Different Immune Responses of Hemocytes from V. parahaemolyticus-Resistant and -Susceptible Shrimp at Early Infection Stage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
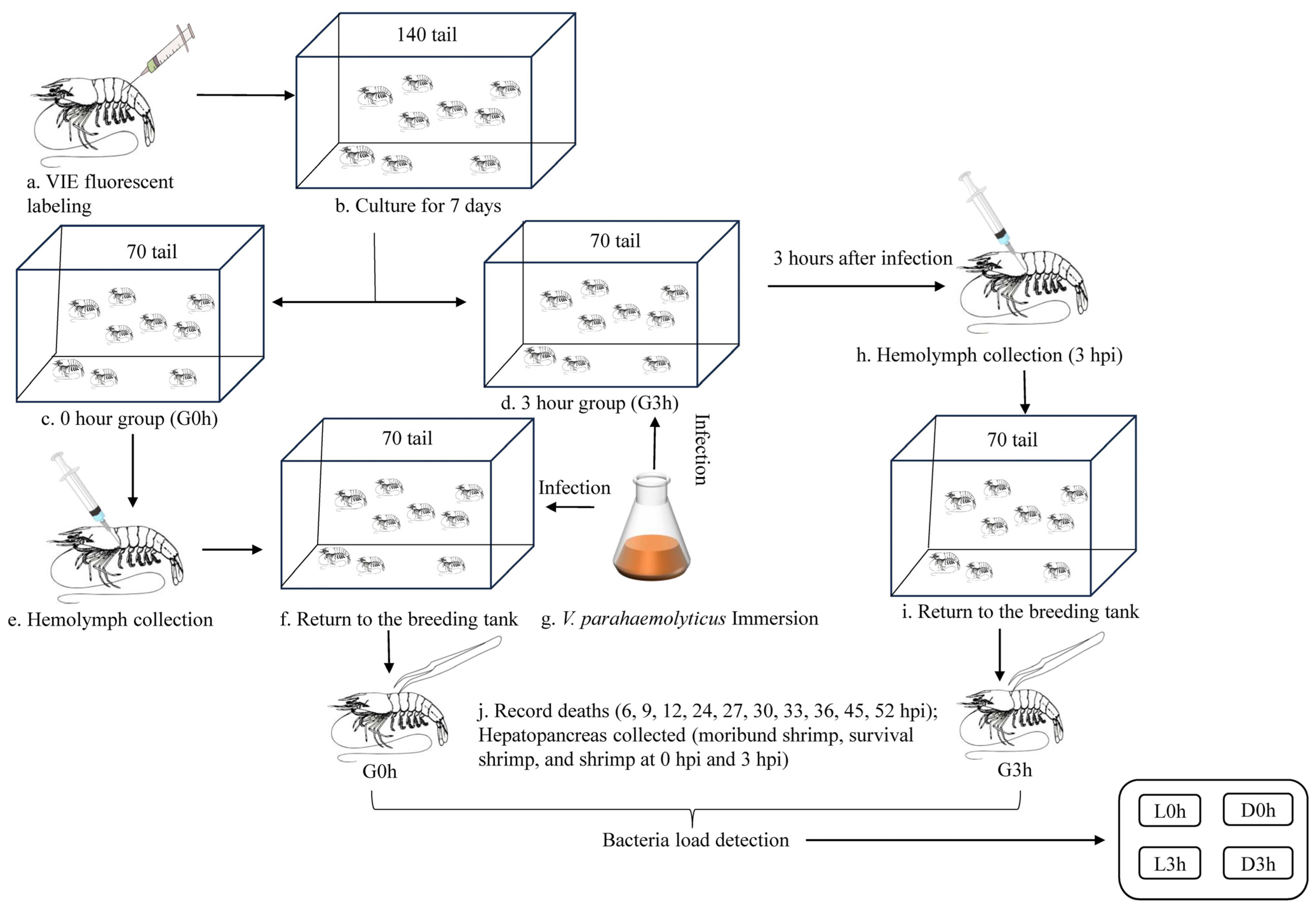
2.2. Experiment Design and Sampling
2.3. DNA Extraction and Bacteria Load Detection
2.4. RNA Extraction and Transcriptome Sequencing
2.5. Reads Mapping and Annotation
2.6. Differential Expression and Enrichment Analysis
2.7. RNA Interference
2.8. Quantitative Real-Time PCR
2.9. Statistical Analysis
3. Results
3.1. The Loads of V. parahaemolyticus in Hepatopancreas of Treated Shrimp
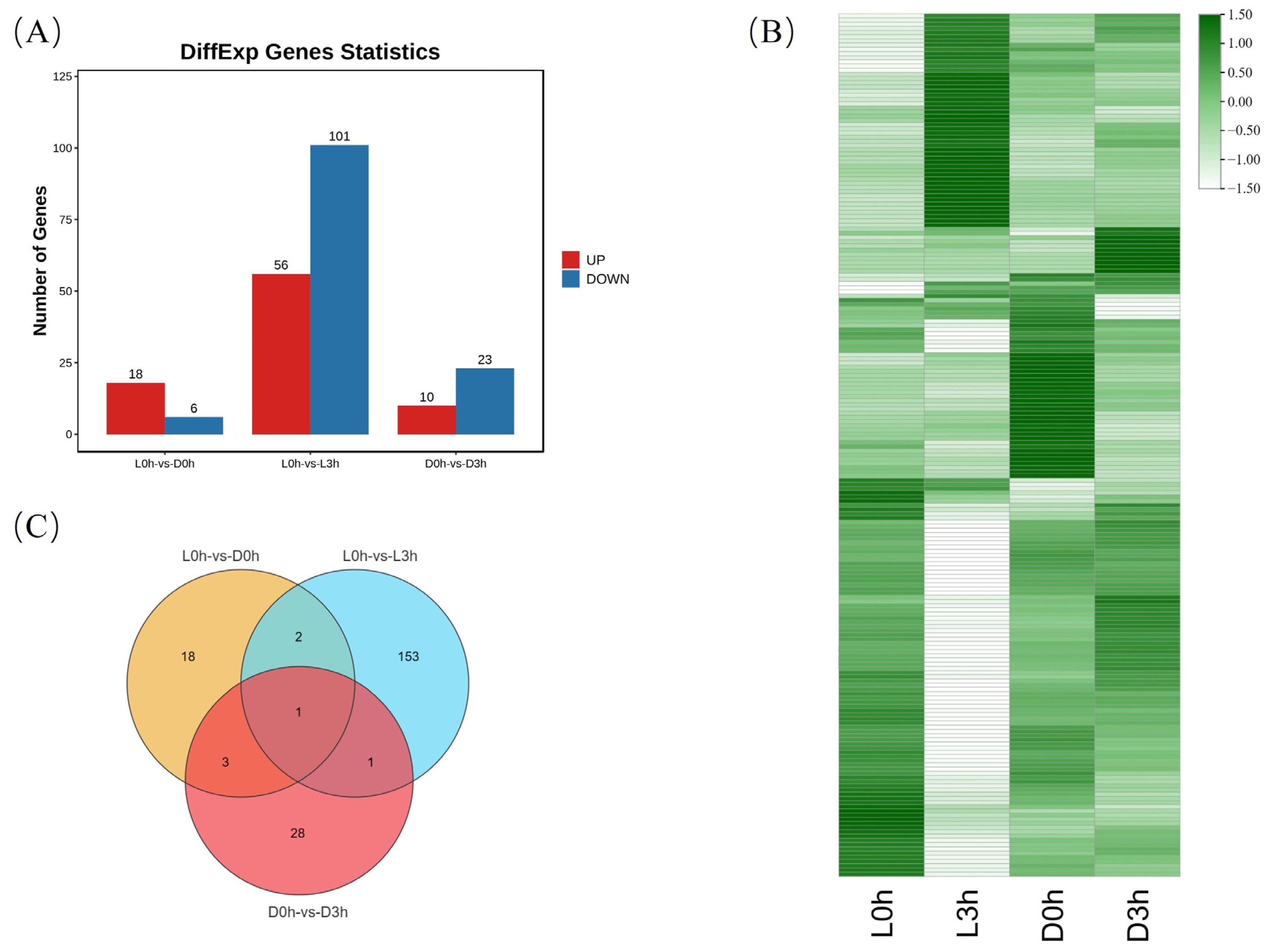
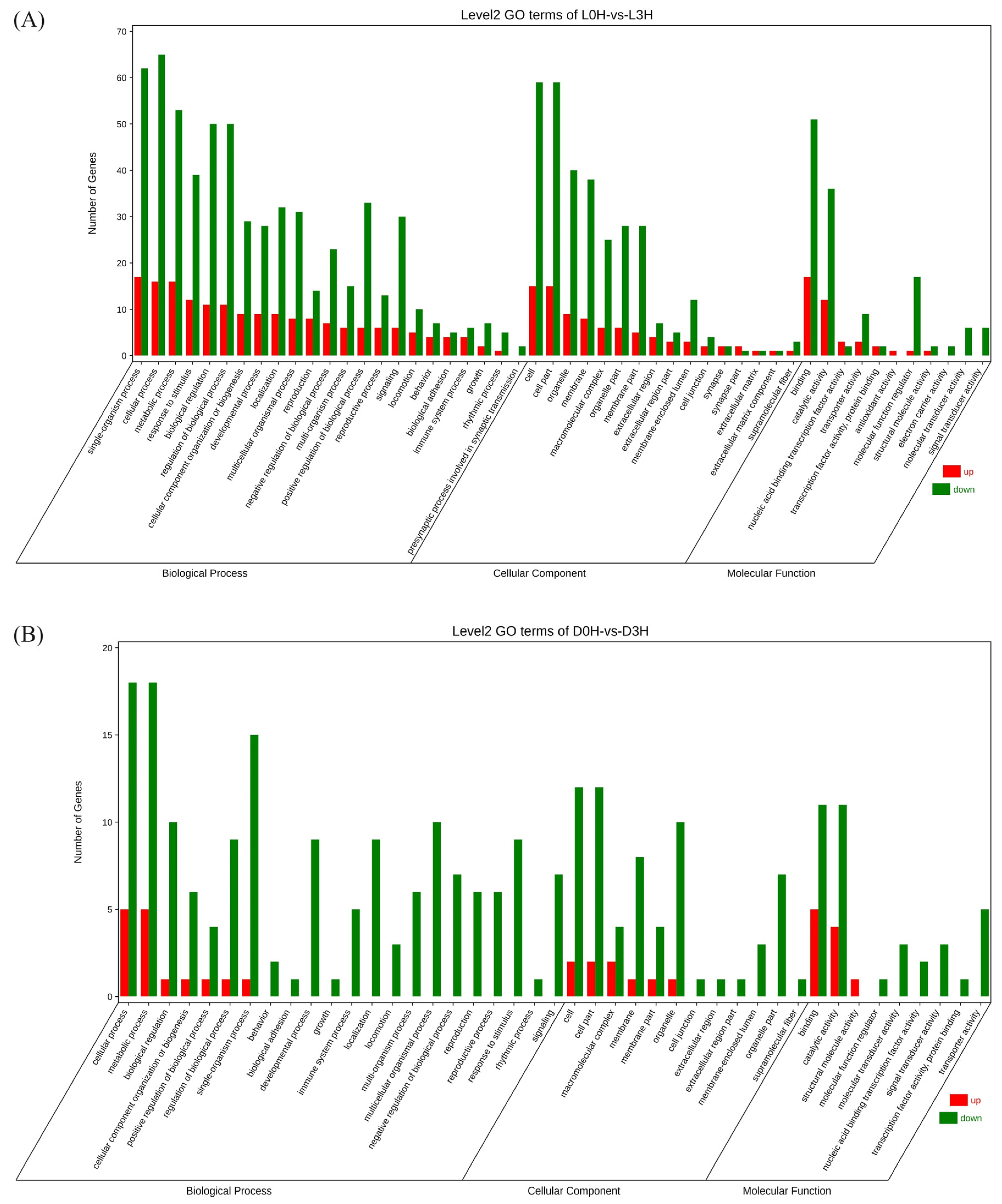
3.2. Differential Immune Responses of Hemocytes against Pathogen Infection between V. parahaemolyticus-Resistant and -Susceptible Shrimp
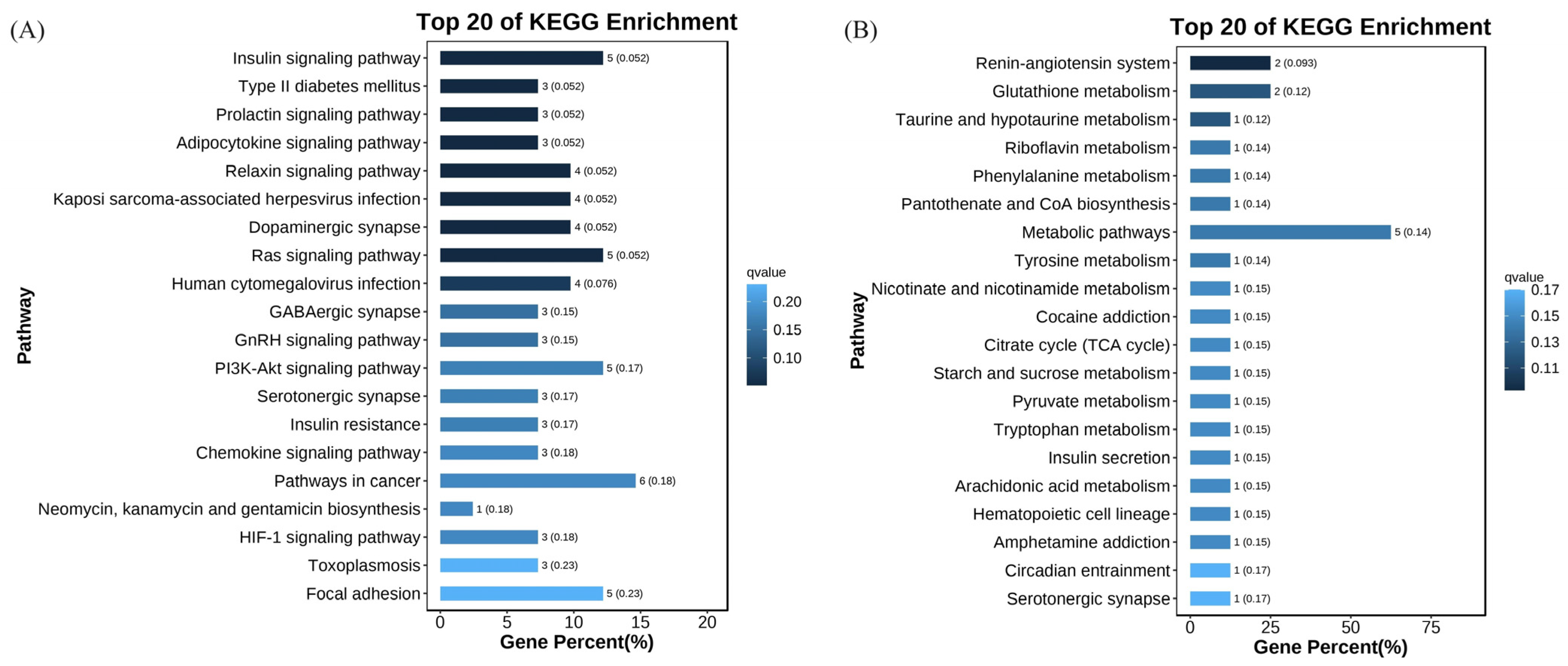
3.3. The Glycolysis Process Showed Different Responses to Pathogen Infection in Hemocytes between V. parahaemolyticus-Resistant and -Susceptible Shrimp
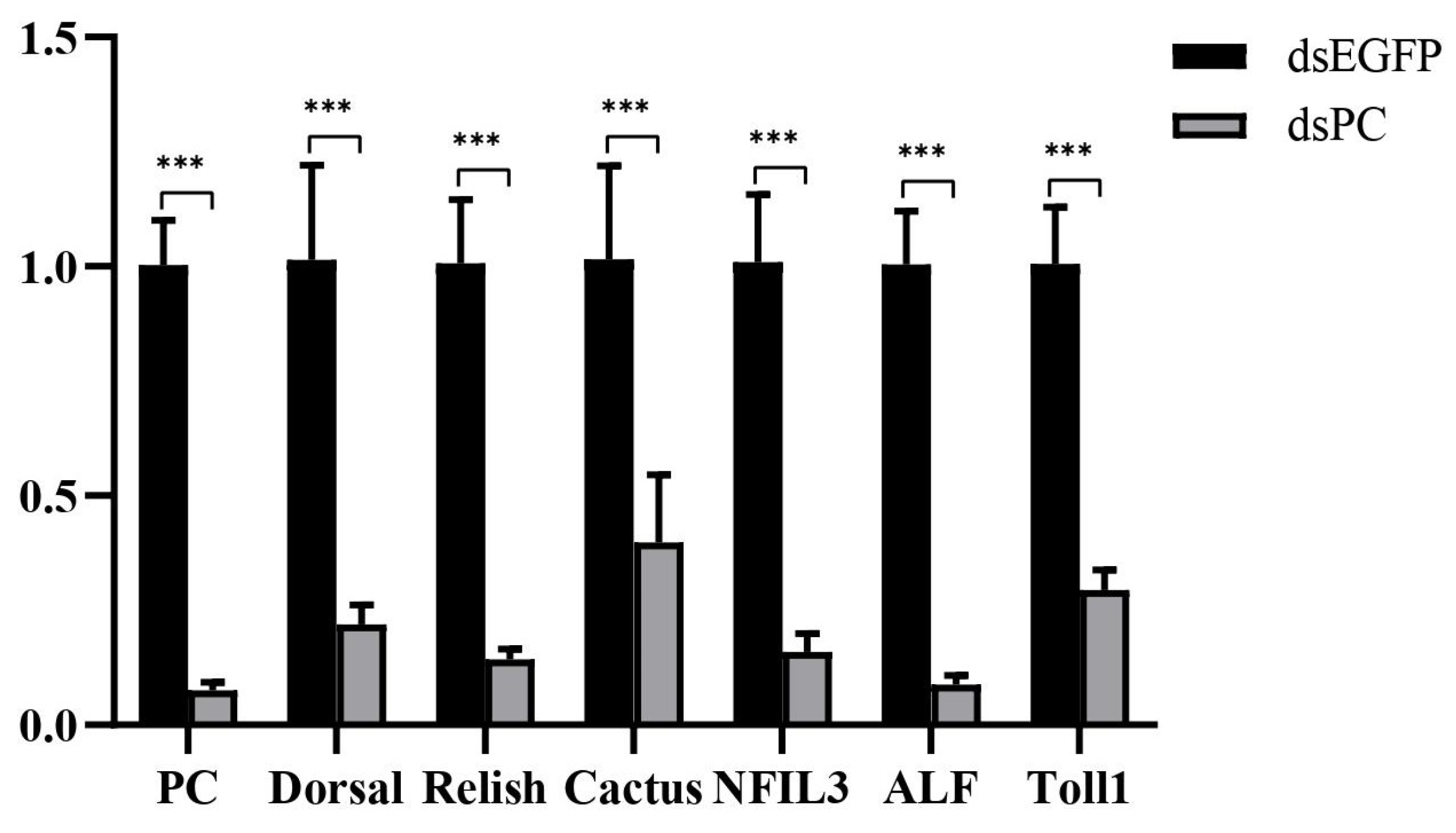
