Morphological and Functional Principles Governing the Plasticity Reserve in the Cerebellum: The Cortico-Deep Cerebellar Nuclei Loop Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Recovery Process and Structural Plasticity in Cerebellar Pathologies
2.1. Reversibility after Acute and Transient Pathology
2.2. Plasticity during Chronic and Progressive Pathologies
2.3. Summary of Clinical Studies
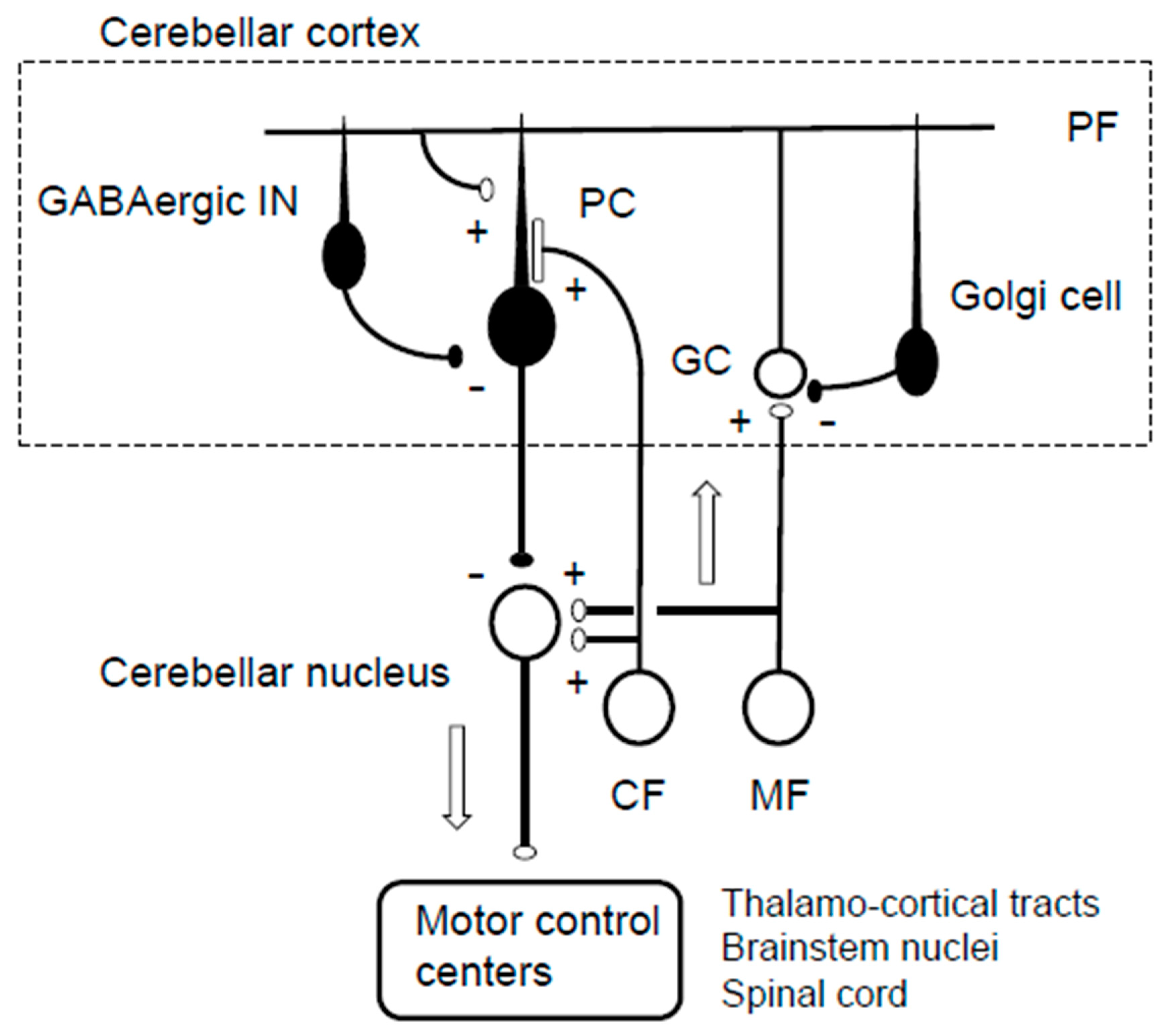
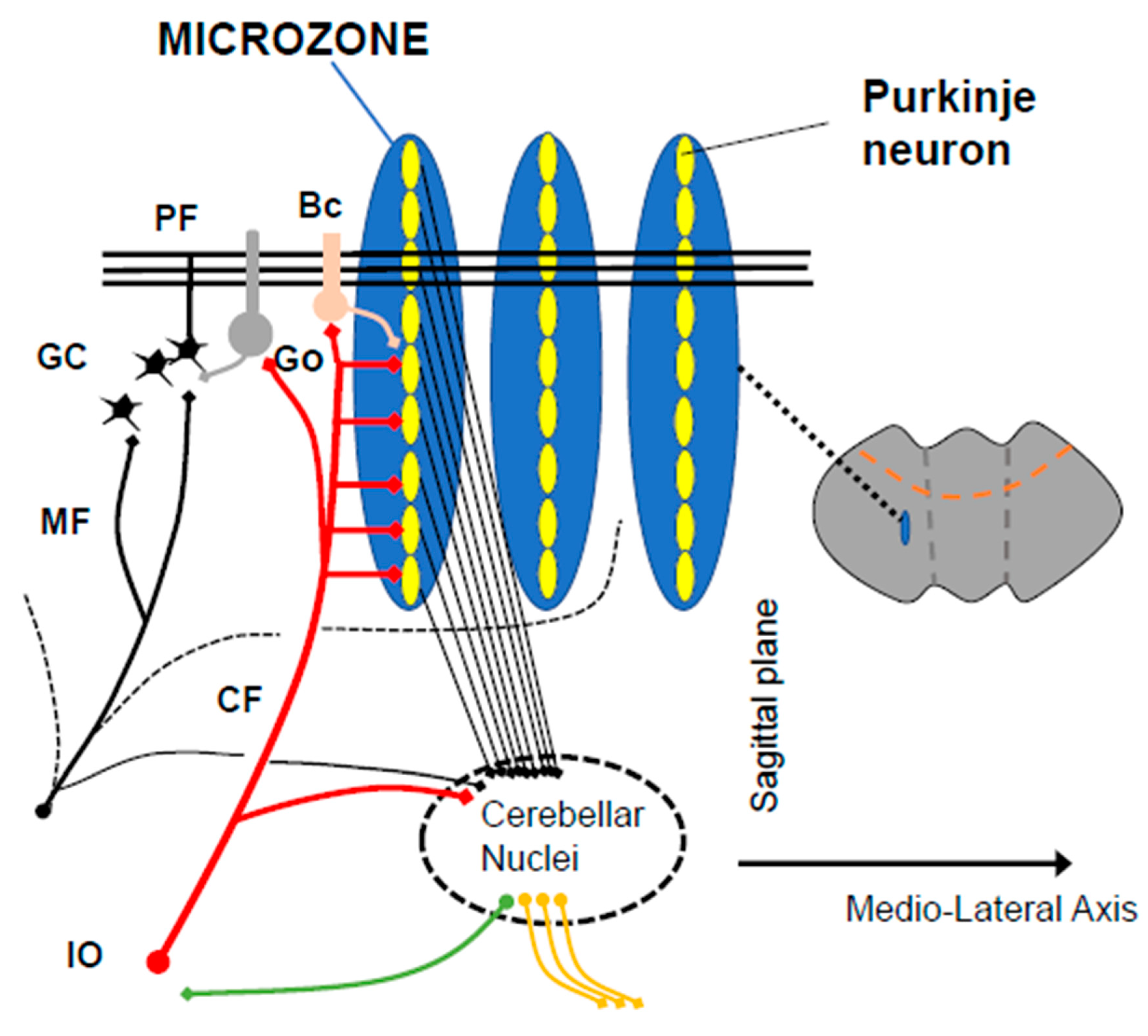
3. Predictive Controller Generating Cerebellar Reserve

3.1. Internal Forward Model and Kalman Filter
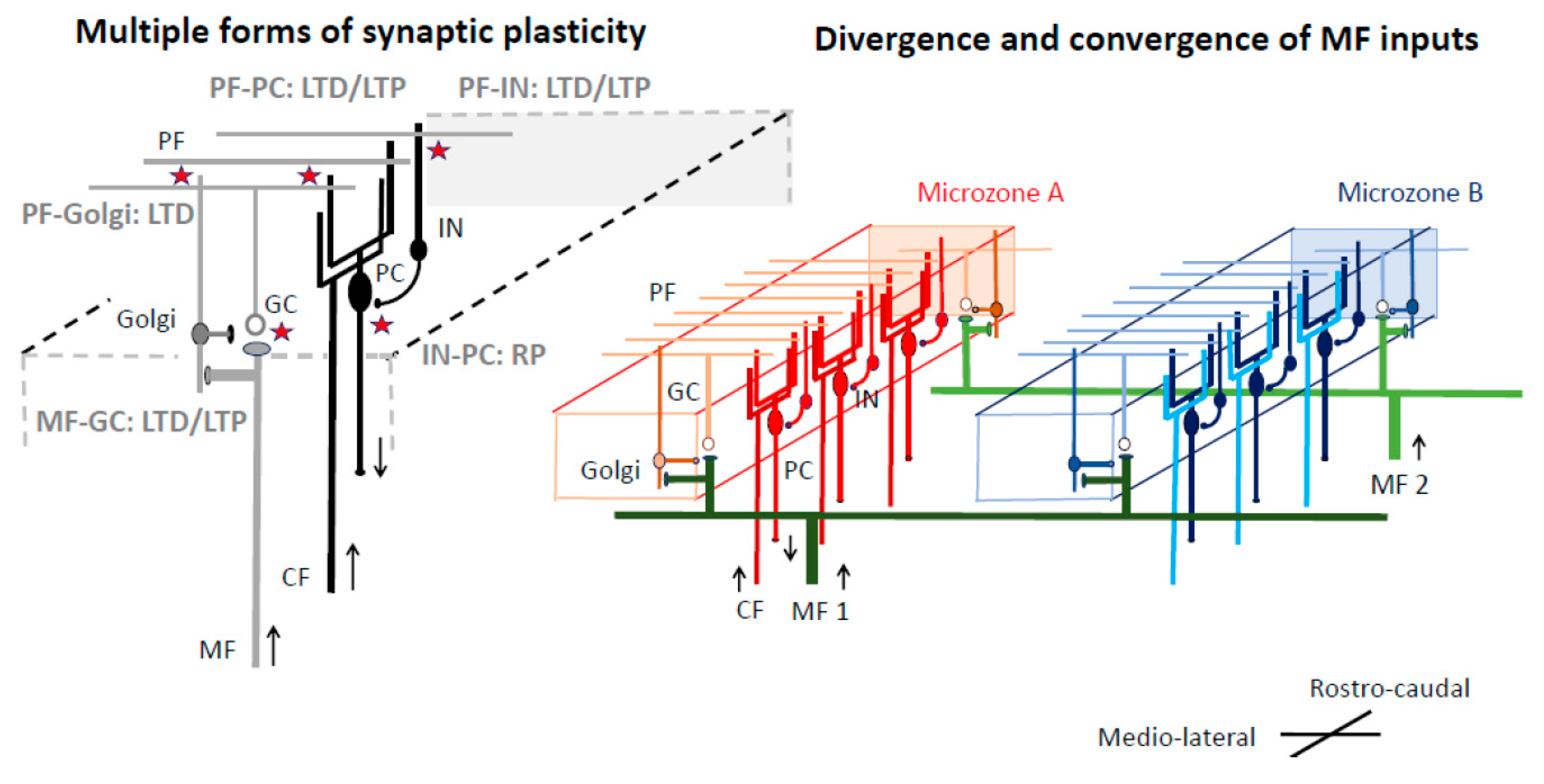
3.2. Reorganization of the Cerebellar Cortex for Predictive Behavior
3.3. Adjustment at Cerebellar Nuclei for Filtering Step
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eccles, J.C.; Ito, M.; Szentagothai, J. The Cerebellum as a Neuronal Machine; Springer: New York, NY, USA, 1967. [Google Scholar]
- Ito, M. The Cerebellum and Neural Control; Raven Press: New York, NY, USA, 1984. [Google Scholar]
- Napper, R.M.; Harvey, R.J. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J. Comp. Neurol. 1988, 274, 168–177. [Google Scholar] [CrossRef]
- Apps, R.; Hawkes, R.; Aoki, S.; Bengtsson, F.; Brown, A.M.; Chen, G.; Ebner, T.J.; Isope, P.; Jörntell, H.; Lackey, E.P.; et al. Cerebellar modules and their role as operational cerebellar processing units: A consensus paper [corrected]. Cerebellum 2018, 17, 654–682. [Google Scholar] [CrossRef]
- Mitoma, H.; Kakei, S.; Yamaguchi, K.; Manto, M. Physiology of cerebellar reserve: Redundancy and plasticity of a modular machine. Int. J. Mol. Sci. 2021, 22, 4777. [Google Scholar] [CrossRef]
- Nagao, S. Ocular reflex adaptation as an experimental model of the cerebellar learning—In memory of Masao Ito. Neuroscience 2021, 462, 191–204. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Lisberger, S.G.; Raymond, J.L. Diversity and dynamics in the cerebellum. Nat. Neurosci. 2021, 24, 160–167. [Google Scholar] [CrossRef]
