Comparative Study of Cardiovascular Effects of Selected Pulmonary Vasodilators in Canine Models of Mitral Valve Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Preparation
2.2. Study Protocol
2.3. Hemodynamic Measurement
2.4. Echocardiography
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.A.; Borgarelli, M.; Scansen, B.A. ACVIM Consensus Statement Guidelines for the Diagnosis, Classification, Treatment, and Monitoring of Pulmonary Hypertension in Dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The Relationship Between the Right Ventricle and Its Load in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Yuchi, Y.; Suzuki, R.; Kanno, H.; Teshima, T.; Matsumoto, H.; Koyama, H. Right Ventricular Myocardial Adaptation Assessed by Two-Dimensional Speckle Tracking Echocardiography in Canine Models of Chronic Pulmonary Hypertension. Front. Vet. Sci. 2021, 8, 727155. [Google Scholar] [CrossRef] [PubMed]
- Feldhütter, E.K.; Domenech, O.; Vezzosi, T.; Tognetti, R.; Eberhard, J.; Friederich, J.; Wess, G. Right Ventricular Size and Function Evaluated by Various Echocardiographic Indices in Dogs with Pulmonary Hypertension. J. Vet. Intern. Med. 2022, 36, 1882–1891. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Gibbs, J.S.R.; Wachter, R.; De Marco, T.; Vonk-Noordegraaf, A.; Vachiéry, J.L. Left Ventricular Heart Failure and Pulmonary Hypertension. Eur. Heart J. 2016, 37, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Borlaug, B.A. Pulmonary Hypertension Due to Left Heart Disease. Circulation 2012, 126, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Kellihan, H.B.; Stepien, R.L. Pulmonary Hypertension in Canine Degenerative Mitral Valve Disease. J. Vet. Cardiol. 2012, 14, 149–164. [Google Scholar] [CrossRef]
- Kellum, H.B.; Stepien, R.L. Sildenafil Citrate Therapy in 22 Dogs with Pulmonary Hypertension. J. Vet. Intern. Med. 2007, 21, 1258–1264. [Google Scholar] [CrossRef]