3.4. Knockdown of PC Inhibited the Expression of Genes in the NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarville, J.L.; Ayres, J.S. Disease tolerance: Concept and mechanisms. Curr. Opin. Immunol. 2018, 50, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yu, Y.B.; Choi, J.H.; Jo, A.H.; Hong, S.M.; Kang, J.C.; Kim, J.H. Viral Shrimp Diseases Listed by the OIE: A Review. Viruses 2022, 14, 585. [Google Scholar] [CrossRef] [PubMed]
- Zorriehzahra, M.J.; Banaederakhshan, R. Early Mortality Syndrome (EMS) as new Emerging Threat in Shrimp Industry. Adv. Anim. Vet. Sci. 2015, 3, 64. [Google Scholar] [CrossRef]
- Lee, C.T.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.N.; Wang, G.H.; Xu, Z.G.; Tu, H.Q.; Hu, F.Q.; Dai, J.; Chang, Y.; Chen, Y.Q.; Lu, Y.J.; Zeng, H.L.; et al. Lactate Is a Natural Suppressor of RLR Signaling by Targeting MAVS. Cell 2019, 178, 176–189.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Y.; Luo, Z.; Xiang, J.; Li, F. Comparison of Gene Expression Between Resistant and Susceptible Families Against VPAHPND and Identification of Biomarkers Used for Resistance Evaluation in Litopenaeus vannamei. Front. Genet. 2021, 12, 772442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Y.; Li, S.; Sun, M.; Li, F. Comparative transcriptomic analysis of gill reveals genes belonging to mTORC1 signaling pathway associated with the resistance trait of shrimp to VPAHPND. Front. Immunol. 