- Streng, M.L.; Popa, L.S.; Ebner, T.J. Complex spike wars: A new hope. Cerebellum 2018, 17, 735–746. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Caplan, D. Cognition, emotion and the cerebellum. Brain 2006, 129, 290–292. [Google Scholar] [CrossRef]
- Zang, Y.; De Schutter, E. Recent data on the cerebellum require new models and theories. Curr. Opin. Neurobiol. 2023, 82, 102765. [Google Scholar] [CrossRef]
- Holmes, G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain 1917, 40, 461–535. [Google Scholar] [CrossRef]
- Mitoma, H.; Manto, M. The physiological basis for therapies of cerebellar ataxias. Ther. Adv. Neurol. Disord. 2016, 9, 396–413. [Google Scholar] [CrossRef]
- Mitoma, H.; Manto, M.; Hampe, C.S. Time is cerebellum. Cerebellum 2018, 17, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Buffo, A.; Gelfo, F.; Guell, X.; Fucà, E.; Kakei, S.; Lee, J.; Manto, M.; Petrosini, L.; Shaikh, A.G.; et al. Consensus paper. Cerebellar reserve: From cerebellar physiology to cerebellar disorders. Cerebellum 2019, 19, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Manto, M.; Kakei, S.; Mitoma, H. The critical need to develop tools assessing cerebellar reserve for the delivery and assessment of non-invasive cerebellar stimulation. Cerebellum Ataxias 2021, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Ishikawa, T.; Lee, J.; Kakei, S. The cerebro-cerebellum as a locus of forward model. Front. Syst. Neurosci. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Konczak, J.; Pierscianek, D.; Hirsiger, S.; Bultmann, U.; Schoch, B.; Gizewski, E.R.; Timmann, D.; Maschke, M.; Frings, M. Recovery of upper limb function after cerebellar stroke lesion symptom mapping and arm kinematics. Stroke 2010, 41, 2191–2200. [Google Scholar] [CrossRef]