- Jaffey, J.A.; Leach, S.B.; Kong, L.R.; Wiggen, K.E.; Bender, S.B.; Reinero, C.R. Clinical Efficacy of Tadalafil Compared to Sildenafil in Treatment of Moderate to Severe Canine Pulmonary Hypertension: A Pilot Study. J. Vet. Cardiol. 2019, 24, 7–19. [Google Scholar] [CrossRef]
- Sastry, B.K.S.; Narasimhan, C.; Reddy, N.K.; Raju, B.S. Clinical Efficacy of Sildenafil in Primary Pulmonary Hypertension: A Randomized, Placebo-Controlled, Double-Blind, Crossover Study. J. Am. Coll. Cardiol. 2004, 43, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Yoshida, T.; Sennba, S.; Uchide, T.; Tanaka, R. Adjunct Ambrisentan Therapy Had Clinical Benefits in 5 Dogs with Sildenafil-Refractory Pulmonary Hypertension. Can. Vet. J. 2022, 63, 497. [Google Scholar] [PubMed]
- Galiè, N.; Olschewski, H.; Oudiz, R.J.; Torres, F.; Frost, A.; Ghofrani, H.A.; Badesch, D.B.; McGoon, M.D.; McLaughlin, V.V.; Roecker, E.B.; et al. Ambrisentan for the Treatment of Pulmonary Arterial Hypertension: Results of the Ambrisentan in Pulmonary Arterial Hypertension, Randomized, Double-Blind, Placebo-Controlled, Multicenter, Efficacy (ARIES) Study 1 and 2. Circulation 2008, 117, 3010–3019. [Google Scholar] [CrossRef]
- Suzuki, R.; Yuchi, Y.; Saito, T.; Yasumura, Y.; Teshima, T.; Matsumoto, H.; Koyama, H. Beraprost Sodium for Pulmonary Hypertension in Dogs: Effect on Hemodynamics and Cardiac Function. Animals 2022, 12, 2078. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Yuchi, Y.; Saito, T.; Teshima, T.; Matsumoto, H.; Koyama, H. Investigation of Beraprost Sodium on Cardiac Function and Hemodynamics in Canine Models of Chronic Pulmonary Hypertension. Front. Vet. Sci. 2022, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiéry, J.-L.; Vizza, C.D.; Kneussl, M.; Manes, A.; Sitbon, O.; Torbicki, A.; Delcroix, M.; Naeije, R.; et al. Effects of Beraprost Sodium, an Oral Prostacyclin Analogue, in Patients with Pulmonary Arterial Hypertension: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Cardiol. 