The Role of Cerebellar Intrinsic Neuronal Excitability, Synaptic Plasticity, and Perineuronal Nets in Eyeblink Conditioning
Simple Summary
Abstract
1. Introduction
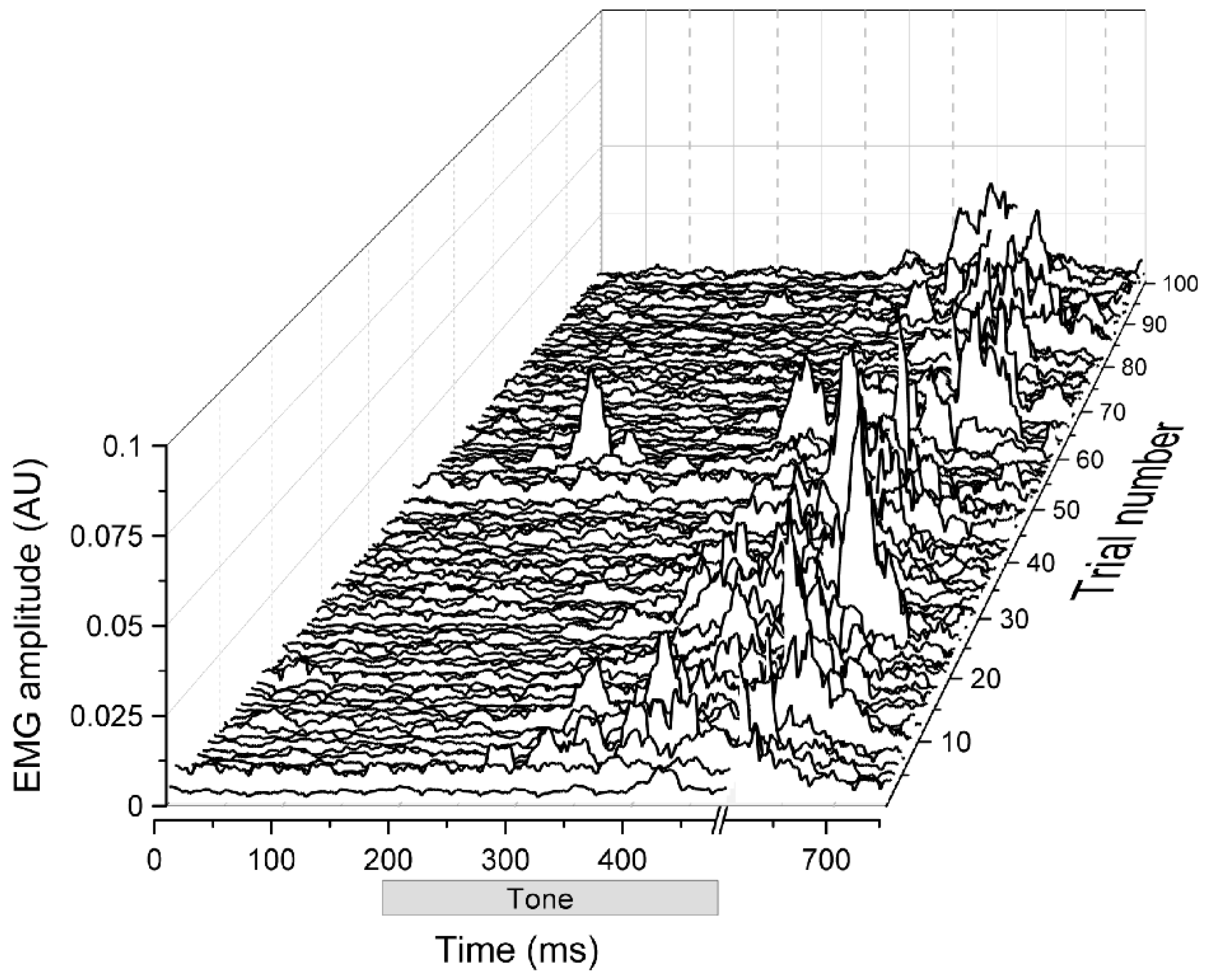
2. Eyeblink Conditioning
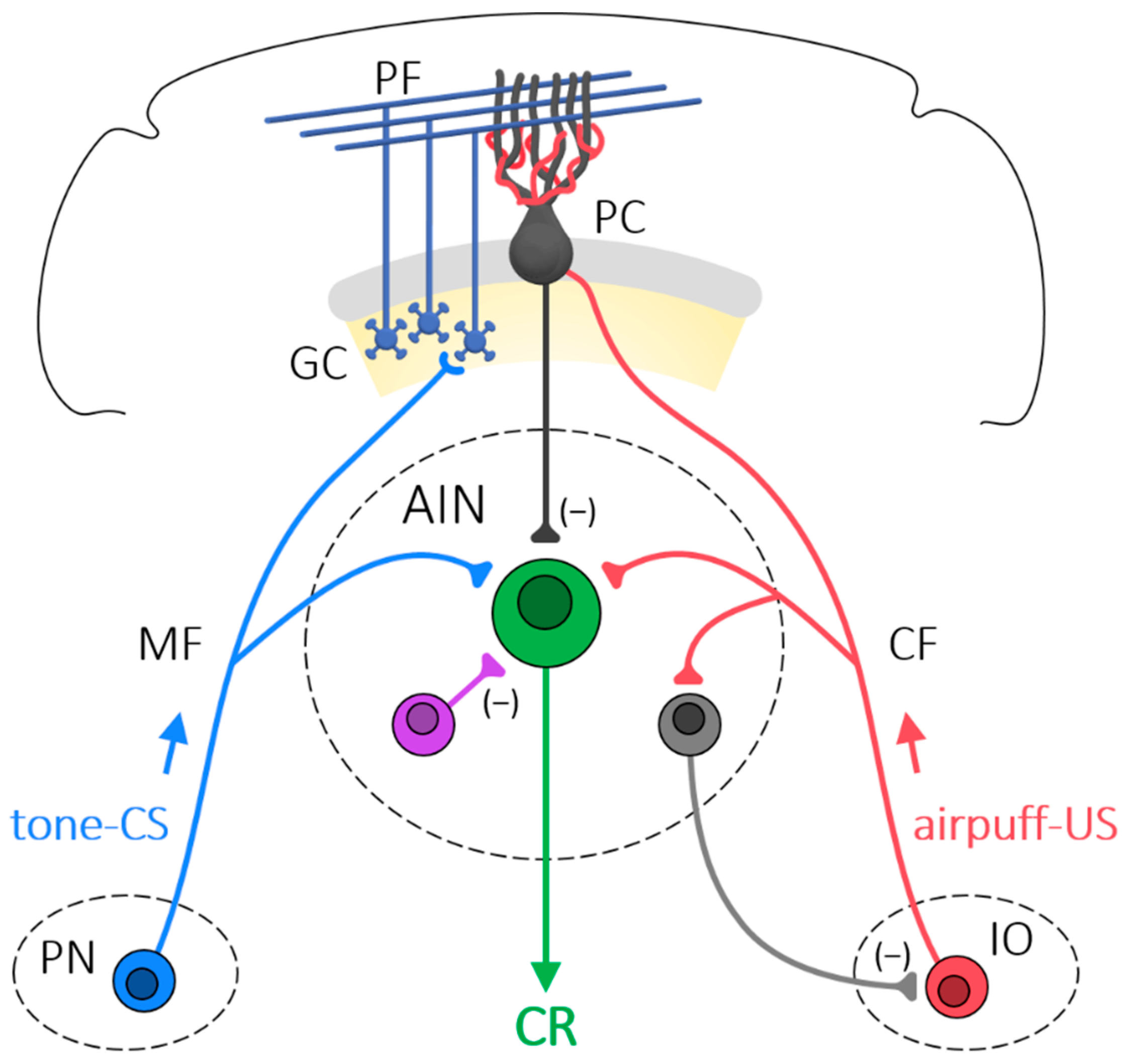
3. The Role of the Cerebellum in Eyeblink Conditioning
4. Intrinsic Membrane Excitability Involved in Eyeblink Conditioning
5. Synaptic Plasticity in the Cerebellum Involved in Eyeblink Conditioning
6. Membrane Excitability and Synaptic Plasticity in Eyeblink Conditioning
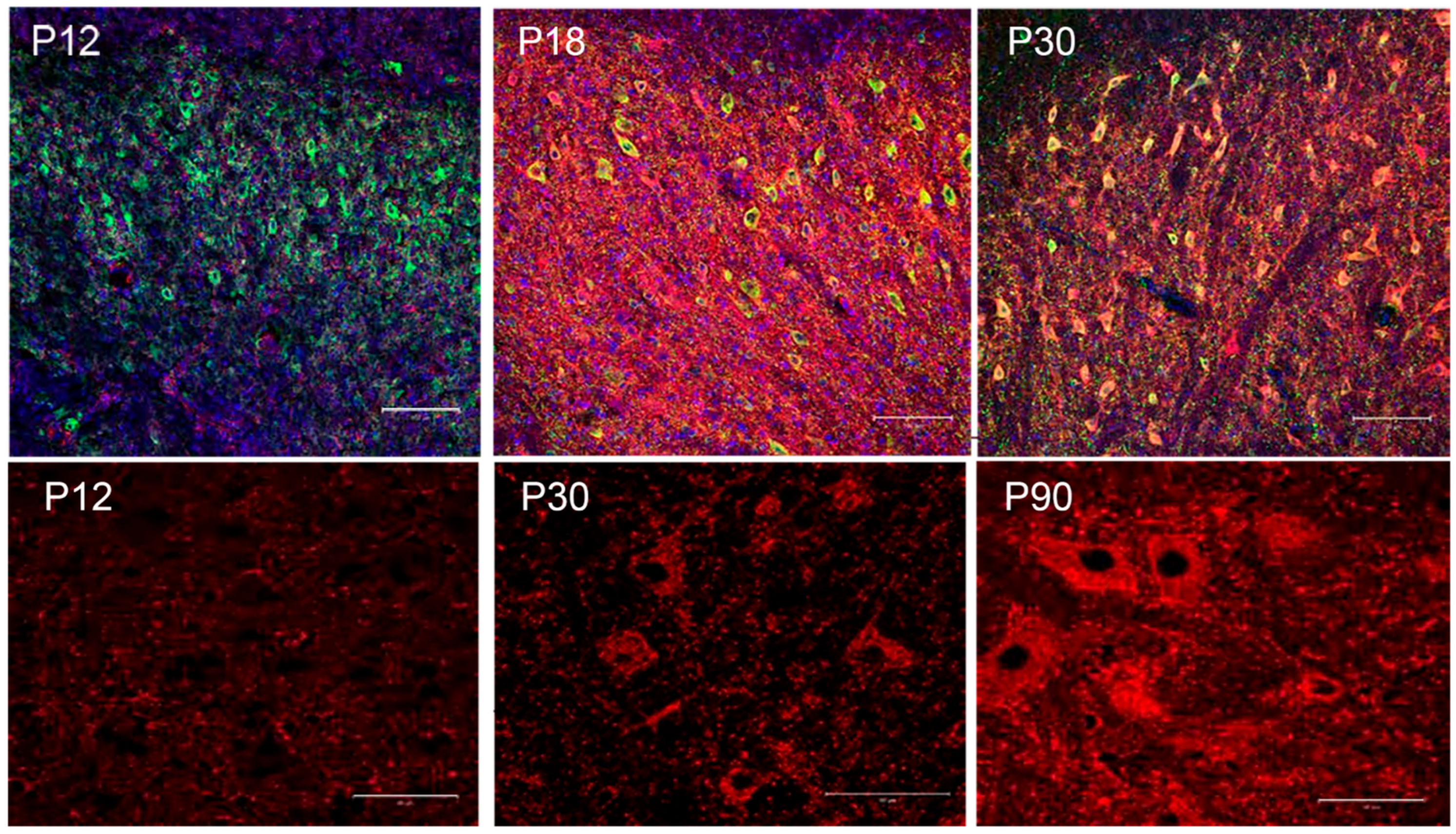
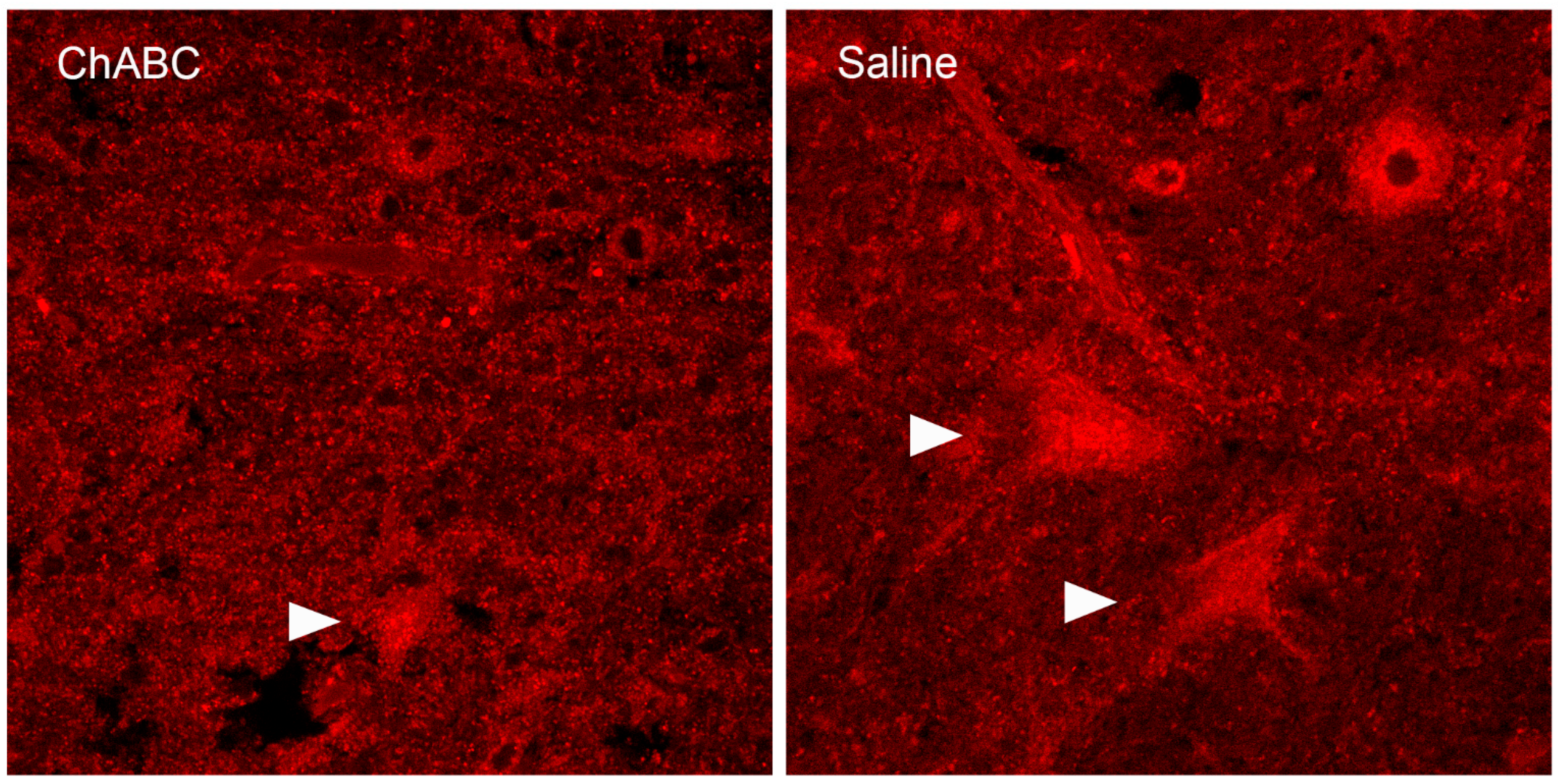
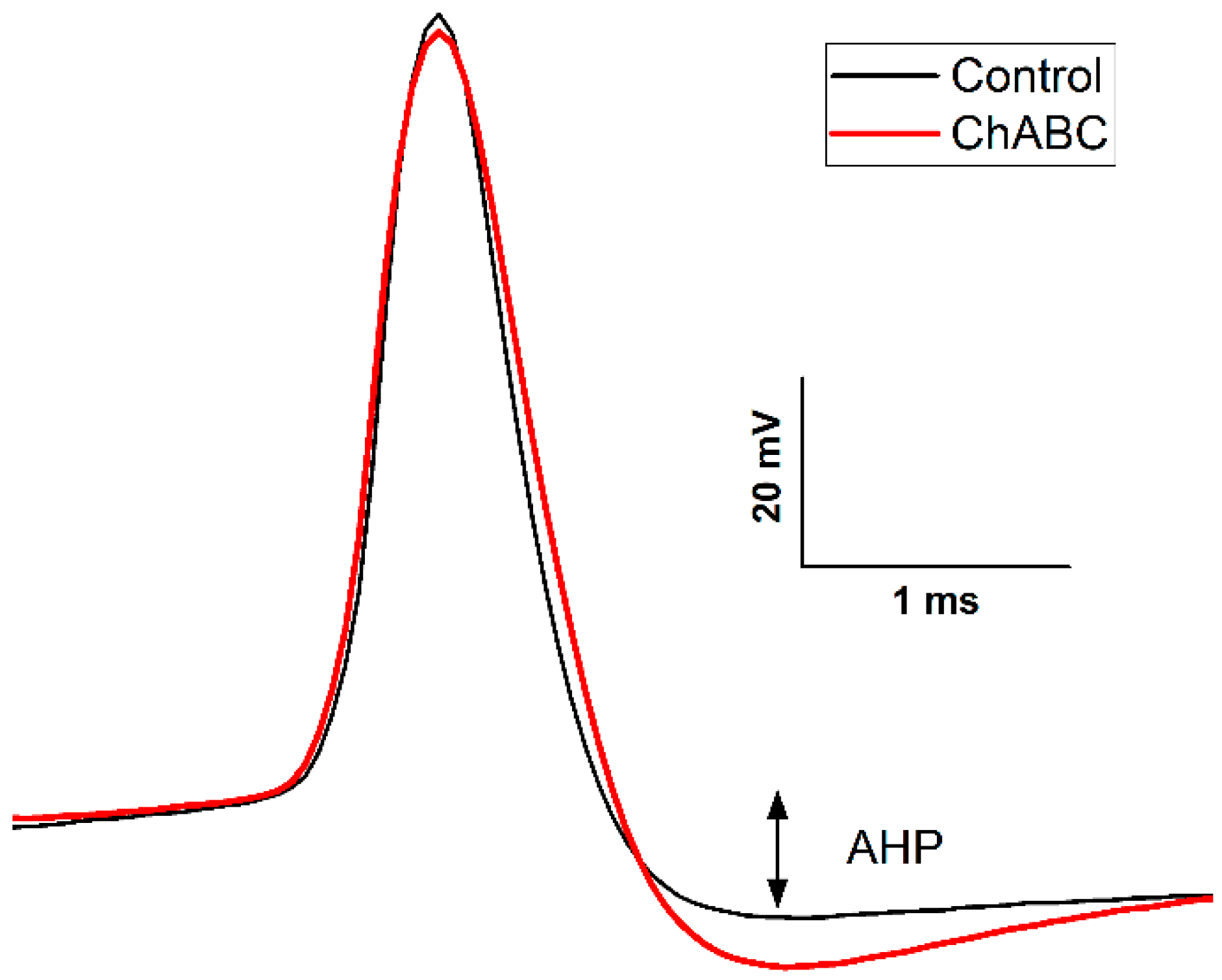
7. The Perineuronal Net
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hilgard, E.R.; Marquis, D.G. Acquisition, extinction, and retention of conditioned responses to light in dogs. J. Comp. Psychol. 1935, 19, 29–58. [Google Scholar] [CrossRef]
- Hilgard, E.R.; Campbell, A.A. The course of acquisition and retention of conditioned eyelid responses in man. J. Exp. Psychol. 1936, 19, 227–247. [Google Scholar] [CrossRef]
- Spence, K.W.; Spence, J.T. Sex and anxiety difference in eyelid conditioning. Psychol. Bull. 1966, 65, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R.; Webster, S. The problem of volition and the conditioned reflex. Part II: Voluntary-responding subjects, 1951–1980. Behaviorism 1988, 16, 17–49. [Google Scholar]
- Coleman, S.R. The problem of volition and the conditioned reflex. Part I: Conceptual background, 1900–1940. Behaviorism 1985, 13, 99–124. [Google Scholar]
- Blaxton, T.A.; Zeffiro, T.A.; Gabrieli, J.D.E.; Bookheimer, S.Y.; Carrillo, M.C.; Theodore, W.H.; Disterhoft, J.F. Functional mapping of human learning: A positron emission tomography activation study of eyeblink conditioning. J. Neurosci. 1996, 16, 4032–4040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Logan, C.G.; Grafton, S.T. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc. Natl. Acad. Sci. USA 1995, 92, 7500–7504. [Google Scholar] [CrossRef]
- Molchan, S.E.; Sunderland, T.; McIntosh, A.R.; Herscovitch, P.; Schreurs, B.G. A functional anatomical study of associative learning in humans. Proc. Natl. Acad. Sci. USA 1994, 91, 8122–8126. [Google Scholar] [CrossRef]
- Kent, J.S.; Kim, D.J.; Newman, S.D.; Bolbecker, A.R.; O’Donnell, B.F.; Hetrick, W.P. Investigating cerebellar neural function in schizophrenia using delay eyeblink conditioning: A pilot fMRI study. Psychiatry Res. Neuroimaging 2020, 304, 111133. [Google Scholar] [CrossRef]
- Dimitrova, A.; Weber, J.; Maschke, M.; Elles, H.-G.; Kolb, F.P.; Forsting, M.; Diener, H.-C.; Timmann, D. Eyeblink-related areas in human cerebellum as shown by fMRI. Hum. Brain Mapp. 2002, 17, 100–115. [Google Scholar] [CrossRef]
- Ramnani, N.; Toni, I.; Josephs, O.; Ashburner, J.; Passingham, R.E. Learning- and expectation-related changes in the human brain during motor learning. J. Neurophysiol. 2000, 84, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Park, P.; Choi, D.I.; Bliss, T.V.P.; Kaang, B.K. The essence of the engram: Cellular or synaptic? Semin. Cell Dev. Biol. 2021, 125, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Brodt, S.; Gais, S. Memory engrams in the neocortex. Neuroscientist 2021, 27, 427–444. [Google Scholar] [CrossRef]
- Josselyn, S.A.; Tonegawa, S. Memory engrams: Recalling the past and imagining the future. Science 2020, 367, eaaw4325. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Hayashi, Y. Catching the engram: Strategies to examine the memory trace. Mol. Brain 2012, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Hubener, M.; Bonhoeffer, T. Searching for engrams. Neuron 2010, 67, 363–371. [Google Scholar] [CrossRef]
- Schafe, G.E.; Doyere, V.; LeDoux, J.E. Tracking the fear engram: The lateral amygdala is an essential locus of fear memory storage. J. Neurosci. 2005, 25, 10010–10015. [Google Scholar] [CrossRef]
- Steinmetz, J.E.; Woodruff-Pak, D.S. Animal models in eyeblink classical conditioning. In Eyeblink Classical Conditioning Volume 2: Animal Models; Woodruff-Pak, D.S., Steinmetz, J.E., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2000; pp. 1–15. [Google Scholar]
- Gormezano, I.; Kehoe, E.J.; Marshall, B.S. Twenty years of classical conditioning research with the rabbit. In Progress in Psychobiology and Physiological Psychology, 10th ed.; Sprague, J.M., Ed.; Academic Press: New York, NY, USA, 1983; pp. 197–275. [Google Scholar]
- Schneiderman, N.; Fuentes, I.; Gormezano, I. Acquisition and extinction of the classically conditioned eyelid response in the albino rabbit. Science 1962, 136, 650–652. [Google Scholar] [CrossRef]
- Gormezano, I.; Kehoe, E.J. Classical Conditioning: Some methodological-conceptual issues. In Handbook of Learning and Cognitive Processes (Volume 2): Conditioning and Behavior Theory; Estes, W.K., Ed.; Earlbaum: Hillsdale, NJ, USA, 1975; pp. 143–179. [Google Scholar]
- Schmajuk, N.A.; Christiansen, B.A. Eyeblink conditioning in rats. Physiol. Behav. 1990, 48, 755–758. [Google Scholar] [CrossRef]
- Weiss, C.; Thompson, R.F. The effects of age on eyeblink conditioning in the freely moving Fischer-344 rat. Neurobiol. Aging 1991, 12, 249–254. [Google Scholar] [CrossRef]
- Shors, T.J.; Servatius, R.J. The contribution of stressor intensity, duration, and context to the stress-induced facilitation of associative learning. Neurobiol. Learn. Mem. 1997, 67, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.E.; Freeman, J.H.; Skelton, R.W. Eyeblink conditioning in the developing rat. Behav. Neurosci. 1992, 106, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Knuttinen, M.-G.; Gamelli, A.E.; Weiss, C.; Power, J.M.; Disterhoft, J.F. Age-related effects on eyeblink conditioning in F344 X BN F1 hybrid rats. Neurobiol. Aging 2001, 22, 1–8. [Google Scholar] [CrossRef]
- Green, J.T.; Rogers, R.F.; Goodlett, C.R.; Steinmetz, J.E. Impairment in eyeblink classical conditioning in adult rats exposed to ethanol as neonates. Alcohol. Clin. Exp. Res. 2000, 24, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Knuttinen, M.-G.; Power, J.M.; Patel, R.I.; O’Connor, M.S.; Disterhoft, J.F. Trace eyeblink conditioning in the freely moving rat: Optimizing the conditioning parameters. Behav. Neurosci. 1999, 113, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Ten Brinke, M.M.; Boele, H.J.; Spanke, J.K.; Potters, J.W.; Kornysheva, K.; Wulff, P.; AC, I.J.; Koekkoek, S.K.; De Zeeuw, C.I. Evolving models of Pavlovian conditioning: Cerebellar cortical dynamics in awake behaving mice. Cell Rep. 2015, 13, 1977–1988. [Google Scholar] [CrossRef]
- Koekkoek, S.K.E.; Den Ouden, W.L.; Perry, G.; Highstein, S.M.; De Zeeuw, C.I. Monitoring kinetic and frequency-domain properties of eyelid responses in mice with magnetic distance measurement technique. J. Neurophysiol. 2002, 88, 2124–2133. [Google Scholar] [CrossRef][Green Version]
- De Zeeuw, C.I.; Van Alphen, A.M.; Koekkoek, S.K.E.; Buharin, E.; Coesmans, M.P.H.; Morpurgo, M.M.; Van Der Burg, J. Recording eye movements in mice: A new approach to investigate the molecular basis of cerebellar control of motor learning and motor timing. Otolaryngol.-Head Neck Surg. 1998, 119, 193–203. [Google Scholar] [CrossRef]
- Miller, L.N.; Weiss, C.; Disterhoft, J.F. Learning-related changes in cellular activity within mouse dentate gyrus during trace eyeblink conditioning. Hippocampus 2022, 32, 776–794. [Google Scholar] [CrossRef]
