Postnatal Growth and Development of the Rumen: Integrating Physiological and Molecular Insights
Abstract
Simple Summary
Abstract
1. Introduction
2. Histology of the Rumen
3. Functions of the Rumen
3.1. Digestion
3.2. Absorption of VFAs
3.3. Metabolism of VFAs
3.4. Barrier Function
4. Prenatal Development of the Rumen
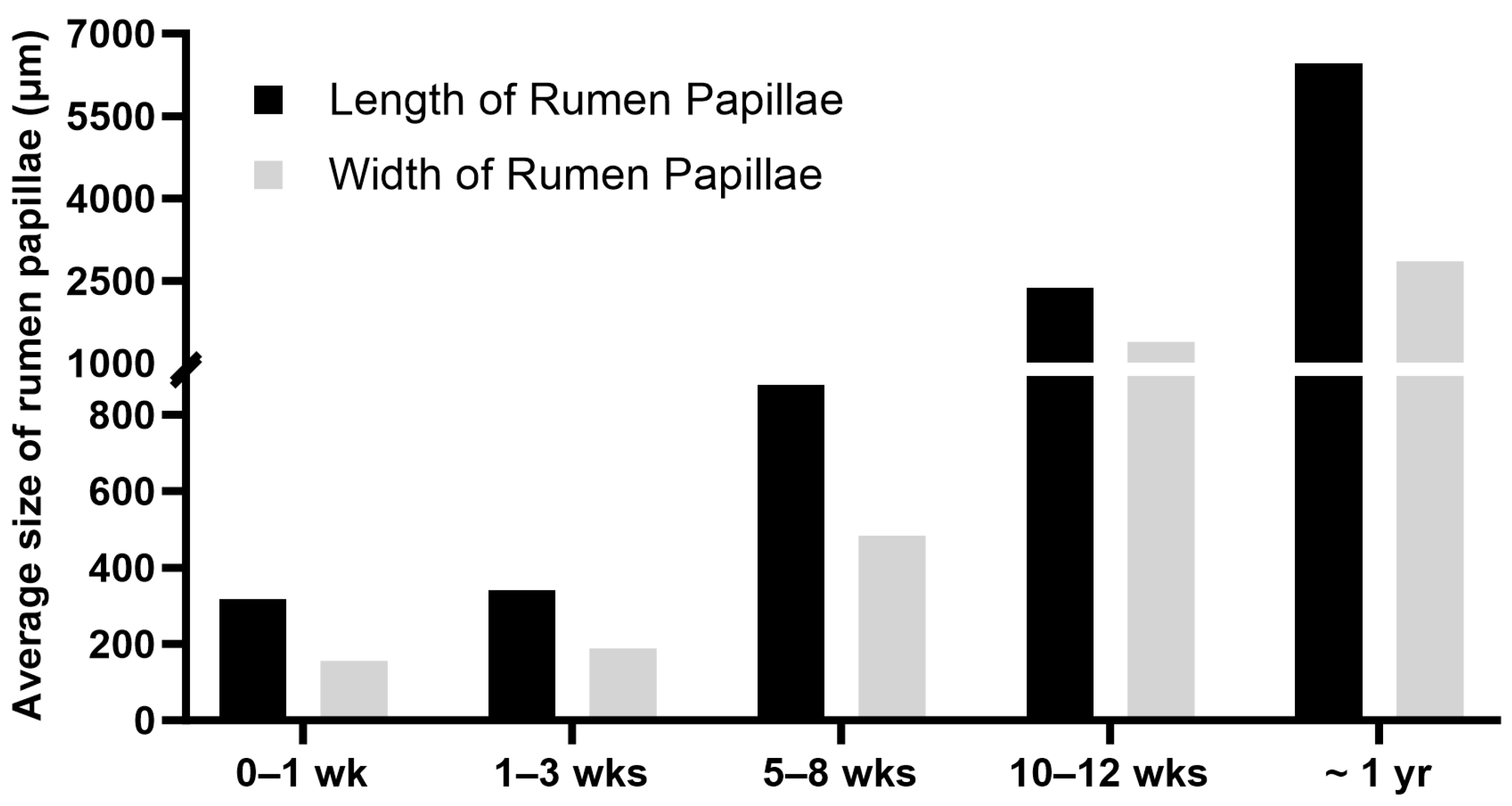
5. Postnatal Development of the Rumen
6. Factors Affecting the Postnatal Development of the Rumen
6.1. Diet
6.2. Volatile Fatty Acids
6.3. Rumen Microbiota
6.4. Hormones
6.5. Weaning
6.6. Other Factors
7. Genes Involved in Rumen Growth and Development
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dehority, B.A. Gastrointestinal Tracts of Herbivores, Particularly the Ruminant: Anatomy, Physiology and Microbial Digestion of Plants. J. Appl. Anim. Res. 2002, 21, 145–160. [Google Scholar] [CrossRef]
- Membrive, C.M.B. Anatomy and physiology of the rumen. In Rumenology; Springer: Cham, Switzerland, 2016; pp. 1–38. [Google Scholar] [CrossRef]
- Harfoot, C.G. Anatomy, physiology and microbiology of the ruminant digestive tract. In Progress in the Chemistry of Fats and Other Lipids; Pergamon Press, Ltd.: London, UK, 1978; Volume 17, pp. 1–19. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Connor, E.E. Rumen Function and Development. In Veterinary Clinics of North America—Food Animal Practice; W.B. Saunders: Philadelphia, PA, USA, 2017; Volume 33, pp. 427–439. [Google Scholar]
- Drackley, J.K. Calf nutrition from birth to breeding. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 55–86. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.; McLeod, K.R.; Klotz, J.L.; Heitmann, R.N. Rumen Development, Intestinal Growth and Hepatic Metabolism In The Pre- and Postweaning Ruminant. J. Dairy Sci. 2004, 87, E55–E65. [Google Scholar] [CrossRef]
- Ji, X.; Tong, H.; Settlage, R.; Yao, W.; Jiang, H. Establishment of a bovine rumen epithelial cell line. J. Anim. Sci. 2021, 99, skab273. [Google Scholar] [CrossRef] [PubMed]
- Dobson, M.J.; Brown, W.C.; Dobson, A.; Phillipson, A.T. A histological study of the organization of the rumen epithelium of sheep. Q. J. Exp. Physiol. Cogn. Med. Sci. 1956, 41, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. Biomed. Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.; Jesse, B.W. Technical note: Isolation and characterization of sheep ruminal epithelial cells. J. Anim. Sci. 1991, 69, 3603–3609. [Google Scholar] [CrossRef]
- Steele, M.A.; Penner, G.B.; Chaucheyras-Durand, F.; Guan, L.L. Development and physiology of the rumen and the lower gut: Targets for improving gut health. J. Dairy Sci. 2016, 99, 4955–4966. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Simmons, N.L. Functional organization of the bovine rumen epithelium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R173–R181. [Google Scholar] [CrossRef]
- van Niekerk, J.K.; Middeldorp, M.; Guan, L.L.; Steele, M.A. Preweaning to postweaning rumen papillae structural growth, ruminal fermentation characteristics, and acute-phase proteins in calves. J. Dairy Sci. 2021, 104, 3632–3645. [Google Scholar] [CrossRef]
- Lavker, R.M.; Matoltsy, A.G. Formation of horny cells: The fate of cell organelles and differentiation products in ruminal epithelium. J. Cell Biol. 1970, 44, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Reece, W.O. Functional Anatomy and Physiology of Domestic Animals, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Liang, Y.; Wang, S.; An, T.; Tarique, I.; Vistro, W.A.; Liu, Y.; Wang, Z.; Zhang, H.; Shi, Y.; Haseeb, A.; et al. Telocytes as a Novel Structural Component in the Muscle Layers of the Goat Rumen. Cell Transpl. 2019, 28, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.; Allison, M.J. Rumen metabolism. J. Anim. Sci. 1983, 57 (Suppl. S2), 461–477. [Google Scholar] [CrossRef]
- Dijkstra, J. Production and absorption of volatile fatty acids in the rumen. Livest. Prod. Sci. 1994, 39, 61–69. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Global Rumen Census, C.; Janssen, P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yin, Y.; Guo, C.; Liu, L.; Mao, S.; Zhu, W.; Liu, J. Transcriptomic analysis reveals the molecular mechanisms of rumen wall morphological and functional development induced by different solid diet introduction in a lamb model. J. Anim. Sci. Biotechnol. 2021, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.; Jami, E. Rumen protozoa: From background actors to featured role in microbiome research. Environ. Microbiol. Rep. 2021, 13, 45–49. [Google Scholar] [CrossRef]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Giger-Reverdin, S.; Domange, C.; Broudiscou, L.P.; Sauvant, D.; Berthelot, V. Rumen function in goats, an example of adaptive capacity. J. Dairy Res. 2020, 87, 45–51. [Google Scholar] [CrossRef]