2002, 39, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Hoendermis, E.S.; Liu, L.C.Y.; Hummel, Y.M.; Van Der Meer, P.; De Boer, R.A.; Berger, R.M.F.; Van Veldhuisen, D.J.; Voors, A.A. Effects of Sildenafil on Invasive Haemodynamics and Exercise Capacity in Heart Failure Patients with Preserved Ejection Fraction and Pulmonary Hypertension: A Randomized Controlled Trial. Eur. Heart J. 2015, 36, 2565–2573. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Chen, H.H.; Borlaug, B.A.; Semigran, M.J.; Lee, K.L.; Lewis, G.; Lewinter, M.M.; Rouleau, J.L.; Bull, D.A.; Mann, D.L.; et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure with Preserved Ejection Fraction A Randomized Clinical Trial. JAMA 2013, 309, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Boilson, B.A.; Schirger, J.A.; Borlaug, B.A. Caveat Medicus! Pulmonary Hypertension in the Elderly: A Word of Caution. Eur. J. Heart Fail. 2010, 12, 89–93. [Google Scholar] [CrossRef]
- Sun, D.; Yang, W.; Wang, Z.; Gao, B. Efficacy of Beraprost Sodium Combined with Sildenafil and Its Effects on Vascular Endothelial Function and Inflammation in Patients Experiencing Left Heart Failure Complicated with Pulmonary Arterial Hypertension. Med. Sci. Monit. 2021, 27, e928413. [Google Scholar] [CrossRef]
- Suzuki, R.; Matsumoto, H.; Teshima, T.; Mochizuki, Y.; Koyama, H. Dobutamine Stress Echocardiography for Assessment of Systolic Function in Dogs with Experimentally Induced Mitral Regurgitation. J. Vet. Intern. Med. 2014, 28, 386–392. [Google Scholar] [CrossRef]
- Akabane, R.; Sato, T.; Sakatani, A.; Ogawa, M.; Nagakawa, M.; Miyakawa, H.; Miyagawa, Y.; Tazaki, H.; Takemura, N. Pharmacokinetics of Single Dose Sildenafil Orally Administered in Canine Models of Chronic Embolic Pulmonary Hypertension. J. Vet. Med. Sci. 2020, 82, 446–451. [Google Scholar] [CrossRef]
- Lewis, J.F.; Kuo, L.C.; Nelson, J.G.; Limacher, M.C.; Quinones, M.A. Pulsed Doppler Echocardiographic Determination of Stroke Volume and Cardiac Output: Clinical Validation of Two New Methods Using the Apical Window. Circulation 1984, 70, 425–431. [Google Scholar] [CrossRef]