- de Oude, N.L.; Hoebeek, F.E.; Ten Brinke, M.M.; de Zeeuw, C.I.; Boele, H.J. Pavlovian eyeblink conditioning is severely impaired in tottering mice. J. Neurophysiol. 2021, 125, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Titley, H.K.; Watkins, G.V.; Lin, C.; Weiss, C.; McCarthy, M.; Disterhoft, J.F.; Hansel, C. Intrinsic excitability increase in cerebellar Purkinje cells following delay eyeblink conditioning in mice. J. Neurosci. 2020, 40, 2038–2046. [Google Scholar] [CrossRef]
- Najafi, F.; Medina, J.F. Bidirectional short-term plasticity during single-trial learning of cerebellar-driven eyelid movements in mice. Neurobiol. Learn. Mem. 2019, 170, 107097. [Google Scholar] [CrossRef] [PubMed]
- López-Ramos, J.C.; Houdek, Z.; Cendelín, J.; Vožeh, F.; Delgado-García, J.M. Timing correlations between cerebellar interpositus neuronal firing and classically conditioned eyelid responses in wild-type and Lurcher mice. Sci. Rep. 2018, 8, 10697. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.W.; Alger, B.; Thompson, R.F. Neuronal substrate of classical conditioning in the hippocampus. Science 1976, 192, 483–485. [Google Scholar] [CrossRef]
- Solomon, P.R.; Moore, J.W. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J. Comp. Physiol. Psychol. 1975, 89, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Thompson, R.F. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. J. Neurosci. 1984, 4, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Thompson, R.F. Cerebellum: Essential involvement in the classically conditioned eyelid response. Science 1984, 223, 296–299. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Clark, G.A.; Lavond, D.G.; Thompson, R.F. Initial localization of the memory trace for a basic form of learning. Proc. Natl. Acad. Sci. USA 1982, 79, 2731–2735. [Google Scholar] [CrossRef]
- Yeo, C.H.; Hardiman, M.J.; Glickstein, M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exp. Brain Res. 1985, 60, 87–98. [Google Scholar] [CrossRef]
- Yeo, C.H.; Hardiman, M.J.; Glickstein, M. Discrete lesions of the cerebellar cortex abolish the classically conditioned nictitating membrane response of the rabbit. Behav. Brain Res. 1984, 13, 261–266. [Google Scholar] [CrossRef]
- Schreurs, B.G. Changes in cerebellar intrinsic neuronal excitability and synaptic plasticity result from eyeblink conditioning. Neurobiol. Learn. Mem. 2019, 166, 107094. [Google Scholar] [CrossRef] [PubMed]
- Baumann, O.; Borra, R.J.; Bower, J.M.; Cullen, K.E.; Habas, C.; Ivry, R.B.; Leggio, M.; Mattingley, J.B.; Molinari, M.; Moulton, E.A.; et al. Consensus paper: The role of the cerebellum in perceptual processes. Cerebellum 2015, 14, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.H.; Steinmetz, A.B. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn. Mem. 2011, 18, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Timmann, D.; Drepper, J.; Frings, M.; Maschke, M.; Richter, S.; Gerwig, M.; Kolb, F.P. The human cerebellum contributes to motor, emotional and cognitive associative learning: A review. Cortex 2010, 46, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Garcia, J.M.; Gruart, A. The role of interpositus nucleus in eyelid conditioned responses. Cerebellum 2002, 1, 289–308. [Google Scholar] [CrossRef]
- Steinmetz, J.E. Brain substrates of classical eyeblink conditioning: A highly localized but also distributed system. Behav. Brain Res. 2000, 110, 13–24. [Google Scholar] [CrossRef]
- Lavond, D.G.; Kim, J.J.; Thompson, R.F. Mammalian brain substrates of aversive classical conditioning. Annu. Rev. Psychol. 1993, 44, 317–342. [Google Scholar] [CrossRef]
- Thurling, M.; Kahl, F.; Maderwald, S.; Stefanescu, R.M.; Schlamann, M.; Boele, H.J.; De Zeeuw, C.I.; Diedrichsen, J.; Ladd, M.E.; Koekkoek, S.K.; et al. Cerebellar cortex and cerebellar nuclei are concomitantly activated during eyeblink conditioning: A 7T fMRI study in humans. J. Neurosci. 2015, 35, 1228–1239. [Google Scholar] [CrossRef]
- Heiney, S.A.; Wohl, M.P.; Chettih, S.N.; Ruffolo, L.I.; Medina, J.F. Cerebellar-dependent expression of motor learning during eyeblink conditioning in head-fixed mice. J. Neurosci. 2014, 34, 14845–14853. [Google Scholar] [CrossRef] [PubMed]
- Boele, H.-J.; Koekkoek, S.K.E.; De Zeeuw, C.I. Cerebellar and extracerebellar involvement in mouse eyeblink conditioning; the ACDC model. Front. Cell. Neurosci. 2010, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Pakaprot, N.; Kim, S.; Thompson, R.F. The role of the cerebellar Interpositus nucleus in short and long term memory for trace eyeblink conditioning. Behav. Neurosci. 2009, 123, 54–61. [Google Scholar] [CrossRef]
- Mojtahedian, S.; Kogan, D.R.; Kanzawa, S.A.; Thompson, R.F.; Lavond, D.G. Dissociation of conditioned eye and limb responses in the cerebellar interpositus. Physiol. Behav. 2007, 91, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.H., Jr.; Halverson, H.E.; Poremba, A. Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. J. Neurosci. 2005, 25, 889–895. [Google Scholar] [CrossRef][Green Version]
- Christian, K.M.; Thompson, R.F. Neural substrates of eyeblink conditioning: Acquisition and retention. Learn. Mem. 2003, 11, 427–455. [Google Scholar] [CrossRef] [PubMed]
- Attwell, P.J.E.; Ivarsson, M.; Millar, L.; Yeo, C.H. Cerebellar mechanisms in eyeblink conditioning. Ann. N. Y. Acad. Sci. 2002, 978, 79–92. [Google Scholar] [CrossRef]
- Wikgren, J.; Korhonen, T. Inactivation of the interpositus nucleus blocks the conditioned response acquired by a somatosensory conditioned stimulus in rabbit eyeblink conditioning. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.P.; Harvey, J.A. Cerebellar lesions and the nictitating membrane reflex: Performance deficits of the conditioned and unconditioned response. J. Neurosci. 1989, 9, 299–311. [Google Scholar] [CrossRef]
- Delgado-Garcia, J.M.; Gruart, A. Building new motor responses: Eyelid conditioning revisited. Trends Neurosci. 2006, 29, 330–338. [Google Scholar] [CrossRef]
- Kelly, T.M.; Zuo, C.C.; Bloedel, J.R. Classical conditioning of the eyelid reflex in the decerebrate-decerebellate rabbit. Behav. Brain Res. 1990, 38, 7–18. [Google Scholar] [CrossRef]
- Perciavalle, V.; Apps, R.; Bracha, V.; Delgado-Garcia, J.M.; Gibson, A.R.; Leggio, M.; Carrel, A.J.; Cerminara, N.; Coco, M.; Gruart, A.; et al. Consensus paper: Current views on the role of cerebellar interpositus nucleus in movement control and emotion. Cerebellum 2013, 12, 738–757. [Google Scholar] [CrossRef]
- Attwell, P.J.E.; Rahman, S.; Yeo, C.H. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. J. Neurosci. 2001, 21, 5715–5722. [Google Scholar] [CrossRef]
- Gruart, A.; Yeo, C.H. Cerebellar cortex and eyeblink conditioning: Bilateral regulation of conditioned responses. Exp. Brain Res. 1995, 104, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Mostofi, A.; Holtzman, T.; Grout, A.S.; Yeo, C.H.; Edgley, S.A. Electrophysiological localization of eyeblink-related microzones in rabbit cerebellar cortex. J. Neurosci. 2010, 30, 8920–8934. [Google Scholar] [CrossRef]
- Nicholson, D.A.; Freeman, J.H., Jr. Developmental changes in eyeblink conditioning and simple spike activity in the cerebellar cortex. Dev. Psychobiol. 2003, 44, 45–57. [Google Scholar] [CrossRef]