- Jenkins, T.C. Lipid metabolism in the rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88 (Suppl. S1), E9–E21. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Kang, J.; Zhang, D. Microbial production of vitamin B(12): A review and future perspectives. Microb. Cell Fact. 2017, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Abdoun, K.; Stumpff, F.; Martens, H. Ammonia and urea transport across the rumen epithelium: A review. Anim. Health Res. Rev. 2006, 7, 43–59. [Google Scholar] [CrossRef]
- Li, Y.; Kreuzer, M.; Clayssen, Q.; Ebert, M.O.; Ruscheweyh, H.J.; Sunagawa, S.; Kunz, C.; Attwood, G.; Amelchanka, S.; Terranova, M. The rumen microbiome inhibits methane formation through dietary choline supplementation. Sci. Rep. 2021, 11, 21761. [Google Scholar] [CrossRef]
- Annison, E.F.; Bryden, W.L. Perspectives on ruminant nutrition and metabolism I. Metabolism in the rumen. Nutr. Res. Rev. 1998, 11, 173–198. [Google Scholar] [CrossRef]
- Beauchemin, K.A. Invited review: Current perspectives on eating and rumination activity in dairy cows. J. Dairy Sci. 2018, 101, 4762–4784. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Penner, G.B.; Stumpff, F.; Gabel, G. Ruminant Nutrition Symposium: Role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 2011, 89, 1092–1107. [Google Scholar] [CrossRef]
- Storm, A.C.; Kristensen, N.B. Effects of particle size and dry matter content of a total mixed ration on intraruminal equilibration and net portal flux of volatile fatty acids in lactating dairy cows. J. Dairy Sci. 2010, 93, 4223–4238. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Pathak, N.N. Fundamentals of Animal Nutrition; Springer: Singapore, 2021; pp. 219–246. [Google Scholar] [CrossRef]
- Gholizade, M.; Fayazi, J.; Zali, H.; Asgari, Y. Transcriptomic Changes in the Rumen Epithelium of Cattle after the Induction of Acidosis. Arch. Razi Inst. 2020, 75, 109–121. [Google Scholar] [CrossRef]
- Dobson, A. Blood flow and absorption from the rumen. Q. J. Exp. Physiol. 1984, 69, 599–606. [Google Scholar] [CrossRef]
- Sehested, J.; Diernaes, L.; Moller, P.D.; Skadhauge, E. Ruminal transport and metabolism of short-chain fatty acids (SCFA) in vitro: Effect of SCFA chain length and pH. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999, 123, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Sehested, J.; Diernaes, L.; Moller, P.D.; Skadhauge, E. Transport of butyrate across the isolated bovine rumen epithelium--interaction with sodium, chloride and bicarbonate. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999, 123, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Zebeli, Q.; Patra, A.K.; Greco, G.; Amasheh, S.; Penner, G.B. Symposium review: The importance of the ruminal epithelial barrier for a healthy and productive cow. J. Dairy Sci. 2019, 102, 1866–1882. [Google Scholar] [CrossRef] [PubMed]
- Bilk, S.; Huhn, K.; Honscha, K.U.; Pfannkuche, H.; Gabel, G. Bicarbonate exporting transporters in the ovine ruminal epithelium. J. Comp. Physiol. B 2005, 175, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Gabel, G.; Aschenbach, J.R.; Muller, F. Transfer of energy substrates across the ruminal epithelium: Implications and limitations. Anim. Health Res. Rev. 2002, 3, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Bilk, S.; Tadesse, G.; Stumpff, F.; Gabel, G. Bicarbonate-dependent and bicarbonate-independent mechanisms contribute to nondiffusive uptake of acetate in the ruminal epithelium of sheep. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1098–G1107. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Gatherar, I.; Haslam, I.; Glanville, M.; Simmons, N.L. Expression and localization of monocarboxylate transporters and sodium/proton exchangers in bovine rumen epithelium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R997–R1007. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.; Aschenbach, J.R.; Gabel, G. Role of Na+/H+ exchange and HCO3− transport in pHi recovery from intracellular acid load in cultured epithelial cells of sheep rumen. J. Comp. Physiol. B 2000, 170, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Beck, U.; Emmanuel, B.; Giesecke, D. The ketogenic effect of glucose in rumen epithelium of ovine (Ovis aries) and bovine (Bos taurus) origin. Comp. Biochem. Physiol. B 1984, 77, 517–521. [Google Scholar] [CrossRef]
- Giesecke, D.; Beck, U.; Wiesmayr, S.; Stangassinger, M. The effect of rumen epithelial development on metabolic activities and ketogenesis by the tissue in vitro. Comp. Biochem. Physiol. B 1979, 62, 459–463. [Google Scholar] [CrossRef]