- Rishniw, M.; Caivano, D.; Dickson, D.; Vatne, L.; Harris, J.; Matos, J.N. Two-Dimensional Echocardiographic Left- Atrial-to-Aortic Ratio in Healthy Adult Dogs: A Reexamination of Reference Intervals. J. Vet. Cardiol. 2019, 26, 29–38. [Google Scholar] [CrossRef]
- Schiller, N.B.; Shah, P.M.; Crawford, M.; DeMaria, A.; Devereux, R.; Feigenbaum, H.; Gutgesell, H.; Reichek, N.; Sahn, D.; Schnittger, I. Recommendations for Quantitation of the Left Ventricle by Two-Dimensional Echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J. Am. Soc. Echocardiogr. 1989, 2, 358–367. [Google Scholar] [CrossRef]
- Ishikawa, T.; Fukushima, R.; Suzuki, S.; Miyaishi, Y.; Nishimura, T.; Hira, S.; Hamabe, L.; Tanaka, R. Echocardiographic Estimation of Left Atrial Pressure in Beagle Dogs with Experimentally-Induced Mitral Valve Regurgitation. J. Vet. Med. Sci. 2011, 73, 1015–1024. [Google Scholar] [CrossRef][Green Version]
- Gentile-Solomon, J.M.; Abbott, J.A. Conventional Echocardiographic Assessment of the Canine Right Heart: Reference Intervals and Repeatability. J. Vet. Cardiol. 2016, 18, 234–247. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography Endorsed by the European Association of Echocardiography, a Registered Branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Visser, L.C.; Sintov, D.J.; Oldach, M.S. Evaluation of Tricuspid Annular Plane Systolic Excursion Measured by Two-Dimensional Echocardiography in Healthy Dogs: Repeatability, Reference Intervals, and Comparison with M-Mode Assessment. J. Vet. Cardiol. 2018, 20, 165–174. [Google Scholar] [CrossRef]
- Visser, L.C.; Scansen, B.A.; Schober, K.E.; Bonagura, J.D. Echocardiographic Assessment of Right Ventricular Systolic Function in Conscious Healthy Dogs: Repeatability and Reference Intervals. J. Vet. Cardiol. 2015, 17, 83–96. [Google Scholar] [CrossRef]