- Miller, M.J.; Chen, N.-K.; Li, L.; Tom, B.; Weiss, C.; Disterhoft, J.F.; Wyrwicz, A.M. fMRI of the conscious rabbit during unilateral classical eyeblink conditioning reveals bilateral cerebellar activation. J. Neurosci. 2003, 23, 11753–11758. [Google Scholar] [CrossRef] [PubMed]
- Lavond, D.G.; Steinmetz, J.E.; Yokaitis, M.H.; Thompson, R.F. Reacquisition of classical conditioning after removal of cerebellar cortex. Exp. Brain Res. 1987, 67, 569–593. [Google Scholar] [CrossRef]
- Harvey, J.A.; Welsh, J.P.; Yeo, C.H.; Romano, A.G. Recoverable and nonrecoverable deficits in conditioned responses after cerebellar cortical lesions. J. Neurosci. 1993, 13, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, F.; Duran, E.; Gomez, A.; Ocanan, F.M.; Alvarez, E.; Jimenez-Moya, F. Cognitive and emotional functions of the teleost fish cerebellum. Brain Res. Bull. 2005, 66, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Keifer, J.; Armstrong, K.E.; Houk, J.C. In vitro classical conditioning of abducens nerve discharge in turtles. J. Neurosci. 1995, 15, 5036–5048. [Google Scholar] [CrossRef] [PubMed]
- Skelton, R.W. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behav. Neurosci. 1988, 102, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bao, S.; Lockard, J.M.; Kim, J.J.; Thompson, R.F. Impaired classical eyeblink conditioning in cerebellar-lesioned and Purkinje cell degeneration (pcd) mutant mice. J. Neurosci. 1996, 16, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Q.; Chu, X.; Li, L.; Li, X.; Li, J.; Yang, Z.; Xu, M.; Luo, C.; Zhang, K. Role of cerebellar cortex in associative learning and memory in guinea pigs. Open Life Sci. 2022, 17, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, L.; Huang, L.-S.; Chen, H.; Zeng, Y.; Feng, H.; Sui, J.-F. Effect of cerebellar reversible inactivations on the acquisition of trace conditioned eyeblink responses in guinea pigs: Comparison of short and long trace intervals. Neurosci. Lett. 2009, 459, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Kotani, S.; Kawahara, S.; Kirino, Y. Purkinje cell activity during classical eyeblink conditioning in decerebrate guinea pigs. Brain Res. 2006, 1068, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Garcia, J.M.; Gruart, A. Firing activities of identified posterior interpositus nucleus neurons during associative learning in behaving cats. Brain Res. Rev. 2005, 49, 367–376. [Google Scholar] [CrossRef]
- Trigo, J.A.; Roa, L.; Gruart, A.; Delgado-Garcia, J.M. A kinetic study of blinking responses in cats. J. Physiol. 2003, 549, 195–205. [Google Scholar] [CrossRef]
- Gruart, A.; Blazquez, P.; Delgado-Garcia, J.M. Kinematics of spontaneous, reflex and conditioned eyelid movements in the alert cat. J. Neurophysiol. 1995, 74, 226–248. [Google Scholar] [CrossRef]
- Gerwig, M.; Dimitrova, A.; Kolb, F.P.; Maschke, M.; Brol, B.; Kunnel, A.; Boring, D.; Thilmann, A.F.; Forsting, M.; Diener, H.C.; et al. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain 2003, 126, 71–94. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Joekes, J. Transsynaptic Delineation of the Rabbit Eyeblink Premotor Pathway: Identifying Anterior Interpositus Neurons and Their Synaptic Changes as a Function of Learning. Unpublished Doctoral Dissertation. Ph.D. Thesis, West Virginia University, Morgantown, WV, USA, 2014. [Google Scholar]
- Freeman, J.H., Jr.; Nicholson, D.A. Developmental changes in eye-blink conditioning and neuronal activity in the cerebellar interpositus nucleus. J. Neurosci. 2000, 20, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.C.W.; Connor, S.; Hinchcliff, R.; LeBoutillier, J.C.; Thompson, R.F.; Petit, T.L. Eye-blink conditioning is associated with changes in synaptic ultrastructure in the rabbit interpositus nuclei. Learn. Mem. 2007, 14, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Freeman, J.H., Jr.; Bruneau, R.; Nolan, B.C.; Cooper, N.R.; Zook, A.; Walters, D. Synapse formation is associated with memory storage in the cerebellum. Proc. Natl. Acad. Sci. USA 2002, 99, 13228–13231. [Google Scholar] [CrossRef]
- Broersen, R.; Albergaria, C.; Carulli, D.; Carey, M.R.; Canto, C.B.; De Zeeuw, C.I. Synaptic mechanisms for associative learning in the cerebellar nuclei. Nat. Commun. 2023, 14, 7459. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Smith-Bell, C.A.; Burhans, L.B.; O’Dell, D.E.; Bell, R.W.; Schreurs, B.G. Changes in membrane properties of rat deep cerebellar nuclear projection neurons during acquisition of eyeblink conditioning. Proc. Natl. Acad. Sci. USA 2018, 115, E9419–E9428. [Google Scholar] [CrossRef] [PubMed]
- Debanne, D.; Inglebert, Y.; Russier, M. Plasticity of intrinsic neuronal excitability. Curr. Opin. Neurobiol. 2019, 54, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.T.; Chin, J.; Leiser, S.C.; Pangalos, M.N.; Randall, A.D. Altered intrinsic neuronal excitability and reduced Na+ currents in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2011, 32, 2109.e1–2109.e14. [Google Scholar] [CrossRef] [PubMed]
- Belmeguenai, A.; Hosy, E.; Bengtsson, F.; Pedroarena, C.M.; Piochon, C.; Teuling, E.; He, Q.; Ohtsuki, G.; De Jeu, M.T.; Elgersma, Y.; et al. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J. Neurosci. 2010, 30, 13630–13643. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, I.; Kaffashian, M.; Shabani, M.; Haghdoost-Yazdi, H.; Behzadi, G.; Janahmadi, M. In vivo 4-aminopyridine treatment alters the neurotoxin 3-acetylpyridine-induced plastic changes in intrinsic electrophysiological properties of rat cerebellar Purkinje neurones. Eur. J. Pharmacol. 2010, 642, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, S.-C.; Clemens, A.M.; Petralia, R.S.; Hoffman, D.A. Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 2007, 54, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Gittis, A.H.; du Lac, S. Intrinsic and synaptic plasticity in the vestibular system. Curr. Opin. Neurobiol. 2006, 16, 385–390. [Google Scholar] [CrossRef]
- Plant, L.D.; Webster, N.J.; Boyle, J.P.; Ramsden, M.; Frier, D.B.; Peers, C.; Pearson, H.A. Amyloid b peptide as a physiological modulator of neuronal “A”-type K+ current. Neurobiol. Aging 2006, 27, 1673–1683. [Google Scholar] [CrossRef]
- Straka, H.; Vibert, N.; Vidal, P.P.; Moore, L.E.; Dutia, M.B. Intrinsic membrane properties of vertebrate vestibular neurons: Function, development and plasticity. Prog. Neurobiol. 2005, 76, 349–392. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.R.; Catterall, W.A. Neuromodulation of Na+ channels: An unexpected form of cellular plasticity. Nat. Rev. Neurosci. 2004, 2, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Strauss, U.; Kole, M.H.P.; Bräuer, A.U.; Pahnke, J.; Bajorat, R.; Rolfs, A.; Nitsch, R.; Deisz, R.A. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur. J. Neurosci. 2004, 19, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Aizenman, C.D.; Huang, E.J.; Linden, D.J. Morphological correlates of intrinsic electrical excitability in neurons of the deep cerebellar nuclei. J. Neurophysiol. 2003, 89, 1738–1747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnston, D.; Christie, B.R.; Frick, A.; Gray, R.; Hoffman, D.A.; Schexnayder, L.K.; Watanabe, S.; Yuan, L.-L. Active dendrites, potassium channels and synaptic plasticity. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 667–674. [Google Scholar] [CrossRef]