- Lane, M.A.; Baldwin, R.L.; Jesse, B.W. Developmental changes in ketogenic enzyme gene expression during sheep rumen development. J. Anim. Sci. 2002, 80, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.S.; Milligan, L.P.; Krishnamurti, C.R. Effects of Propionate on Ketogenesis from Butyrate by Bovine Tissues. Can. J. Anim. Sci. 1970, 50, 210. [Google Scholar] [CrossRef]
- Goosen, P.C. Metabolism in rumen epithelium oxidation of substrates and formation of ketone bodies by pieces of rumen epithelium. Z. Tierphysiol. Tierernahr. Futtermittelkunde 1976, 37, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Weekes, T.E. The in vitro metabolism of propionate and glucose by the rumen epithelium. Comp. Biochem. Physiol. B 1974, 49, 393–406. [Google Scholar] [CrossRef]
- Urrutia, N.; Bomberger, R.; Matamoros, C.; Harvatine, K.J. Effect of dietary supplementation of sodium acetate and calcium butyrate on milk fat synthesis in lactating dairy cows. J. Dairy Sci. 2019, 102, 5172–5181. [Google Scholar] [CrossRef] [PubMed]
- Black, A.L.; Luick, J.; Moller, F.; Anand, R.S. Pyruvate and propionate metabolism in lactating cows. Effect of butyrate on pyruvate metabolism. J. Biol. Chem. 1966, 241, 5233–5237. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, Y.; Zhang, H.; Sun, Y.; Mao, Y.; Yang, Z.; Li, M. Transcriptional regulation of milk fat synthesis in dairy cattle. J. Funct. Foods 2022, 96, 105208. [Google Scholar] [CrossRef]
- Stumpff, F.; Georgi, M.I.; Mundhenk, L.; Rabbani, I.; Fromm, M.; Martens, H.; Gunzel, D. Sheep rumen and omasum primary cultures and source epithelia: Barrier function aligns with expression of tight junction proteins. J. Exp. Biol. 2011, 214, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Malago, J.J. Contribution of microbiota to the intestinal physicochemical barrier. Benef. Microbes 2015, 6, 295–311. [Google Scholar] [CrossRef]
- Shen, H.; Xu, Z.; Shen, Z.; Lu, Z. The Regulation of Ruminal Short-Chain Fatty Acids on the Functions of Rumen Barriers. Front. Physiol. 2019, 10, 1305. [Google Scholar] [CrossRef]
- Liu, J.H.; Xu, T.T.; Liu, Y.J.; Zhu, W.Y.; Mao, S.Y. A high-grain diet causes massive disruption of ruminal epithelial tight junctions in goats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R232–R241. [Google Scholar] [CrossRef]
- Naydenov, N.G.; Hopkins, A.M.; Ivanov, A.I. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle 2009, 8, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Takai, Y. Recent advances in understanding tight junctions. Fac. Rev. 2021, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2018, 9, 1942. [Google Scholar] [CrossRef]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef]
- Raleigh, D.R.; Marchiando, A.M.; Zhang, Y.; Shen, L.; Sasaki, H.; Wang, Y.; Long, M.; Turner, J.R. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol. Biol. Cell 2010, 21, 1200–1213. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, M.; Gao, L.; Tu, Y.; Bai, Y. Rumen-derived lipopolysaccharide induced ruminal epithelium barrier damage in goats fed a high-concentrate diet. Microb. Pathog. 2019, 131, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, R.L.t.; Wu, S.; Li, W.; Li, C.; Bequette, B.J.; Li, R.W. Quantification of Transcriptome Responses of the Rumen Epithelium to Butyrate Infusion using RNA-seq Technology. Gene Regul. Syst. Bio 2012, 6, 67–80. [Google Scholar] [CrossRef]
- Meissner, S.; Hagen, F.; Deiner, C.; Gunzel, D.; Greco, G.; Shen, Z.; Aschenbach, J.R. Key role of short-chain fatty acids in epithelial barrier failure during ruminal acidosis. J. Dairy Sci. 2017, 100, 6662–6675. [Google Scholar] [CrossRef]
- Kandel, A.; Masello, M.; Xiao, Z. CD4+ T Cell Responses to Pathogens in Cattle. In Bovine Science—Challenges and Advances; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Spiljar, M.; Merkler, D.; Trajkovski, M. The Immune System Bridges the Gut Microbiota with Systemic Energy Homeostasis: Focus on TLRs, Mucosal Barrier, and SCFAs. Front. Immunol. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004, 127, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gao, S.; Ding, J.; He, J.; Ma, L.; Bu, D. Effects of Heat Stress on the Ruminal Epithelial Barrier of Dairy Cows Revealed by Micromorphological Observation and Transcriptomic Analysis. Front. Genet. 2021, 12, 768209. [Google Scholar] [CrossRef] [PubMed]
- Kalenberg, C.A.; Stoffel, M.H. The embryonic development of the bovine stomach revisited. Anat. Histol. Embryol. 2020, 49, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Warner, E.D. The organogenesis and early histogenesis of the bovine stomach. Am. J. Anat. 1958, 102, 33–63. [Google Scholar] [CrossRef] [PubMed]