- Suzuki, R.; Matsumoto, H.; Teshima, T.; Koyama, H. Clinical Assessment of Systolic Myocardial Deformations in Dogs with Chronic Mitral Valve Insufficiency Using Two-Dimensional Speckle-Tracking Echocardiography. J. Vet. Cardiol. 2013, 15, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM Consensus Guidelines for the Diagnosis and Treatment of Myxomatous Mitral Valve Disease in Dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Suzuki, S.; Fukushima, R.; Ishikawa, T.; Yamamoto, Y.; Hamabe, L.; Kim, S.; Yoshiyuki, R.; Machida, N.; Tanaka, R. Comparative Effects of Amlodipine and Benazepril on Left Atrial Pressure in Dogs with Experimentally-Induced Mitral Valve Regurgitation. BMC Vet. Res. 2012, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Matsuura, H.; Kanai, N.; Fukatsu, Y.; Hirano, T.; Nishikawa, N.; Kameoka, K.; Umetsu, T. The in Vitro and Ex Vivo Antiplatelet Effect of TRK-100, a Stable Prostacyclin Analog, in Several Species. Jpn. J. Pharmacol. 1988, 47, 1–10. [Google Scholar] [CrossRef]
- Braunwald, E. Heart Disease: A Textbook of Cardiovascular Medicine, 3rd ed.; Braunwald, E., Ed.; Saunders: Philadelphia, PA, USA, 1988; ISBN 0721619541. [Google Scholar]
- Akiba, T.; Miyazaki, M.; Toda, N. Vasodilator Actions of TRK-100, a New Prostaglandin I2 Analogue. Br. J. Pharmacol. 1986, 89, 703–711. [Google Scholar] [CrossRef]
- Tamura, M.; Kurumatani, H.; Matsushita, T. Comparative Effects of Beraprost, a Stable Analogue of Prostacyclin, with PGE(1), Nitroglycerin and Nifedipine on Canine Model of Vasoconstrictive Pulmonary Hypertension. Prostaglandins Leukot. Essent. Fatty Acids 2001, 64, 197–202. [Google Scholar] [CrossRef]
- Koh, E.; Morimoto, S.; Jiang, B.; Inoue, T.; Nabata, T.; Kitano, S.; Yasuda, O.; Fukuo, K.; Ogihara, T. Effects of Beraprost Sodium, a Stable Analogue of Prostacyclin, on Hyperplasia, Hypertrophy and Glycosaminoglycan Synthesis of Rat Aortic Smooth Muscle Cells. Artery 1993, 20, 242–252. [Google Scholar]
- McLaughlin, V.V.; McGoon, M.D. Pulmonary Arterial Hypertension. Circulation 2006, 114, 1417–1431. [Google Scholar] [CrossRef]
- Akabane, R.; Sakatani, A.; Ogawa, M.; Nagakawa, M.; Miyakawa, H.; Miyagawa, Y.; Takemura, N. The Effect of Sildenafil on Pulmonary Haemodynamics in a Canine Model of Chronic Embolic Pulmonary Hypertension. Res. Vet. Sci. 2020, 133, 106–110. [Google Scholar] [CrossRef]