- Nelson, A.B.; Krispel, C.M.; Sekirnjak, C.; du Lac, S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron 2003, 40, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Daoudal, G.; Debanne, D. Long-term plasticity of intrinsic excitability: Learning rules and mechanisms. Learn. Mem. 2003, 10, 456–465. [Google Scholar] [CrossRef]
- Schrader, L.A.; Anderson, A.E.; Varga, A.W.; Levy, M.; Sweatt, J.D. The other half of Hebb: K+ channels and the regulation of neuronal excitability in the hippocampus. Mol. Neurobiol. 2002, 25, 51–66. [Google Scholar]
- Bekkers, J.M.; Delaney, A.J. Modulation of excitability by a-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. J. Neurosci. 2001, 21, 6553–6560. [Google Scholar] [CrossRef]
- Armano, S.; Rossi, P.; D’Angelo, E. Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J. Neurosci. 2000, 20, 5208–5216. [Google Scholar] [CrossRef]
- Pongs, O. Voltage-gated potassium channels: From hyperexcitability to excitement. FEBS Lett. 1999, 452, 31–35. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Hille, B. Voltage-gated ion channels and electrical excitability. Neuron 1998, 20, 371–380. [Google Scholar] [CrossRef]
- Gruol, D.L.; Dionne, V.E.; Yool, A.J. Multiple voltage-sensitive K+ channels regulate dendritic excitability in cerebellar Purkinje neurons. Neurosci. Lett. 1989, 97, 97–102. [Google Scholar] [CrossRef]
- Adams, P.R.; Galvan, M. Voltage-dependent currents of vertebrate neurons and their role in membrane excitability. In Advances in Neurology, 44th ed.; Delgado-Escueta, A.V., Ed.; Raven Press: New York, NY, USA, 1986; pp. 137–170. [Google Scholar]
- dos-Santos, R.C.; Sweeten, B.L.; Stelly, C.E.; Tasker, J.G. The Neuroendocrine Impact of Acute Stress on Synaptic Plasticity. Endocrinology 2023, 164, bqad149. [Google Scholar] [CrossRef]
- Huang, H.; Shakkottai, V.G. Targeting ion channels and purkinje neuron intrinsic membrane excitability as a therapeutic strategy for cerebellar ataxia. Life 2023, 13, 1350. [Google Scholar] [CrossRef]
- Carzoli, K.L.; Kogias, G.; Fawcett-Patel, J.; Liu, S.J. Cerebellar interneurons control fear memory consolidation via learning-induced HCN plasticity. Cell Rep. 2023, 42, 113057. [Google Scholar] [CrossRef]
- Daou, A.; Margoliash, D. Intrinsic plasticity and birdsong learning. Neurobiol. Learn. Mem. 2021, 180, 107407. [Google Scholar] [CrossRef]
- Hansel, C.; Disterhoft, J.F. Why is synaptic plasticity not enough? Neurobiol. Learn. Mem. 2020, 176, 107336. [Google Scholar] [CrossRef] [PubMed]
- Titley, H.K.; Brunel, N.; Hansel, C. Toward a neurocentric view of learning. Neuron 2017, 95, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Llinas, R.R. Intrinsic electrical properties of mammalian neurons and CNS function: A historical perspective. Front. Cell. Neurosci. 2014, 8, 320. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, R.; Camp, A.J. Intrinsic neuronal excitability: Implications for health and disease. Biomol. Concepts 2011, 2, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Linden, D.J. The other side of the engram: Experience-driven changes in neuronal intrinsic excitability. Nat. Rev. Neurosci. 2003, 4, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.C.; Chung, G.; Kim, S.K.; Kim, S.J. Dynamic alteration of intrinsic properties of the cerebellar Purkinje cell during the motor memory consolidation. Mol. Brain 2023, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Viet, N.M.; Wang, T.; Tran-Anh, K.; Sugihara, I. Heterogeneity of intrinsic plasticity in cerebellar Purkinje cells linked with cortical molecular zones. iScience 2022, 25, 103705. [Google Scholar] [CrossRef]
- Johansson, F. Intrinsic memory of temporal intervals in cerebellar Purkinje cells. Neurobiol. Learn. Mem. 2019, 166, 107103. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.C.; Kim, S.J. Plasticity leading to cerebellum-dependent learning: Two different regions, two different types. Pflüg. Arch.-Eur. J. Physiol. 2019, 471, 927–934. [Google Scholar] [CrossRef]
- Shim, H.G.; Lee, Y.S.; Kim, S.J. The emerging concept of intrinsic plasticity: Activity-dependent modulation of intrinsic excitability in cerebellar purkinje cells and motor learning. Exp. Neurobiol. 2018, 27, 139–154. [Google Scholar] [CrossRef]
- Yang, Z.; Santamaria, F. Purkinje cell intrinsic excitability increases after synaptic long term depression. J. Neurophysiol. 2016, 116, 1208–1217. [Google Scholar] [CrossRef]
- Kim, S.J.; Linden, D.J. Ubiquitous plasticity and memory storage. Neuron 2007, 56, 582–592. [Google Scholar] [CrossRef]
- Zhu, L.; Scelfo, B.; Tempia, F.; Sacchetti, B.; Strata, P. Membrane excitability and fear conditioning in cerebellar Purkinje cell. Neuroscience 2006, 140, 801–810. [Google Scholar] [CrossRef]
- Grasselli, G.; Boele, H.J.; Titley, H.K.; Bradford, N.; van Beers, L.; Jay, L.; Beekhof, G.C.; Busch, S.E.; De Zeeuw, C.I.; Schonewille, M.; et al. SK2 channels in cerebellar Purkinje cells contribute to excitability modulation in motor-learning-specific memory traces. PLoS Biol. 2020, 18, e3000596. [Google Scholar] [CrossRef]
- Schreurs, B.G.; Gusev, P.A.; Tomsic, D.; Alkon, D.L.; Shi, T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J. Neurosci. 1998, 18, 5498–5507. [Google Scholar] [CrossRef]
- Schreurs, B.G.; Tomsic, D.; Gusev, P.A.; Alkon, D.L. Dendritic excitability microzones and occluded long-term depression after classical conditioning of the rabbit’s nictitating membrane response. J. Neurophysiol. 1997, 77, 86–92. [Google Scholar] [CrossRef]
- Schreurs, B.G.; Sanchez-Andres, J.V.; Alkon, D.L. Learning-specific differences in Purkinje-cell dendrites of lobule HVI (lobulus simplex): Intracellular recording in a rabbit cerebellar slice. Brain Res. 1991, 548, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Schreurs, B.G. Characteristics of IA currents in adult rabbit cerebellar Purkinje cells. Brain Res. 2006, 1096, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Disterhoft, J.F.; Coulter, D.A.; Alkon, D.L. Conditioning-specific membrane changes of rabbit hippocampal neurons measured in vitro. Proc. Natl. Acad. Sci. USA 1986, 83, 2733–2737. [Google Scholar] [CrossRef]
- Oh, M.M.; Disterhoft, J.F. Increased excitability of both principal neurons and interneurons during associative learning. Neuroscientist 2015, 21, 372–384. [Google Scholar] [CrossRef]
- Hirano, T. Regulation and interaction of multiple types of synaptic plasticity in a Purkinje neuron and their contribution to motor learning. Cerebellum 2018, 17, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.G.; Jang, D.C.; Lee, J.; Chung, G.; Lee, S.; Kim, Y.G.; Jeon, D.E.; Kim, S.J. Long-term depression of intrinsic excitability accompanied by synaptic depression in cerebellar Purkinje cells. J. Neurosci. 2017, 37, 5659–5669. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, E.; Tempia, F.; Lippiello, P.; Miniaci, M.C. Modulation, plasticity and pathophysiology of the parallel fiber-Purkinje cell synapse. Front. Synaptic Neurosci. 2016, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Jorntell, H. Cerebellar synaptic plasticity and the credit assignment problem. Cerebellum 2016, 15, 104–111. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Mapelli, L.; Casellato, C.; Garrido, J.A.; Luque, N.; Monaco, J.; Prestori, F.; Pedrocchi, A.; Ros, E. Distributed circuit plasticity: New clues for the cerebellar mechanisms of learning. Cerebellum 2015, 15, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Hesslow, G.; Jirenhed, D.-A.; Rasmusson, A.; Johansson, F. Classical conditioning of motor responses: What is the learning mechanism? Neural Netw. 2013, 47, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yamaguchi, K.; Nagao, S.; Yamzaki, T. Long-term depression as a model of cerebellar plasticity. Prog. Brain Res. 2014, 210, 1–30. [Google Scholar]