- Al Masri, S.; Reincke, R.; Huenigen, H.; Gemeinhardt, O.; Richardson, K.C.; Plendl, J. Computed tomography study of the fetal development of the dairy cow stomach complex. J. Dairy Sci. 2018, 101, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Cabrera, R.; Valencia, A. Observations on the histological development of the bovine rumen papillae. Morphological changes due to age. Anat. Histol. Embryol. 1978, 7, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Masot, J.; Franco, A.; Gazquez, A.; Redondo, E. Histomorphometric and immunohistochemical study of the goat rumen during prenatal development. Anat. Rec. 2012, 295, 776–785. [Google Scholar] [CrossRef]
- Stallcup, O.T.; Kreider, D.L.; Rakes, J.M. Histological development and histochemical localization of enzymes in rumen and reticulum in bovine fetuses. J. Anim. Sci. 1990, 68, 1773–1789. [Google Scholar] [CrossRef]
- Diao, Q.; Zhang, R.; Fu, T. Review of strategies to promote rumen development in calves. Animals 2019, 9, 490. [Google Scholar] [CrossRef]
- Kaba, T.; Abera, B.; Kassa, T. Esophageal groove dysfunction: A cause of ruminal bloat in newborn calves. BMC Vet. Res. 2018, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- White, R.G.; Leng, R.A. Glucose metabolism in feeding and postabsorptive lambs and mature sheep. Comp. Biochem. Physiol. Part A Physiol. 1980, 67, 223–229. [Google Scholar] [CrossRef]
- Lesmeister, K.E.; Tozer, P.R.; Heinrichs, A.J. Development and analysis of a rumen tissue sampling procedure. J. Dairy Sci. 2004, 87, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, Z.; Li, B.; Zhao, C.; Wang, Y.; Chen, Y.; Jiang, Y. Dynamics of rumen gene expression, microbiome colonization, and their interplay in goats. BMC Genom. 2021, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.L.; Drackley, J.K. The Development, Nutrition, and Management of the Young Calf; Iowa State University Press: Ames, IA, USA, 1998; pp. 1–50. [Google Scholar]
- Diao, Q.-y.; Zhang, R.; Tu, Y. Current research progresses on calf rearing and nutrition in China. J. Integr. Agric. 2017, 16, 2805–2814. [Google Scholar] [CrossRef]
- Alves Costa, N.; Pansani, A.P.; de Castro, C.H.; Basile Colugnati, D.; Xaxier, C.H.; Guimaraes, K.C.; Antas Rabelo, L.; Nunes-Souza, V.; Souza Caixeta, L.F.; Nassar Ferreira, R. Milk restriction or oligosaccharide supplementation in calves improves compensatory gain and digestive tract development without changing hormone levels. PLoS ONE 2019, 14, e0214626. [Google Scholar] [CrossRef] [PubMed]
- Arne, A.; Ilgaza, A. Prebiotic and synbiotic effect on rumen papilla length development and rumen pH in 12-week-old calves. Vet. World 2021, 14, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Gorka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Jagusiak, W.; Holst, J.J.; Guilloteau, P.; Zabielski, R. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J. Dairy Sci. 2011, 94, 5578–5588. [Google Scholar] [CrossRef] [PubMed]
- Kern, R.J.; Lindholm-Perry, A.K.; Freetly, H.C.; Kuehn, L.A.; Rule, D.C.; Ludden, P.A. Rumen papillae morphology of beef steers relative to gain and feed intake and the association of volatile fatty acids with kallikrein gene expression. Livest. Sci. 2016, 187, 24–30. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Liang, G.; Guan, L.L. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019, 20, 172. [Google Scholar] [CrossRef]
- Novak, T.E.; Rodriguez-Zas, S.L.; Southey, B.R.; Starkey, J.D.; Stockler, R.M.; Alfaro, G.F.; Moisa, S.J. Jersey steer ruminal papillae histology and nutrigenomics with diet changes. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Yohe, T.T.; Dennis, T.S.; Buss, L.N.; Croft, E.J.D.; Quigley, J.D.; Hill, T.M.; Suarez-Mena, F.X.; Aragona, K.M.; Laarman, A.H.; Costa, J.H.C.; et al. Performance and visceral tissue growth and development of Holstein calves fed differing milk replacer allowances and starch concentrations in pelleted starter. J. Dairy Sci. 2022, 105, 4099–4115. [Google Scholar] [CrossRef]
- Yohe, T.T.; O’Diam, K.M.; Daniels, K.M. Growth, ruminal measurements, and health characteristics of Holstein bull calves fed an Aspergillus oryzae fermentation extract. J. Dairy Sci. 2015, 98, 6163–6175. [Google Scholar] [CrossRef]
- Lane, M.A.; Baldwin, R.L.t.; Jesse, B.W. Sheep rumen metabolic development in response to age and dietary treatments. J. Anim. Sci. 2000, 78, 1990–1996. [Google Scholar] [CrossRef]
- Nishihara, K.; Suzuki, Y.; Kim, D.; Roh, S. Growth of rumen papillae in weaned calves is associated with lower expression of insulin-like growth factor-binding proteins 2, 3, and 6. Anim. Sci. J. 2019, 90, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Tamate, H.; McGilliard, A.D.; Jacobson, N.L.; Getty, R. Effect of Various Dietaries on the Anatomical Development of the Stomach in the Calf. J. Dairy Sci. 1962, 45, 408–420. [Google Scholar] [CrossRef]