- Baier, B.; Müller, N.; Rhode, F.; Dieterich, M. Vestibular compensation in cerebellar stroke patients. Eur. J. Neurol. 2015, 22, 416–418. [Google Scholar] [CrossRef]
- Shuvaev, A.N.; Horiuchi, H.; Seki, T.; Goenawan, H.; Irie, T.; Iizuka, A.; Sakai, N.; Hirai, H. Mutant PKCγ in spinocerebellar ataxia type 14 disrupts synapse elimination and long-term depression in Purkinje cells in vivo. J. Neurosci. 2011, 31, 14324–14334. [Google Scholar] [CrossRef]
- Mark, M.D.; Krause, M.; Boele, H.J.; Kruse, W.; Pollok, S.; Kuner, T.; Dalkara, D.; Koekkoek, S.; De Zeeuw, C.I.; Herlitze, S. Spinocerebellar ataxia type 6 protein aggregates cause deficits in motor learning and cerebellar plasticity. J. Neurosci. 2015, 35, 8882–8895. [Google Scholar] [CrossRef]
- Shuvaev, A.N.; Belozor, O.S.; Mozhei, O.I.; Shuvaev, A.N.; Fritsler, Y.V.; Khilazheva, E.D.; Mosyagina, A.I.; Hirai, H.; Teschemacher, A.G.; Kasparov, S. Indirect negative effect of mutant ataxin-1 on short- and long-term synaptic plasticity in mouse models of spinocerebellar ataxia type 1. Cells 2022, 11, 2247. [Google Scholar] [CrossRef]
- Draganova, R.; Pfaffenrot, V.; Steiner, K.M.; Göricke, S.L.; Elangovan, N.; Timmann, D.; Konczak, J. Neurostructural changes and declining sensorimotor function due to cerebelalr cortical degeneration. J. Neurophysiol. 2021, 125, 1735–1745. [Google Scholar] [CrossRef]
- Burciu, R.G.; Fritsche, N.; Granert, O.; Schmitz, L.; Spönemann, N.; Konczak, J.; Theysohn, N.; Gerwig, M.; van Eimeren, T.; Timmann, D. Brain changes associated with postural training in patients with cerebellar degeneration: A voxel-based morphometry study. J. Neurosci. 2013, 33, 4594–4604. [Google Scholar] [CrossRef] [PubMed]