- Ohtsuki, G.; Shishikura, M.; Ozaki, A. Synergistic excitability plasticity in cerebellar functioning. FEBS J. 2020, 287, 4557–4593. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.R.; Raman, I.M. Nothing can be coincidence; synaptic inhibition and plasticity in the cerebellar nuclei. Trends Neurosci. 2009, 32, 170–177. [Google Scholar] [CrossRef]
- Pedroarena, C.M.; Schwarz, C. Efficacy and short-term plasticity at GABAergic synapses between Purkinje and cerebellar nuclei neurons. J. Neurophysiol. 2003, 89, 704–715. [Google Scholar] [CrossRef]
- Aizenman, C.D.; Linden, D.J. Rapid, synaptically driven increases in the excitability of cerebellar deep nuclear neurons. Nat. Neurosci. 2000, 3, 109–111. [Google Scholar] [CrossRef]
- Morishita, W.; Sastry, B.R. Postsynaptic mechanisms underlying long-term depression of GABAergic transmission in neurons of the deep cerebellar nuclei. J. Neurophysiol. 1996, 76, 59–68. [Google Scholar] [CrossRef]
- Najac, M.; Raman, I.M. Integration of Purkinje cell inhibition by cerebellar nucleo-olivary neurons. J. Neurosci. 2015, 35, 544–549. [Google Scholar] [CrossRef]
- Zheng, N.; Raman, I.M. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum 2010, 9, 56–66. [Google Scholar] [CrossRef]
- Pugh, J.R.; Raman, I.M. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 2006, 51, 113–123. [Google Scholar] [CrossRef]
- Telgkamp, P.; Raman, I.M. Depression of inhibitory synaptic transmission between Purkinje cells and neurons of the cerebellar nuclei. J. Neurosci. 2002, 22, 8447–8457. [Google Scholar] [CrossRef] [PubMed]
- Raman, I.M.; Gustafson, A.E.; Padgett, D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J. Neurosci. 2000, 20, 9004–9016. [Google Scholar] [CrossRef]
- Marr, D. A theory of cerebellar cortex. J. Physiol. 1969, 202, 437–470. [Google Scholar] [CrossRef] [PubMed]
- Albus, J.S. A theory of cerebellar function. Math. Biosci. 1971, 10, 25–61. [Google Scholar] [CrossRef]
- Ito, M.; Sakurai, M.; Tongroach, P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J. Physiol. 1982, 324, 113–134. [Google Scholar] [CrossRef]
- Ito, M.; Kano, M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci. Lett. 1982, 33, 253–258. [Google Scholar] [CrossRef]
- Gilbert, P.F.C.; Thach, W.T. Purkinje cell activity during motor learning. Brain Res. 1977, 128, 309–328. [Google Scholar] [CrossRef]
- Pugh, J.R.; Raman, I.M. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J. Neurosci. 2008, 28, 10549–10560. [Google Scholar] [CrossRef]
- Aizenman, C.D.; Huang, E.J.; Manis, P.B.; Linden, D.J. Use-Dependent Changes in Synaptic Strength at the Purkinje Cell to Deep Nuclear Synapse; Gerrits, N.M., Ruigrok, T.J.H., De Zeeuw, C.I., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2000; Volume 124, pp. 257–273. [Google Scholar]
- Aizenman, C.D.; Manis, P.B.; Linden, D.J. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron 1998, 21, 827–835. [Google Scholar] [CrossRef]
- Ouardouz, M.; Sastry, B.R. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J. Neurophysiol. 2000, 84, 1414–1421. [Google Scholar] [CrossRef]
- Ito, M. Cerebellar learning in the vestibulo-ocular reflex. Trends Cogn. Sci. 1998, 2, 313–321. [Google Scholar] [CrossRef]
- Takehara-Nishiuchi, K. The anatomy and physiology of eyeblink classical conditioning. Curr. Top. Behav. Neurosci. 2018, 37, 297–323. [Google Scholar] [CrossRef]
- Johansson, F.; Jirenhed, D.A.; Rasmussen, A.; Zucca, R.; Hesslow, G. Absence of parallel fibre to purkinje cell LTD during eyeblink conditioning. Sci. Rep. 2018, 8, 14777. [Google Scholar] [CrossRef]
- Thompson, R.F.; Steinmetz, J.E. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience 2009, 162, 732–755. [Google Scholar] [CrossRef] [PubMed]
- Koekkoek, S.K.E.; Hulscher, H.C.; Dortland, B.R.; Hensbroek, R.A.; Elgersma, Y.; Ruigrok, T.J.H.; De Zeeuw, C.I. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science 2003, 301, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiol. Rev. 2001, 81, 1143–1195. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.D.; Garcia, K.S.; Medina, J.F.; Steele, P.M. Does cerebellar LTD mediate motor learning? Toward a resolution without a smoking gun. Neuron 1998, 20, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cajal, S.R. The Croonian lecture—La fine structure des centres nerveux. Proc. R. Soc. Lond. 1894, 55, 444–468. [Google Scholar]
- Yuste, R.; Tank, D.W. Dendritic integrations in mammalian neurons, a century after Cajal. Neuron 1996, 16, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, C. Viewing the cerebellum through the eyes of Ramon Y Cajal. Cerebellum 2008, 7, 517–522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heysieattalab, S.; Lee, K.H.; Liu, Y.; Wang, Y.; Foy, M.R.; Bi, X.; Baudry, M. Impaired cerebellar plasticity and eye-blink conditioning in calpain-1 knock-out mice. Neurobiol. Learn. Mem. 2020, 170, 106995. [Google Scholar] [CrossRef] [PubMed]
- Connor, S.; LeBoutillier, J.C.; Petit, T.L.; Bloomfield, J.; Thompson, R.F.; Weeks, A.C.W. Eyeblink conditioning leads to fewer synapses in the rabbit cerebellar cortex. Behav. Neurosci. 2009, 123, 856–862. [Google Scholar] [CrossRef]
- Miyata, M.; Kim, H.-T.; Hashimoto, K.; Lee, T.-K.; Cho, S.-Y.; Jiang, H.; Wu, Y.; Jun, K.; Wu, D.; Kano, M.; et al. Deficient long-term synaptic depression in the rostral cerebellum correlated with impaired motor learning in phospholipase C b4 mutant mice. Eur. J. Neurosci. 2001, 13, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Schonewille, M.; Belmeguenai, A.; Koekkoek, S.K.; Houtman, S.H.; Boele, H.J.; van Beugen, B.J.; Gao, Z.; Badura, A.; Ohtsuki, G.; Amerika, W.E.; et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron 2010, 67, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.P.; Yamaguchi, H.; Zeng, X.-H.; Kojo, M.; Nakada, Y.; Takagi, A.; Sugimori, M.; Llinas, R.R. Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc. Natl. Acad. Sci. USA 2006, 102, 17166–17171. [Google Scholar] [CrossRef]
- Carey, M.R.; Lisberger, S.G. Embarrassed, but not depressed: Eye opening lessons for cerebellar learning. Neuron 2002, 35, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Schonewille, M.; Gao, Z.; Boele, H.J.; Vinueza Veloz, M.F.; Amerika, W.E.; Simek, A.A.; De Jeu, M.T.; Steinberg, J.P.; Takamiya, K.; Hoebeek, F.E.; et al. Reevaluating the Role of LTD in Cerebellar Motor Learning. Neuron 2011, 70, 43–50. [Google Scholar] [CrossRef]
- Tseng, W.; Guan, R.; Disterhoft, J.F.; Weiss, C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus 2004, 14, 58–65. [Google Scholar] [CrossRef]
- McEchron, M.D.; Tseng, W.; Disterhoft, J.F. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J. Neurosci. 2003, 23, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Zola, S. Trace eyeblink classical conditioning in the monkey: A nonsurgical method and behavioral analysis. Behav. Neurosci. 1998, 112, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- McEchron, M.D.; Bouwmeester, H.; Tseng, W.; Weiss, C.; Disterhoft, J.F. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus 1998, 8, 638–646. [Google Scholar] [CrossRef]