| Variables | BPS (n = 6) | Sildenafil (n = 6) | BPS + Sil (n = 6) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Heart rate (bpm) | 86 (80, 89) | 83 (75, 92) | 86 (76, 87) | 88 (81, 92) | 72 (72, 85) | 84 (74, 89) |
| SAP (mmHg) | 120 (102, 125) | 113 (104, 122) | 114 (101, 117) | 111 (99, 122) | 115 (111, 126) | 113 (107, 116) |
| MAP (mmHg) | 82 (68, 96) | 86 (73, 93) | 79 (70, 87) | 70 (61, 84) | 84 (80, 92) | 80 (74, 86) |
| PCWP (mmHg) | 10.7 (8.8, 11.8) | 10.2 (3.7, 11.2) | 8.4 (5.8, 11.6) | 11.3 (7.6, 12.7) * | 9.4 (5.6, 12.6) | 8.9 (5.2, 13.1) |
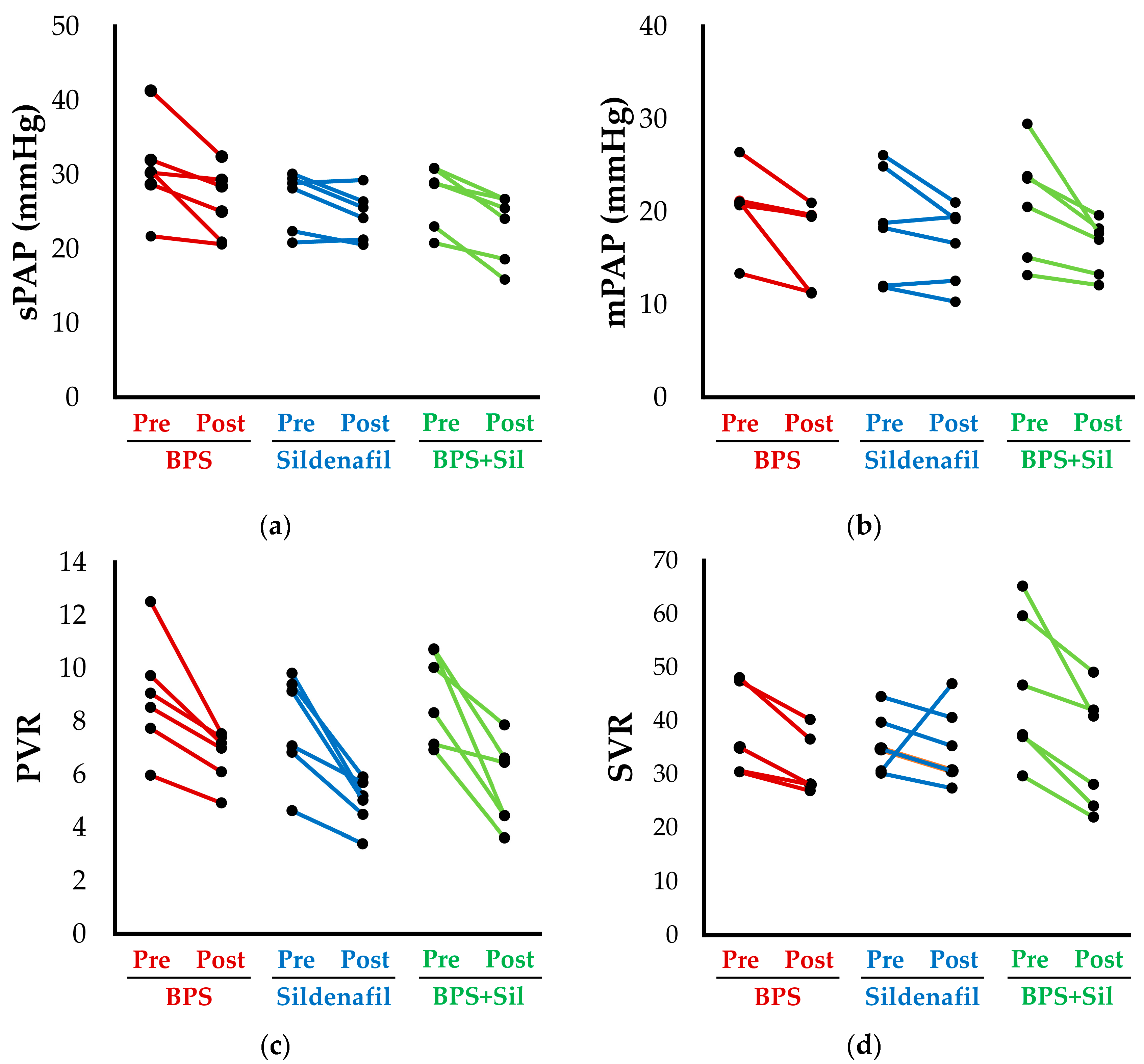
| sPAP (mmHg) | 30.4 (27.0, 34.4) | 26.8 (20.9, 30.1) * | 28.5 (22.0, 29.7) | 24.9 (21.1, 27.1) * | 28.9 (22.5, 30.9) | 24.8 (18.0, 26.7) * |
| mPAP (mmHg) | 21.0 (18.9, 22.5) | 19.6 (11.3, 20.0) * | 18.6 (12.0, 25.2) | 17.9 (12.0, 19.9) * | 22.1 (14.6, 25.3) | 17.4 (13.0, 18.6) * |
| dPAP (mmHg) | 13.8 (11.6, 15.1) | 12.8 (6.6, 13.0) | 11.0 (6.4, 20.3) | 8.2 (4.6, 12.4) | 12.7 (9.3, 15.4) | 11.0 (9.5, 11.5) |
| sRVP (mmHg) | 28.8 (26.6, 37.0) | 23.5 (20.2, 32.4) * | 23.3 (21.5, 30.1) | 21.8 (16.3, 28.8) | 23.9 (18.4, 28.9) | 22.9 (19.3, 27.3) |
| dRVP (mmHg) | 1.0 (0.6, 3.0) | 1.1 (0.4, 4.6) | 2.3 (0.2, 3.8) | 1.5 (0.3, 3.2) | 2.1 (0.9, 4.0) | 1.6 (0.8, 2.2) |
| dP/dtmax (mmHg/s) | 377 (258, 490) | 429 (350, 476) | 350 (309, 408) | 395 (342, 445) * | 298 (256, 360) | 379 (290, 424) * |
| dP/dtmin (mmHg/s) | −327 (−427, −229) | −382 (−396, −248) | −242 (−303, −169) | −317 (−356, −258) * | −261 (−317, −219) | −327 (−370, −261) |
| RAP (mmHg) | 2.3 (0.7, 4.0) | 3.3 (1.5, 5.7) | 1.3 (0.7, 2.5) | 1.7 (0, 2.9) | 2.7 (1.9, 3.1) | 2.7 (1.9, 3.5) |
| CVP (mmHg) | 2.9 (0.7, 4.4) | 2.1 (0.9, 3.6) | 1.5 (0.4, 2.5) | 2.3 (0.2, 3.7) | 2.7 (1.3, 4.0) | 1.5 (0.5, 4.4) |
| RV CO (L/min) | 1.2 (1.0, 1.3) | 1.3 (1.1, 1.5) | 1.2 (1.1, 1.5) | 1.4 (1.2, 1.5) * | 1.1 (1.0, 1.6) | 1.4 (1.1, 1.7) * |
| RV SV (mL) | 14.0 (12.1, 15.6) | 15.5 (14.1, 17.5) * | 15.6 (12.8, 17.6) | 15.9 (13.1, 17.3) | 15.4 (12.4, 20.6) | 17.8 (14.8, 19.5) |