- Yohe, T.T.; Schramm, H.; Parsons, C.L.M.; Tucker, H.L.M.; Enger, B.D.; Hardy, N.R.; Daniels, K.M. Form of calf diet and the rumen. I: Impact on growth and development. J. Dairy Sci. 2019, 102, 8486–8501. [Google Scholar] [CrossRef]
- Gentile, A.; Sconza, S.; Lorenz, I.; Otranto, G.; Rademacher, G.; Famigli-Bergamini, P.; Klee, W. D-Lactic acidosis in calves as a consequence of experimentally induced ruminal acidosis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2004, 51, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Dieho, K.; Bannink, A.; Geurts, I.A.L.; Schonewille, J.T.; Gort, G.; Dijkstra, J. Morphological adaptation of rumen papillae during the dry period and early lactation as affected by rate of increase of concentrate allowance. J. Dairy Sci. 2016, 99, 2339–2352. [Google Scholar] [CrossRef]
- Connor, E.E.; Baldwin, R.L.t.; Li, C.J.; Li, R.W.; Chung, H. Gene expression in bovine rumen epithelium during weaning identifies molecular regulators of rumen development and growth. Funct. Integr. Genom. 2013, 13, 133–142. [Google Scholar] [CrossRef]
- Rosenboom, R.W. Rumen Transition from Weaning to 400 Pounds. Vet. Clin. N. Am. Food Anim. Pract. 2022, 38, 153–164. [Google Scholar] [CrossRef]
- Niwinska, B.; Hanczakowska, E.; Arciszewski, M.B.; Klebaniuk, R. Review: Exogenous butyrate: Implications for the functional development of ruminal epithelium and calf performance. Animal 2017, 11, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, D.; Mao, S.; Zhu, W.; Liu, J. Infusion of sodium butyrate promotes rumen papillae growth and enhances expression of genes related to rumen epithelial VFA uptake and metabolism in neonatal twin lambs. J. Anim. Sci. 2019, 97, 909–921. [Google Scholar] [CrossRef]
- Sakata, T.; Tamate, H. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J. Dairy Sci. 1978, 61, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.A.; Jesse, B.W. Effect of volatile fatty acid infusion on development of the rumen epithelium in neonatal sheep. J. Dairy Sci. 1997, 80, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Mentschel, J.; Leiser, R.; Mulling, C.; Pfarrer, C.; Claus, R. Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Arch. Tierernahr. 2001, 55, 85–102. [Google Scholar] [CrossRef]
- Chai, J.; Liu, Z.; Wu, J.; Kang, Y.; Abdelsattar, M.M.; Zhao, W.; Wang, S.; Yang, S.; Deng, F.; Li, Y.; et al. Dietary beta-hydroxybutyric acid improves the growth performance of young ruminants based on rumen microbiota and volatile fatty acid biosynthesis. Front. Microbiol. 2023, 14, 1296116. [Google Scholar] [CrossRef]
- Flatt, W.P.; Warner, R.G.; Loosli, J.K. Influence of purified materials on the development of the ruminant stomach. J. Dairy Sci. 1958, 41, 1593–1600. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Li, P.F. PPARalpha: An emerging target of metabolic syndrome, neurodegenerative and cardiovascular diseases. Front. Endocrinol. 2022, 13, 1074911. [Google Scholar] [CrossRef]
- Malhi, M.; Gui, H.; Yao, L.; Aschenbach, J.R.; Gabel, G.; Shen, Z. Increased papillae growth and enhanced short-chain fatty acid absorption in the rumen of goats are associated with transient increases in cyclin D1 expression after ruminal butyrate infusion. J. Dairy Sci. 2013, 96, 7603–7616. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zhang, J.; Liang, Z.; Du, M.; Yang, Y.; Zheng, J.; Yan, P.; Long, R.; Tong, B.; Han, J.; et al. Age-dependent variations in rumen bacterial community of Mongolian cattle from weaning to adulthood. BMC Microbiol. 2022, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- Sujani, S.; Seresinhe, R.T. Exogenous Enzymes in Ruminant Nutrition: A Review. Asian J. Anim. Sci. 2015, 9, 85–99. [Google Scholar] [CrossRef]
- Li, K.; Shi, B.; Na, R. The Colonization of Rumen Microbiota and Intervention in Pre-Weaned Ruminants. Animals 2023, 13, 994. [Google Scholar] [CrossRef] [PubMed]
- Minato, H.; Otsuka, M.; Shirasaka, S.; Itabashi, H.; Mitsumori, M. Colonization of microorganisms in the rumen of young calves. J. Gen. Appl. Microbiol. 1992, 38, 447–456. [Google Scholar] [CrossRef]
- Hu, R.; Zou, H.; Wang, Z.; Cao, B.; Peng, Q.; Jing, X.; Wang, Y.; Shao, Y.; Pei, Z.; Zhang, X.; et al. Nutritional Interventions Improved Rumen Functions and Promoted Compensatory Growth of Growth-Retarded Yaks as Revealed by Integrated Transcripts and Microbiome Analyses. Front. Microbiol. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xie, F.; Sun, D.; Liu, J.; Zhu, W.; Mao, S. Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model. Microbiome 2019, 7, 83. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Y.; Yu, Z.; Xu, Q.; Zheng, N.; Zhao, S.; Huang, G.; Wang, J. Ruminal microbiota-host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Na, S.W.; Guan, L.L. Understanding the role of rumen epithelial host-microbe interactions in cattle feed efficiency. Anim. Nutr. 2022, 10, 41–53. [Google Scholar] [CrossRef]