- McGlinchey-Berroth, R.; Carillo, M.C.; Gabrieli, J.D.E.; Brawn, C.M.; Disterhoft, J.F. Impaired trace eyeblink conditioning in bilateral, medial-temporal lobe amnesia. Behav. Neurosci. 1997, 111, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Moye, T.B.; Rudy, J.W. Ontogenesis of trace conditioning in young rats: Dissociation of associative and memory processes. Dev. Psychobiol. 1987, 20, 405–414. [Google Scholar] [CrossRef]
- Solomon, P.R.; Vander Schaaf, E.R.; Thompson, R.F.; Weisz, D.J. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 1986, 100, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, N. Interstimulus interval function of the nictitating membrane response of the rabbit under delay versus trace conditioning. J. Comp. Physiol. Psychol. 1966, 62, 397–402. [Google Scholar] [CrossRef]
- Thompson, R.F. The neurobiology of learning and memory. Science 1986, 233, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M.; Liu, G. Electrophysiological mapping of the auditory areas in the cerebellum of the cat. Brain Res. 1985, 335, 121–129. [Google Scholar] [CrossRef]
- Gould, T.J.; Sears, L.L.; Steinmetz, J.E. Possible CS and US pathways for rabbit classical eyelid conditioning: Electrophysiological evidence for projections from the pontine nuclei and inferior olive to cerebellar cortex and nuclei. Behav. Neural Biol. 1993, 60, 172–185. [Google Scholar] [CrossRef]
- Siegel, J.J.; Mauk, M.D. Persistent activity in prefrontal cortex during trace eyelid conditioning: Dissociating responses that reflect cerebellar output from those that do not. J. Neurosci. 2013, 33, 15272–15284. [Google Scholar] [CrossRef]
- Hattori, S.; Yoon, T.; Disterhoft, J.F.; Weiss, C. Functional reorganization of a prefrontal cortical network mediating consolidation of trace eyeblink conditioning. J. Neurosci. 2014, 34, 1432–1445. [Google Scholar] [CrossRef]
- Yu, X.T.; Yu, J.; Choi, A.; Takehara-Nishiuchi, K. Lateral entorhinal cortex supports the development of prefrontal network activity that bridges temporally discontiguous stimuli. Hippocampus 2021, 31, 1285–1299. [Google Scholar] [CrossRef]
- Kehoe, E.J.; Ludvig, E.A.; Sutton, R.S. Timing and cue competition in conditioning of the nictitating membrane response of the rabbit (Oryctolagus cuniculus). Learn. Mem. 2013, 20, 97–102. [Google Scholar] [CrossRef][Green Version]
- Gormezano, I.; Kehoe, E.J. Associative transfer in classical conditioning to serial compounds. In Quantitative Analyses of Behavior; Commons, M.L., Herrnstein, R.J., Wagner, A.R., Eds.; Ballinger: Cambridge, MA, USA, 1984; pp. 297–322. [Google Scholar]
- Kehoe, E.J.; Feyer, A.M.; Moses, J.L. Second-order conditioning of the rabbit’s nictitating membrane response as a function of the CS2-CS1 and CS1-US intervals. Anim. Learn. Behav. 1981, 9, 304–314. [Google Scholar] [CrossRef]
- Kehoe, E.J.; Gibbs, C.M.; Garcia, E.; Gormezano, I. Associative transfer and stimulus selection in classical conditioning of the rabbit’s nictitating membrane response to serial compound CSs. J. Exp. Psychol. Anim. Behav. Process. 1979, 5, 1–18. [Google Scholar] [CrossRef]
- Kehoe, E.J.; Graham-Clarke, P.; Schreurs, B.G. Temporal patterns of the rabbit’s nictitating membrane response to compound and component stimuli under mixed CS-US intervals. Behav. Neurosci. 1989, 103, 283–295. [Google Scholar] [CrossRef]
- Schreurs, B.G.; Alkon, D.L. US-US conditioning of the rabbit’s nictitating membrane response: Emergence of a conditioned response without alpha conditioning. Psychobiology 1990, 18, 312–320. [Google Scholar] [CrossRef]
- Schreurs, B.G.; Burhans, L.B.; Smith-Bell, C.A.; Mrowka, S.W.; Wang, D. Ontogeny of trace eyeblink conditioning to shock-shock pairings in the rat pup. Behav. Neurosci. 2013, 127, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Schmolesky, M.T.; De Zeeuw, C.I.; Hansel, C. Climbing fiber synaptic plasticity and modifications in Purkinje cell excitability. Prog. Brain Res. 2005, 148, 81–94. [Google Scholar] [PubMed]
- Hansel, C.; Linden, D.J. Long-term depression of the cerebellar climbing fiber-Purkinje neuron synapse. Neuron 2000, 26, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Mozzachiodi, R.; Byrne, J.H. More than synaptic plasticity: Role of nonsynaptic plasticity in learning and memory. Trends Neurosci. 2010, 33, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hirono, M.; Karube, F.; Yanagawa, Y. Modulatory effects of monoamines and perineuronal nets on output of cerebellar Purkinje cells. Front. Neural Circuits 2021, 15, 661899. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, D.E.; Schreurs, B.G.; Smith-Bell, C.; Wang, D. Disruption of rat deep cerebellar perineuronal net alters eyeblink conditioning and neuronal electrophysiology. Neurobiol. Learn. Mem. 2021, 177, 107358. [Google Scholar] [CrossRef]
- Carulli, D.; Broersen, R.; de Winter, F.; Muir, E.M.; Meskovic, M.; de Waal, M.; de Vries, S.; Boele, H.J.; Canto, C.B.; De Zeeuw, C.I.; et al. Cerebellar plasticity and associative memories are controlled by perineuronal nets. Proc. Natl. Acad. Sci. USA 2020, 117, 6855–6865. [Google Scholar] [CrossRef]
- Hirono, M.; Watanabe, S.; Karube, F.; Fujiyama, F.; Kawahara, S.; Nagao, S.; Yanagawa, Y.; Misonou, H. Perineuronal nets in the deep cerebellar nuclei regulate GABAergic transmission and delay eyeblink conditioning. J. Neurosci. 2018, 38, 6130–6144. [Google Scholar] [CrossRef]
- Celio, M.R.; Blümcke, I. Perineuronal nets--a specialized form of extracellular matrix in the adult nervous system. Brain Res. Rev. 1994, 19, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Celio, M.R.; Spreafico, R.; De Biasi, S.; Vitellaro-Zuccarello, L. Perineuronal nets: Past and present. Trends Neurosci. 1998, 21, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, C. The History of the Synapse. Anat. Rec. 2020, 303, 1252–1279. [Google Scholar] [CrossRef] [PubMed]
- Fellin, T. Communication between neurons and astrocytes: Relevance to the modulation of synaptic and network activity. J. Neurochem. 2009, 108, 533–544. [Google Scholar] [CrossRef]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. Tripartite synapses: Roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 2009, 57, 343–346. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Sorg, B.A.; Berretta, S.; Blacktop, J.M.; Fawcett, J.W.; Kitagawa, H.; Kwok, J.C.F.; Miquel, M. Casting a wide net: Role of perineuronal nets in neural plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef]
- Tewari, B.P.; Chaunsali, L.; Prim, C.E.; Sontheimer, H. A glial perspective on the extracellular matrix and perineuronal net remodeling in the central nervous system. Front. Cell. Neurosci. 2022, 16, 1022754. [Google Scholar] [CrossRef]
- Crapser, J.D.; Arreola, M.A.; Tsourmas, K.I.; Green, K.N. Microglia as hackers of the matrix: Sculpting synapses and the extracellular space. Cell. Mol. Immunol. 2021, 18, 2472–2488. [Google Scholar] [CrossRef]
- Ferrer-Ferrer, M.; Dityatev, A. Shaping synapses by the neural extracellular matrix. Front. Neuroanat. 2018, 12, 40. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Kwok, J.C.F. Proteoglycan sulphation in the function of the mature central nervous system. Front. Integr. Neurosci. 2022, 16, 895493. [Google Scholar] [CrossRef] [PubMed]
