. [Google Scholar] [CrossRef]

| Primer | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | GCCGTGGTGCTCAACAAAACATC | ATGCCATTCCAGTCAGCTTGCC |
| siwi1 | ACTCCCCTGGATCTGCTTGCC | TCCTCCGAGTGTTTTGCTGTGAAC |
| siwi2 | GCACACGTCGTTTGAGCGAAAG | ATGTTGGCGTCCGAAGCGAATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Tong, L. The Impact of Diabetes on Male Silkworm Reproductive Health. Biology 2024, 13, 557. https://doi.org/10.3390/biology13080557
Zeng X, Tong L. The Impact of Diabetes on Male Silkworm Reproductive Health. Biology. 2024; 13(8):557. https://doi.org/10.3390/biology13080557
Chicago/Turabian StyleZeng, Xiaoyan, and Li Tong. 2024. "The Impact of Diabetes on Male Silkworm Reproductive Health" Biology 13, no. 8: 557. https://doi.org/10.3390/biology13080557
APA StyleZeng, X., & Tong, L. (2024). The Impact of Diabetes on Male Silkworm Reproductive Health. Biology, 13(8), 557. https://doi.org/10.3390/biology13080557