2023, 14, 1150628. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Li, S.H.; Yu, Y.; Liu, Y.; Li, F.H. Comparative transcriptome analysis of hepatopancreas reveals the potential mechanism of shrimp resistant to Vibrio parahaemolyticus infection. Fish Shellfish Immun. 2024, 144, 109282. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, Y.; Li, S.; Liu, Y.; Zhang, X.; Li, F. Integrated application of transcriptomics and metabolomics provides insights into acute hepatopancreatic necrosis disease resistance of Pacific white shrimp Litopenaeus vannamei. mSystems 2023, 8, e0006723. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kang, C. Advancements in the study of the classification and immune function of shrimp hemocytes. Sheng Wu Gong Cheng Xue Bao 2021, 37, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, K.; Du, W.; Li, F. Two Independently Comparative Transcriptome Analyses of Hemocytes Provide New Insights into Understanding the Disease-Resistant Characteristics of Shrimp against Vibrio Infection. Biology 2023, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, S.H.; Yu, Y.; Yuan, J.B.; Yu, K.J.; Li, F.H. Pathogenicity of a Vibrio owensii strain isolated from Fenneropenaeus chinensis carrying pirAB genes and causing AHPND. Aquaculture 2021, 530, 735747. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yuan, J.B.; Sun, Y.M.; Li, S.H.; Gao, Y.; Yu, Y.; Liu, C.Z.; Wang, Q.C.; Lv, X.J.; Zhang, X.X.; et al. Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat. Commun. 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Landmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Y.; Wang, P.S.; Song, X.R.; Zhang, H.; Ma, S.S.; Wang, J.T.; Li, W.W.; Lv, R.X.; Liu, X.Q.; Ma, S.; et al. Typhimurium reprograms macrophage metabolism via T3SS effector SopE2 to promote intracellular replication and virulence. Nat. Commun. 2021, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Challagundla, N.; Phadnis, D.; Gupta, A.; Agrawal-Rajput, R. Host Lipid Manipulation by Intracellular Bacteria: Moonlighting for Immune Evasion. J. Membr. Biol. 2023, 256, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, X.; Wu, T.Z.; Jiang, J.; Jiang, Y.P.; Zhang, S.; Ruan, Y.Y.; Zhang, H.P.; Zhang, C.; Chen, P.; et al. Integrative analysis of metabolomics and proteomics reveals amino acid metabolism disorder in sepsis (vol 20, 123, 2022). J. Transl. Med. 2022, 20, 366. [Google Scholar] [CrossRef] [PubMed]