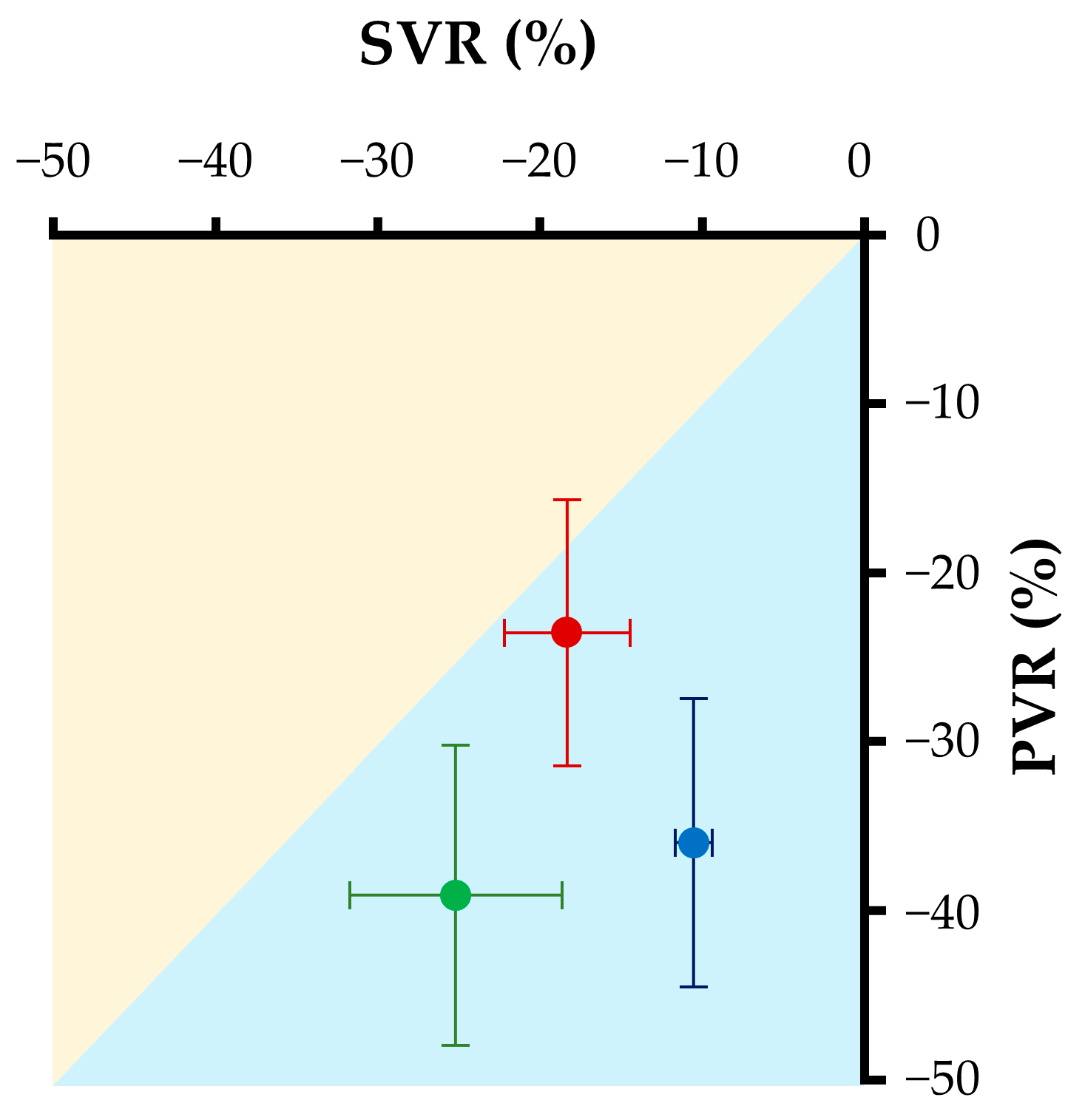
| PVR | 8.8 (7.3, 10.4) | 7.1 (5.8, 7.4) * | 8.1 (6.3, 9.5) | 5.4 (4.2, 5.9) * | 9.2 (7.8, 10.7) | 5.4 (4.2, 6.9) * |
| SVR | 35.1 (33.6, 47.6) | 28.1 (27.8, 37.5) * | 37.2 (33.6, 43.0) | 33.0 (29.8, 38.7) | 42.0 (35.2, 61.1) | 34.5 (23.5, 43.8) * |
| Variables | BPS (n = 6) | Sildenafil (n = 6) | BPS + Sil (n = 6) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| LA/Ao | 1.6 (1.5, 1.7) | 1.6 (1.5, 1.8) | 1.6 (1.6, 1.7) | 1.6 (1.6, 1.7) | 1.7 (1.5, 1.7) | 1.6 (1.6, 1.7) |
| LVEDV (mL/m2) | 42.1 (35.7, 52.4) | 37.3 (33.3, 44.1) | 41.6 (37.9, 48.2) | 47.0 (42.1, 54.1) * | 41.3 (35.7, 55.1) | 39.3 (34.9, 52.3) |
| LVESV (mL/m2) | 18.4 (15.5, 24.2) | 15.5 (13.3, 18.2) | 17.2 (13.8, 22.0) | 18.7 (15.2, 25.5) * | 18.5 (14.5, 29.3) | 16.8 (10.8, 23.9) * |
| EF (%) | 51.4 (45.6, 64.6) | 61.3 (50.1, 68.4) | 60.5 (50.4, 65.4) | 62.1 (49.5, 65.4) | 54.2 (35.3, 66.0) | 61.1 (42.7, 71.0) * |
| E (m/s) | 0.9 (0.8, 1.1) | 1.0 (0.9, 1.1) | 1.0 (0.8, 1.3) | 1.0 (0.9, 1.4) | 0.9 (0.8, 1.1) | 0.9 (0.9, 1.2) |
| A (m/s) | 0.3 (0.3, 0.4) | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.4) | 0.3 (0.3, 0.4) | 0.4 (0.3, 0.4) | 0.3 (0.3, 0.4) |
| E/A | 2.9 (2.3, 3.5) | 2.3 (2.1, 3.5) | 2.9 (2.3, 3.0) | 3.1 (2.5, 4.2) | 2.9 (2.1, 3.6) | 2.7 (2.2, 4.5) |
| E/e’ | 10.8 (9.2, 12.0) | 10.2 (8.5, 11.3) | 11.7 (11.0, 12.3) | 13.5 (10.0, 16.5) * | 10.3 (8.4, 11.3) | 11.1 (9.0, 13.2) |
| LV CO (mL) | 2.0 (1.8, 2.3) | 2.5 (2.0, 3.0) * | 2.0 (1.6, 2.2) | 2.3 (2.1, 2.5) | 1.9 (1.5, 2.3) | 2.4 (1.7, 3.7) * |
| LV-SL (%) | 18.5 (16.5, 19.6) | 20.1 (18.6, 23.4) | 18.7 (17.0, 20.3) | 20.7 (18.0, 22.0) * | 17.5 (15.7, 18.7) | 20.4 (17.6, 22.8) |
| LV-SC (%) | 19.1 (17.6, 21.5) | 18.8 (17.7, 22.2) | 18.0 (17.5, 22.0) | 18.9 (17.2, 25.4) | 21.0 (18.7, 23.9) | 20.9 (16.6, 27.0) |
| Variables | BPS (n = 6) | Sildenafil (n = 6) | BPS + Sil (n = 6) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| RVEDA (cm2) | 4.9 (4.4, 5.2) | 4.6 (4.1, 5.1) | 4.8 (4.3, 5.3) | 4.4 (4.2, 5.0) | 4.6 (4.2, 5.0) | 4.6 (4.5, 5.2) |