- Carceller, H.; Gramuntell, Y.; Klimczak, P.; Nacher, J. Perineuronal nets: Subtle structures with large implications. Neuroscientist 2022, 29, 569–590. [Google Scholar] [CrossRef] [PubMed]
- Carulli, D.; Verhaagen, J. An extracellular perspective on CNS maturation: Perineuronal nets and the control of plasticity. Int. J. Mol. Sci. 2021, 22, 2434. [Google Scholar] [CrossRef] [PubMed]
- Testa, D.; Prochiantz, A.; Di Nardo, A.A. Perineuronal nets in brain physiology and disease. Semin. Cell Dev. Biol. 2019, 89, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Dityatev, A. Crosstalk between glia, extracellular matrix and neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef]
- Van’t Spijker, H.M.; Kwok, J.C.F. A sweet talk: The molecular systems of perineuronal nets in controlling neuronal communication. Front. Integr. Neurosci. 2017, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.D.; Omar, M.H.; Koleske, A.J. Extracellular matrix control of dendritic spine and synapse structure and plasticity in adulthood. Front. Neuroanat. 2014, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Thach, W.T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J. Neurophysiol. 1968, 31, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Wu, S.; Chen, C.H.; Regehr, W.G. Unusually slow spike frequency adaptation in deep cerebellar nuclei neurons preserves linear transformations on the subsecond timescale. J. Neurosci. 2022, 42, 7581–7593. [Google Scholar] [CrossRef]
- Murakami, M.; Kosaka, M.; Sato, H.; Ohtsuka, A.; Taguchi, T. The intensely positively charged perineuronal net in the adult rat brain, with special reference to its reactions to oxine, chondroitinase ABC, hyaluronidase and collagenase. Arch. Histol. Cytol. 2001, 64, 313–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mueller, A.L.; Davis, A.; Sovich, S.; Carlson, S.S.; Robinson, F.R. Distribution of N-acetylgalactosamine-positive perineuronal nets in the macaque brain: Anatomy and implications. Neural Plast. 2016, 2016, 6021428. [Google Scholar] [CrossRef] [PubMed]
- Starkey, J.; Horstick, E.J.; Ackerman, S.D. Glial regulation of critical period plasticity. Front. Cell. Neurosci. 2023, 17, 1247335. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Oohashi, T.; Pizzorusso, T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci. 2019, 20, 451–465. [Google Scholar] [CrossRef]
- Foscarin, S.; Raha-Chowdhury, R.; Fawcett, J.W.; Kwok, J.C.F. Brain ageing changes proteoglycan sulfation, rendering perineuronal nets more inhibitory. Aging 2017, 9, 1607–1622. [Google Scholar] [CrossRef]
- Ueno, H.; Suemitsu, S.; Okamoto, M.; Matsumoto, Y.; Ishihara, T. Sensory experience-dependent formation of perineuronal nets and expression of Cat-315 immunoreactive components in the mouse somatosensory cortex. Neuroscience 2017, 355, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Nabel, E.; Morishita, H. Regulating critical period plasticity: Insight from the visual system to fear circuitry for therapeutic interventions. Front. Psychiatry 2013, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Carulli, D.; Rhodes, K.E.; Fawcett, J.W. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J. Comp. Neurol. 2007, 501, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Köppe, G.; Brückner, G.; Brauer, K.; Härtig, W.; Bigl, V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res. 1997, 288, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Miao, Q.L. Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 2013, 32, 352–363. [Google Scholar] [CrossRef]
- Wang, D.; Schreurs, B.G. Maturation of membrane properties of neurons in the rat deep cerebellar nuclei. Dev. Neurobiol. 2014, 74, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, G.; Piochon, C.; Hansel, C. Climbing fiber signaling and cerebellar gain control. Front. Cell. Neurosci. 2009, 3, 548. [Google Scholar] [CrossRef] [PubMed]
- Goldsberry, M.E.; Freeman, J.H. Sensory system development influences the ontogeny of trace eyeblink conditioning. Dev. Psychobiol. 2017, 59, 70–76. [Google Scholar] [CrossRef]
- Claflin, D.I.; Garrett, T.; Buffington, M.L. A developmental comparison of trace and delay eyeblink conditioning in rats using matching interstimulus intervals. Dev. Psychobiol. 2005, 47, 77–88. [Google Scholar] [CrossRef]
- Freeman, J.H., Jr.; Nicholson, D.A.; Muckler, A.S.; Rabinak, C.A.; DiPietro, N.T. Ontogeny of eyeblink conditioned response timing in rats. Behav. Neurosci. 2003, 117, 283–291. [Google Scholar] [CrossRef]
- Ivkovich, D.; Paczkowski, C.; Stanton, M.E. Ontogeny of delay versus trace eyeblink conditioning in the rat. Dev. Psychobiol. 2000, 36, 148–160. [Google Scholar] [CrossRef]
- Stanton, M.E.; Fox, G.D.; Carter, C.S. Ontogeny of the conditioned eyeblink response in rats: Acquisition or expression. Neuropharmacology 1998, 37, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.H., Jr.; Rabinak, C.A.; Campolattaro, M.M. Pontine stimulation overcomes developmental limitations in the neural mechanisms of eyeblink conditioning. Learn. Mem. 2005, 12, 255–259. [Google Scholar] [CrossRef][Green Version]
- Wingert, J.C.; Sorg, B.A. Impact of perineuronal nets on electrophysiology of parvalbumin interneurons, principal neurons, and brain oscillations: A review. Front. Synaptic Neurosci. 2021, 13, 673210. [Google Scholar] [CrossRef]
- Hayani, H.; Song, I.; Dityatev, A. Increased excitability and reduced excitatory synaptic input into fast-spiking Ca2 interneurons after enzymatic attenuation of extracellular matrix. Front. Cell. Neurosci. 2018, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Carulli, D.; Rhodes, K.E.; Brown, D.J.; Bonnert, T.P.; Pollack, S.J.; Oliver, K.; Strata, P.; Fawcett, J.W. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J. Comp. Neurol. 2006, 494, 559–577. [Google Scholar] [CrossRef]
- Lafarga, M.; Berciano, M.T.; Blanco, M. The perineuronal net in the fastigial nucleus of the rat cerebellum. A Golgi and quantitative study. Anat. Embryol. 1984, 170, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, I.R. Axonal endings in the cat medial superior olive: Coated vesicles and intercellular substance. Brain Res. 1972, 46, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y. Very long-term memories may be stored in the pattern of holes in the perineuronal net. Proc. Natl. Acad. Sci. USA 2013, 110, 12456–12461. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, S.; Badura, A.; Lutzu, S.; Pathak, S.S.; Thieme, A.; Verpeut, J.L.; Wagner, M.J.; Yang, Y.M.; Fioravante, D. Cognitive-affective functions of the cerebellum. J. Neurosci. 2023, 43, 7554–7564. [Google Scholar] [CrossRef] [PubMed]
- Frischknecht, R.; Heine, M.; Perrais, D.; Seidenbecher, C.I.; Choquet, D.; Gundelfinger, E.D. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat. Neurosci. 2009, 12, 897–904. [Google Scholar] [CrossRef]
- Favuzzi, E.; Marques-Smith, A.; Deogracias, R.; Winterflood, C.M.; Sánchez-Aguilera, A.; Mantoan, L.; Maeso, P.; Fernandes, C.; Ewers, H.; Rico, B. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron 2017, 95, 639–655.e610. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Tucic, M.; Blazikova, M.; Korenic, A.; Missirlis, Y.; Stamenkovic, V.; Andjus, P. Structural and functional modulation of perineuronal nets: In search of important players with highlight on tenascins. Cells 2021, 10, 1345. [Google Scholar] [CrossRef]