- Draganova, R.; Konietschke, F.; Steiner, K.M.; Elangovan, N.; Gümüs, M.; Göricke, S.M.; Ernst, T.M.; Deistung, A.; van Eimeren, T.; Konczak, J.; et al. Motor training-related brain reorganization in patients with cerebellar degeneration. Hum. Brain Mapp. 2022, 43, 1611–1629. [Google Scholar] [CrossRef] [PubMed]
- Manto, M.; Jacquy, J.; Hildebrand, J.; Godaux, E. Recovery of hypermetria after a cerebellar stroke occurs as a multistage process. Ann. Neurol. 1995, 38, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Konczak, J.; Schoch, B.; Dimitrova, A.; Gizewski, E.; Timmann, D. Functional recovery of children and adolescents after cerebellar tumour resection. Brain 2005, 128, 1428–1441. [Google Scholar] [CrossRef]
- Ilg, W.; Synofzik, M.; Brötz, D.; Burkard, S.; Giese, M.A.; Schöls, L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology 2009, 73, 1823–1830. [Google Scholar] [CrossRef]
- Shuvaev, A.N.; Hosoi, N.; Sato, Y.; Yanagihara, D.; Hirai, H. Progressive impairment of cerebellar mGluR signaling and its therapeutic potential for cerebellar ataxia in spinocerebellar ataxia type 1 model mice. J. Physiol. 2017, 595, 141–164. [Google Scholar] [CrossRef]
- Mitoma, H.; Honnorat, J.; Yamaguchi, K.; Manto, M. LTDpathies: A novel clinical concept. Cerebellum 2022, 20, 948–951. [Google Scholar] [CrossRef]
- Linnemann, C.; Sultan, F.; Pedroarena, C.M.; Schwarz, C.; Their, P. Lurcher mice exhibit potentiation of GABA(A)-receptor mediated conductance in cerebellar nucleus neurons in close temporal relationship to Purkinje cell death. J. Neurophysiol. 2004, 91, 1102–1107. [Google Scholar] [CrossRef][Green Version]