- Holmskov, U.; Thiel, S.; Jensenius, J.C. Collections and ficolins: Humoral lectins of the innate immune defense. Annu. Rev. Immunol. 2003, 21, 547–578. [Google Scholar] [CrossRef]
- Kane, E.I.; Spratt, D.E. Structural Insights into Ankyrin Repeat-Containing Proteins and Their Influence in Ubiquitylation. Int. J. Mol. Sci. 2021, 22, 609. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Fujimura, Y.; Titani, K. Snake venom proteases affecting hemostasis and thrombosis. Biochim. Biophys. Acta 2000, 1477, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17r–27r. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chung, J.S. Trehalose metabolism in the blue crab: Isolation of multiple structural cDNA isoforms of trehalose-6-phosphate synthase and their expression in muscles. Gene 2014, 536, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.S. A trehalose 6-phosphate synthase gene of the hemocytes of the blue crab, Callinectes sapidus: Cloning, the expression, its enzyme activity and relationship to hemolymph trehalose levels. Saline Syst. 2008, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Habarou, F.; Brassier, A.; Rio, M.; Chrétien, D.; Monnot, S.; Barbier, V.; Barouki, R.; Bonnefont, J.P.; Boddaert, N.; Chadefaux-Vekemans, B.; et al. Pyruvate carboxylase deficiency: An underestimated cause of lactic acidosis. Mol. Genet. Metab. Rep. 2015, 2, 25–31. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, W.; Li, S.; Li, F. Different Immune Responses of Hemocytes from V. parahaemolyticus-Resistant and -Susceptible Shrimp at Early Infection Stage. Biology 2024, 13, 300. https://doi.org/10.3390/biology13050300
Du W, Li S, Li F. Different Immune Responses of Hemocytes from V. parahaemolyticus-Resistant and -Susceptible Shrimp at Early Infection Stage. Biology. 2024; 13(5):300. https://doi.org/10.3390/biology13050300
Chicago/Turabian StyleDu, Wenran, Shihao Li, and Fuhua Li. 2024. "Different Immune Responses of Hemocytes from V. parahaemolyticus-Resistant and -Susceptible Shrimp at Early Infection Stage" Biology 13, no. 5: 300. https://doi.org/10.3390/biology13050300
APA StyleDu, W., Li, S., & Li, F. (2024). Different Immune Responses of Hemocytes from V. parahaemolyticus-Resistant and -Susceptible Shrimp at Early Infection Stage. Biology, 13(5), 300. https://doi.org/10.3390/biology13050300