- Burket, J.A.; Webb, J.D.; Deutsch, S.I. Perineuronal nets and metal cation concentrations in the microenvironments of fast-spiking, parvalbumin-expressing gabaergic interneurons: Relevance to neurodevelopment and neurodevelopmental disorders. Biomolecules 2021, 11, 1235. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Andrini, O.; Clerici, M.; Negrini, D.; Passi, A.; Moriondo, A. Chondroitin sulfates act as extracellular gating modifiers on voltage-dependent ion channels. Cell. Physiol. Biochem. 2008, 22, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.B.; Islam, A.; Constanti, A. The fate of interneurons, GABA(A) receptor sub-types and perineuronal nets in Alzheimer’s disease. Brain Pathol. 2022, 33, e13129. [Google Scholar] [CrossRef]
- Härtig, W.; Derouiche, A.; Welt, K.; Brauer, K.; Grosche, J.; Mäder, M.; Reichenbach, A.; Brückner, G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999, 842, 15–29. [Google Scholar] [CrossRef]
- Härtig, W.; Singer, A.; Grosche, J.; Brauer, K.; Ottersen, O.P.; Brückner, G. Perineuronal nets in the rat medial nucleus of the trapezoid body surround neurons immunoreactive for various amino acids, calcium-binding proteins and the potassium channel subunit Kv3.1b. Brain Res. 2001, 899, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Morawski, M.; Reinert, T.; Meyer-Klaucke, W.; Wagner, F.E.; Troger, W.; Reinert, A.; Jager, C.; Bruckner, G.; Arendt, T. Ion exchanger in the brain: Quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci. Rep. 2015, 5, 16471. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Fyhn, M.; Jendelova, P.; Kwok, J.C.F.; Ruzicka, J.; Sorg, B.A. The extracellular matrix and perineuronal nets in memory. Mol. Psychiatry 2022, 27, 3192–3203. [Google Scholar] [CrossRef]
- Ross, M.T.; Flores, D.; Bertram, R.; Johnson, F.; Wu, W.; Hyson, R.L. Experience-dependent intrinsic plasticity during auditory learning. J. Neurosci. 2019, 39, 1206–1221. [Google Scholar] [CrossRef]
- Song, C.; Ehlers, V.L.; Moyer, J.R., Jr. Trace fear conditioning differentially modulates intrinsic excitability of medial prefrontal cortex-basolateral complex of amygdala projection neurons in infralimbic and prelimbic cortices. J. Neurosci. 2015, 35, 13511–13524. [Google Scholar] [CrossRef]
- Sehgal, M.; Song, C.; Ehlers, V.L.; Moyer, J.R. Learning to learn—Intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol. Learn. Mem. 2013, 105, 186–189. [Google Scholar] [CrossRef]
- Bekisz, M.; Garkun, Y.; Wabno, J.; Hess, G.; Wrobel, A.; Kossut, M. Increased excitability of cortical neurons induced by associative learning: An ex vivo study. Eur. J. Neurosci. 2010, 32, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, F.D.; Mozzachiodi, R.; Baxter, D.A.; Byrne, J.H. Classical and operant conditioning differentially modify the intrinsic properties of an identified neuron. Nat. Neurosci. 2006, 9, 17–19. [Google Scholar] [CrossRef]
- Alkon, D.L. Calcium-mediated reduction of ionic currents: A biophysical memory trace. Science 1984, 226, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J.; Dalecka, M.; Safrankova, K.; Peretti, D.; Jendelova, P.; Kwok, J.C.F.; Fawcett, J.W. Perineuronal nets affect memory and learning after synapse withdrawal. Transl. Psychiatry 2022, 12, 480. [Google Scholar] [CrossRef]
- Lasek, A.W.; Chen, H.; Chen, W.Y. Releasing addiction memories trapped in perineuronal nets. Trends Genet. 2018, 34, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Paylor, J.W.; Wendlandt, E.; Freeman, T.S.; Greba, Q.; Marks, W.N.; Howland, J.G.; Winship, I.R. Impaired cognitive function after perineuronal net degradation in the medial prefrontal cortex. eNeuro 2018, 5, ENEURO.0253-0218.2018. [Google Scholar] [CrossRef]
- Xue, Y.X.; Xue, L.F.; Liu, J.F.; He, J.; Deng, J.H.; Sun, S.C.; Han, H.B.; Luo, Y.X.; Xu, L.Z.; Wu, P.; et al. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J. Neurosci. 2014, 34, 6647–6658. [Google Scholar] [CrossRef]
- Knapska, E.; Lioudyno, V.; Kiryk, A.; Mikosz, M.; Górkiewicz, T.; Michaluk, P.; Gawlak, M.; Chaturvedi, M.; Mochol, G.; Balcerzyk, M.; et al. Reward learning requires activity of matrix metalloproteinase-9 in the central amygdala. J. Neurosci. 2013, 33, 14591–14600. [Google Scholar] [CrossRef]
- Wright, J.W.; Brown, T.E.; Harding, J.W. Inhibition of hippocampal matrix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plast. 2007, 2007, 073813. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, B.; Kraszewski, P.; Lee, S.; Cope, E.C. From molecules to behavior: Implications for perineuronal net remodeling in learning and memory. J. Neurochem. 2023, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Terstege, D.J.; Scott, G.A.; Tsutsui, M.; Epp, J.R. Neurogenesis mediated plasticity is associated with reduced neuronal activity in CA1 during context fear memory retrieval. Sci. Rep. 2022, 12, 7016. [Google Scholar] [CrossRef] [PubMed]
- Lesnikova, A.; Casarotto, P.; Moliner, R.; Fred, S.M.; Biojone, C.; Castren, E. Perineuronal net receptor PTPsigma regulates retention of memories. Front. Synaptic Neurosci. 2021, 13, 672475. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.H.; Lensjø, K.K.; Wigestrand, M.B.; Malthe-Sørenssen, A.; Hafting, T.; Fyhn, M. Removal of perineuronal nets disrupts recall of a remote fear memory. Proc. Natl. Acad. Sci. USA 2018, 115, 607–612. [Google Scholar] [CrossRef]
- Banerjee, S.B.; Gutzeit, V.A.; Baman, J.; Aoued, H.S.; Doshi, N.K.; Liu, R.C.; Ressler, K.J. Perineuronal nets in the adult sensory cortex are necessary for fear learning. Neuron 2017, 95, 169–179. [Google Scholar] [CrossRef]
- Morikawa, S.; Ikegaya, Y.; Narita, M.; Tamura, H. Activation of perineuronal net-expressing excitatory neurons during associative memory encoding and retrieval. Sci. Rep. 2017, 7, 46024. [Google Scholar] [CrossRef]
- Hylin, M.J.; Orsi, S.A.; Moore, A.N.; Dash, P.K. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn. Mem. 2013, 20, 267–273. [Google Scholar] [CrossRef]
- Karpova, N.N.; Pickenhagen, A.; Lindholm, J.; Tiraboschi, E.; Kulesskaya, N.; Ágústsdóttir, A.; Antila, H.; Popova, D.; Akamine, Y.; Sullivan, R.; et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 2011, 334, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Gogolla, N.; Caroni, P.; Luthi, A.; Herry, C. Perineuronal nets protect fear memories from erasure. Science 2009, 325, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreurs, B.G.; O’Dell, D.E.; Wang, D. The Role of Cerebellar Intrinsic Neuronal Excitability, Synaptic Plasticity, and Perineuronal Nets in Eyeblink Conditioning. Biology 2024, 13, 200. https://doi.org/10.3390/biology13030200
Schreurs BG, O’Dell DE, Wang D. The Role of Cerebellar Intrinsic Neuronal Excitability, Synaptic Plasticity, and Perineuronal Nets in Eyeblink Conditioning. Biology. 2024; 13(3):200. https://doi.org/10.3390/biology13030200
Chicago/Turabian StyleSchreurs, Bernard G., Deidre E. O’Dell, and Desheng Wang. 2024. "The Role of Cerebellar Intrinsic Neuronal Excitability, Synaptic Plasticity, and Perineuronal Nets in Eyeblink Conditioning" Biology 13, no. 3: 200. https://doi.org/10.3390/biology13030200
APA StyleSchreurs, B. G., O’Dell, D. E., & Wang, D. (2024). The Role of Cerebellar Intrinsic Neuronal Excitability, Synaptic Plasticity, and Perineuronal Nets in Eyeblink Conditioning. Biology, 13(3), 200. https://doi.org/10.3390/biology13030200