- Sakata, T.; Hikosaka, K.; Shiomura, Y.; Tamate, H. Stimulatory effect of insulin on ruminal epithelium cell mitosis in adult sheep. Br. J. Nutr. 1980, 44, 325–331. [Google Scholar] [CrossRef]
- Kato, S.; Sato, K.; Chida, H.; Roh, S.G.; Ohwada, S.; Sato, S.; Guilloteau, P.; Katoh, K. Effects of Na-butyrate supplementation in milk formula on plasma concentrations of GH and insulin, and on rumen papilla development in calves. J. Endocrinol. 2011, 211, 241–248. [Google Scholar] [CrossRef]
- Gálfi, P.; Neogrády, S.; Sakata, T. Effects of Volatile Fatty Acids on the Epithelial Cell Proliferation of the Digestive Tract and Its Hormonal Mediation. In Physiological Aspects of Digestion and Metabolism in Ruminants; Elsevier: Amsterdam, The Netherlands, 1991; pp. 49–59. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Guo, G.; Huo, W.J.; Yang, W.Z.; Wang, H. Effects of isobutyrate supplementation in pre- and post-weaned dairy calves diet on growth performance, rumen development, blood metabolites and hormone secretion. Animal 2017, 11, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Zitnan, R.; Kuhla, S.; Sanftleben, P.; Bilska, A.; Schneider, F.; Zupcanova, M.; Voigt, J. Diet induced ruminal papillae development in neonatal calves not correlating with rumen butyrate. Veterinární Medicína 2005, 50, 472–479. [Google Scholar] [CrossRef]
- Shen, Z.; Seyfert, H.M.; Lohrke, B.; Schneider, F.; Zitnan, R.; Chudy, A.; Kuhla, S.; Hammon, H.M.; Blum, J.W.; Martens, H.; et al. An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J. Nutr. 2004, 134, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, K.; Suzuki, Y.; Roh, S. Ruminal epithelial insulin-like growth factor-binding proteins 2, 3, and 6 are associated with epithelial cell proliferation. Anim. Sci. J. 2020, 91, e13422. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Ahluwalia, A. The Critical Role of Growth Factors in Gastric Ulcer Healing: The Cellular and Molecular Mechanisms and Potential Clinical Implications. Cells 2021, 10, 1964. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Kuhla, S.; Zitnan, R.; Seyfert, H.M.; Schneider, F.; Hagemeister, H.; Chudy, A.; Lohrke, B.; Blum, J.W.; Hammon, H.M.; et al. Intraruminal infusion of n-butyric acid induces an increase of ruminal papillae size independent of IGF-1 system in castrated bulls. Arch. Anim. Nutr. 2005, 59, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Pico, C.; Palou, M.; Pomar, C.A.; Rodriguez, A.M.; Palou, A. Leptin as a key regulator of the adipose organ. Rev. Endocr. Metab. Disord. 2022, 23, 13–30. [Google Scholar] [CrossRef]
- Hayashi, H.; Yamakado, M.; Yamaguchi, M.; Kozakai, T. Leptin and ghrelin expressions in the gastrointestinal tracts of calves and cows. In Journal of Veterinary Medical Science; Japanese Society of Veterinary Science: Tokyo, Japan, 2020; Volume 82, pp. 475–478. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, L.; Guo, J.; Ma, Y.; Li, F. Expression of Ghrelin in gastrointestinal tract and the effect of early weaning on Ghrelin expression in lambs. Mol. Biol. Rep. 2014, 41, 909–914. [Google Scholar] [CrossRef]
- Greco, G.; Amasheh, S.; Shen, Z.; Lu, Z.; Aschenbach, J.R. Effects of glucagon-like peptides 1 and 2 and epidermal growth factor on the epithelial barrier of the rumen of adult sheep. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1727–1738. [Google Scholar] [CrossRef]
- Baldwin, R.L. The proliferative actions of insulin, insulin-like growth factor-I, epidermal growth factor, butyrate and propionate on ruminal epithelial cells in vitro. Small Rumin. Res. 1999, 32, 261–268. [Google Scholar] [CrossRef]
- Connor, E.E.; Baldwin, R.L.t.; Walker, M.P.; Ellis, S.E.; Li, C.; Kahl, S.; Chung, H.; Li, R.W. Transcriptional regulators transforming growth factor-beta1 and estrogen-related receptor-alpha identified as putative mediators of calf rumen epithelial tissue development and function during weaning. J. Dairy Sci. 2014, 97, 4193–4207. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, M.; Zhang, L.; Li, L.; Lian, H.; Li, M.; Gao, T.; Fu, T.; Tu, Y. Integration of Long Non-Coding RNA and mRNA Profiling Reveals the Mechanisms of Different Dietary NFC/NDF Ratios Induced Rumen Development in Calves. Animals 2022, 12, 650. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, H.; Li, M.; Zhao, K.; Xia, S.; Chen, Y.; Shao, J.; Tang, T.; Bai, X.; Liu, Z.; et al. Integration of Non-Coding RNA and mRNA Profiles Reveals the Mechanisms of Rumen Development Induced by Different Types of Diet in Calves. Genes 2023, 14, 1093. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Weary, D.M.; von Keyserlingk, M.A. Invited review: Effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. J. Dairy Sci. 2011, 94, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, D.; Hotzel, M.J.; Ungerfeld, R. Minimising the stress of weaning of beef calves: A review. Acta Vet. Scand. 2011, 53, 28. [Google Scholar] [CrossRef] [PubMed]