| RVESA (cm2) | 2.7 (2.4, 3.0) | 2.5 (2.1, 2.7) | 2.8 (2.5, 3.1) | 2.4 (2.2, 2.7) * | 2.4 (2, 2.9) | 2.5 (2.2, 3.4) |
| RV FACn (%/kg−0.097) | 57.1 (51.4, 64.7) | 60.2 (50.0, 67.9) | 52.0 (46.9, 60.3) | 59.4 (54.3, 65.3) * | 61 (52.4, 64.9) | 61.0 (56.3, 66.0) |
| TAPSEn (mm/kg0.284) | 5.5 (5.3, 5.7) | 6.4 (5.6, 6.7) | 5.8 (5.1, 6.2) | 6.1 (5.9, 6.8) | 5.8 (5.1, 6.4) | 6.5 (5.1, 7.3) * |
| RV s’ (cm/s) | 9.5 (8.6, 10.3) | 9.2 (8.2, 10.0) | 9.6 (8.9, 10.1) | 10.1 (9.7, 10.7) | 9.6 (8.3, 10.3) | 9.1 (8.5, 10.6) |
| RV-SL3seg (%) | 21.1 (18.6, 27.1) | 22.9 (18.6, 24.9) | 22.1 (20.9, 25.5) | 24.1 (23.1, 26.4) | 21.3 (19.5, 24.0) | 25.7 (20.9, 27.8) * |
| RV-SL6seg (%) | 19.3 (16.6, 21.6) | 20.8 (17.5, 21.9) | 18.7 (17.7, 21.3) | 20.6 (19.1, 21.6) | 20.2 (16.8, 22.3) | 21.7 (20.0, 25.5) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuchi, Y.; Suzuki, R.; Ishida, N.; Satomi, S.; Saito, T.; Teshima, T.; Matsumoto, H. Comparative Study of Cardiovascular Effects of Selected Pulmonary Vasodilators in Canine Models of Mitral Valve Disease. Biology 2024, 13, 311. https://doi.org/10.3390/biology13050311
Yuchi Y, Suzuki R, Ishida N, Satomi S, Saito T, Teshima T, Matsumoto H. Comparative Study of Cardiovascular Effects of Selected Pulmonary Vasodilators in Canine Models of Mitral Valve Disease. Biology. 2024; 13(5):311. https://doi.org/10.3390/biology13050311
Chicago/Turabian StyleYuchi, Yunosuke, Ryohei Suzuki, Narumi Ishida, Shuji Satomi, Takahiro Saito, Takahiro Teshima, and Hirotaka Matsumoto. 2024. "Comparative Study of Cardiovascular Effects of Selected Pulmonary Vasodilators in Canine Models of Mitral Valve Disease" Biology 13, no. 5: 311. https://doi.org/10.3390/biology13050311
APA StyleYuchi, Y., Suzuki, R., Ishida, N., Satomi, S., Saito, T., Teshima, T., & Matsumoto, H. (2024). Comparative Study of Cardiovascular Effects of Selected Pulmonary Vasodilators in Canine Models of Mitral Valve Disease. Biology, 13(5), 311. https://doi.org/10.3390/biology13050311