- Carballo, O.C.; Khan, M.A.; Knol, F.W.; Lewis, S.J.; Stevens, D.R.; Laven, R.A.; McCoard, S.A. Impact of weaning age on rumen development in artificially reared lambs1. J. Anim. Sci. 2019, 97, 3498–3510. [Google Scholar] [CrossRef] [PubMed]
- Mikus, T.; Marzel, R.; Mikus, O. Early weaning: New insights on an ever-persistent problem in the dairy industry. J. Dairy Res. 2020, 87, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, S.; Kinoshita, A.; Huther, L.; Salm, L.; Kehraus, S.; Sudekum, K.H.; Huber, K.; Danicke, S.; Frahm, J. Weaning age influences indicators of rumen function and development in female Holstein calves. BMC Vet. Res. 2022, 18, 102. [Google Scholar] [CrossRef]
- Liu, S.; Ma, J.; Li, J.; Alugongo, G.M.; Wu, Z.; Wang, Y.; Li, S.; Cao, Z. Effects of Pair Versus Individual Housing on Performance, Health, and Behavior of Dairy Calves. Animals 2019, 10, 50. [Google Scholar] [CrossRef]
- Eckert, E.; Brown, H.E.; Leslie, K.E.; DeVries, T.J.; Steele, M.A. Weaning age affects growth, feed intake, gastrointestinal development, and behavior in Holstein calves fed an elevated plane of nutrition during the preweaning stage. J. Dairy Sci. 2015, 98, 6315–6326. [Google Scholar] [CrossRef] [PubMed]
- Agustinho, B.C.; Wolfe, A.; Tsai, C.Y.; Pereira, L.M.; Konetchy, D.E.; Laarman, A.H.; Rezamand, P. Effect of weaning age and pace on blood metabolites, cortisol concentration, and the mRNA abundance of inflammation-related genes in gastrointestinal, adipose, and liver tissue of Holstein dairy calves. J. Dairy Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Lee, H.J.; Lee, W.S.; Kim, H.S.; Ki, K.S.; Hur, T.Y.; Suh, G.H.; Kang, S.J.; Choi, Y.J. Structural growth, rumen development, and metabolic and immune responses of Holstein male calves fed milk through step-down and conventional methods. J. Dairy Sci. 2007, 90, 3376–3387. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, S.; Kinoshita, A.; Kluess, J.; Kersten, S.; Meyer, U.; Huber, K.; Danicke, S.; Frahm, J. Weaning Holstein Calves at 17 Weeks of Age Enables Smooth Transition from Liquid to Solid Feed. Animals 2019, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.H.; Kertz, A.F. Review: Effects of different forms of calf starters on feed intake and growth rate: A systematic review and Bayesian meta-analysis of studies from 1938 to 2021. Appl. Anim. Sci. 2021, 37, 273–293. [Google Scholar] [CrossRef]
- Kertz, A.F.; Hill, T.M.; Quigley, J.D., 3rd; Heinrichs, A.J.; Linn, J.G.; Drackley, J.K. A 100-Year Review: Calf nutrition and management. J. Dairy Sci. 2017, 100, 10151–10172. [Google Scholar] [CrossRef] [PubMed]
- Armengol, R.; Fraile, L. Colostrum and milk pasteurization improve health status and decrease mortality in neonatal calves receiving appropriate colostrum ingestion. J. Dairy Sci. 2016, 99, 4718–4725. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Khan, M.Z.; Xiao, J.; Alugongo, G.M.; Chen, X.; Li, S.; Wang, Y.; Cao, Z. An Overview of Waste Milk Feeding Effect on Growth Performance, Metabolism, Antioxidant Status and Immunity of Dairy Calves. Front. Vet. Sci. 2022, 9, 898295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, W.B.; Bi, Y.L.; Tu, Y.; Beckers, Y.; Du, H.C.; Diao, Q.Y. Early Feeding Regime of Waste Milk, Milk, and Milk Replacer for Calves Has Different Effects on Rumen Fermentation and the Bacterial Community. Animals 2019, 9, 443. [Google Scholar] [CrossRef]
- Arowolo, M.A.; He, J. Use of probiotics and botanical extracts to improve ruminant production in the tropics: A review. Anim. Nutr. 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Fomenky, B.E.; Do, D.N.; Talbot, G.; Chiquette, J.; Bissonnette, N.; Chouinard, Y.P.; Lessard, M.; Ibeagha-Awemu, E.M. Direct-fed microbial supplementation influences the bacteria community composition of the gastrointestinal tract of pre- and post-weaned calves. Sci. Rep. 2018, 8, 14147. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.A.; Chethan, H.S.; Srivastava, R.; Gabbur, A.B. Role of probiotics in ruminant nutrition as natural modulators of health and productivity of animals in tropical countries: An overview. Trop. Anim. Health Prod. 2022, 54, 110. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.C.; Elolimy, A.A.; Loor, J.J. Rumen Microbiome, Probiotics, and Fermentation Additives. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Baran, M.; Kmet, V. Effect of pectinase on rumen fermentation in sheep and lambs. Arch. Tierernahr. 1987, 37, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.T.; Anderson, K.L.; Yoon, I.; Scott, M.F.; Carlson, S.A. Amelioration of salmonellosis in pre-weaned dairy calves fed Saccharomyces cerevisiae fermentation products in feed and milk replacer. Vet. Microbiol. 2014, 172, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Laksesvela, B.; Ommundsen, A.; Landsverk, T. Indigestion in young calves. V. The influence of grass silage and fine hay. Acta Vet. Scand. 1978, 19, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Chester-Jones, H.; Fontenot, J.P.; Veit, H.P. Physiological and pathological effects of feeding high levels of magnesium to steers. J. Anim. Sci. 1990, 68, 4400–4413. [Google Scholar] [CrossRef]
- Mu, Y.; Qi, W.; Zhang, T.; Zhang, J.; Mao, S. Multi-omics Analysis Revealed Coordinated Responses of Rumen Microbiome and Epithelium to High-Grain-Induced Subacute Rumen Acidosis in Lactating Dairy Cows. mSystems 2022, 7, e0149021. [Google Scholar] [CrossRef]
- Pan, X.; Ma, Z.; Sun, X.; Li, H.; Zhang, T.; Zhao, C.; Wang, N.; Heller, R.; Hung Wong, W.; Wang, W.; et al. CNEReg Interprets Ruminant-specific Conserved Non-coding Elements by Developmental Gene Regulatory Network. Genom. Proteom. Bioinform. 2023, 21, 632–648. [Google Scholar] [CrossRef] [PubMed]
- Do, D.N.; Dudemaine, P.L.; Fomenky, B.E.; Ibeagha-Awemu, E.M. Integration of miRNA weighted gene co-expression network and miRNA-mRNA co-expression analyses reveals potential regulatory functions of miRNAs in calf rumen development. Genomics 2019, 111, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Wray, G.A. The evolutionary significance of cis-regulatory mutations. Nat. Rev. Genet. 2007, 8, 206–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, W.; Li, Q.; Wang, Y.; Wang, Z.; Zhang, Q.; Xu, L.; Xu, L.; Hu, X.; Zhu, B.; et al. Transcriptome Analysis of Bovine Rumen Tissue in Three Developmental Stages. Front. Genet. 2022, 13, 821406. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, K.; Kato, D.; Suzuki, Y.; Kim, D.; Nakano, M.; Yajima, Y.; Haga, S.; Nakano, M.; Ishizaki, H.; Kawahara-Miki, R.; et al. Comparative transcriptome analysis of rumen papillae in suckling and weaned Japanese Black calves using RNA sequencing. J. Anim. Sci. 2018, 96, 2226–2237. [Google Scholar] [CrossRef]
- Chen, C.; Yin, Y.; Li, H.; Zhou, B.; Zhou, J.; Zhou, X.; Li, Z.; Liu, G.; Pan, X.; Zhang, R.; et al. Ruminant-specific genes identified using high-quality genome data and their roles in rumen evolution. Sci. Bull. 2022, 67, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, C. ILK: A pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr. Opin. Cell Biol. 2012, 24, 607–613. [Google Scholar] [CrossRef]
- Sha, Y.; He, Y.; Liu, X.; Zhao, S.; Hu, J.; Wang, J.; Li, S.; Li, W.; Shi, B.; Hao, Z. Rumen Epithelial Development- and Metabolism-Related Genes Regulate Their Micromorphology and VFAs Mediating Plateau Adaptability at Different Ages in Tibetan Sheep. Int. J. Mol. Sci. 2022, 23, 16078. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Andreyev, O.; Hoyt, R.F., Jr.; Singh, A.; Hunt, T.; Horvath, K.A. Overexpression of FABP3 inhibits human bone marrow derived mesenchymal stem cell proliferation but enhances their survival in hypoxia. Exp. Cell Res. 2014, 323, 56–65. [Google Scholar] [CrossRef]
- Kato, D.; Suzuki, Y.; Haga, S.; So, K.; Yamauchi, E.; Nakano, M.; Ishizaki, H.; Choi, K.; Katoh, K.; Roh, S.G. Utilization of digital differential display to identify differentially expressed genes related to rumen development. Anim. Sci. J. 2016, 87, 584–590. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, H.; Xu, J.; Zhang, L.; Yan, L.; Shen, Z. Elevated cyclin D1 expression is governed by plasma IGF-1 through Ras/Raf/MEK/ERK pathway in rumen epithelium of goats supplying a high metabolizable energy diet. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhrel, B.; Jiang, H. Postnatal Growth and Development of the Rumen: Integrating Physiological and Molecular Insights. Biology 2024, 13, 269. https://doi.org/10.3390/biology13040269
Pokhrel B, Jiang H. Postnatal Growth and Development of the Rumen: Integrating Physiological and Molecular Insights. Biology. 2024; 13(4):269. https://doi.org/10.3390/biology13040269
Chicago/Turabian StylePokhrel, Binod, and Honglin Jiang. 2024. "Postnatal Growth and Development of the Rumen: Integrating Physiological and Molecular Insights" Biology 13, no. 4: 269. https://doi.org/10.3390/biology13040269
APA StylePokhrel, B., & Jiang, H. (2024). Postnatal Growth and Development of the Rumen: Integrating Physiological and Molecular Insights. Biology, 13(4), 269. https://doi.org/10.3390/biology13040269