- Klintsova, A.Y.; Scamra, C.; Hoffman, M.; Napper, R.M.; Goodlett, C.R.; Greenough, W.T. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum. Brain Res. 2002, 937, 83–93. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Fields, R.D.; Johansen-Berg, H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012, 15, 528–536. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z.; Jordan, M.I. An internal model for sensorimotor integration. Science 1995, 269, 1880–1882. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Miall, R.C. Forward models for physiological motor control. Neural. Netw. 1996, 9, 1265–1279. [Google Scholar]
- Todorov, E. Optimality principles in sensorimotor control. Nat. Neurosci. 2004, 7, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.S.; Hewitt, A.L.; Ebner, T.J. Predictive and feedback performance errors are signaled in the simple spike discharge of individual Purkinje cells. J. Neurosci. 2012, 32, 15345–15358. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.W.; Diedrichsen, J.; Krakauer, J.W.; Shadmehr, R.; Bastian, A.J. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J. Neurophysiol. 2007, 98, 54–62. [Google Scholar] [CrossRef]
- Cabaraux, P.; Gandini, J.; Kakei, S.; Manto, M.; Mitoma, H.; Tanaka, H. Dysmetria and errors in predictions: The role of internal forward model. Int. J. Mol. Sci. 2020, 21, 6900. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishikawa, T.; Kakei, S. Neural evidence of the cerebellum as a state predictor. Cerebellum 2019, 18, 349–371. [Google Scholar] [CrossRef]
- Colin, F.; Ris, L.; Godaux, E. Neuroanatomy of the cerebellum. In The Cerebellum and Its Disorders; Manto, M., Pandolfo, M., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 6–29. [Google Scholar]
- Walloe, S.; Pakkenberg, B.; Fabriciusm, K. Stereological estimation of total cell numbers in the human cerebral and cerebellar cortex. Front. Hum. Neurosci. 2014, 8, 508. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The human brains in numbers: A linearly scaled-up primate brain. Front. Hum. Neurosci. 2009, 3, 31. [Google Scholar] [CrossRef]
- D’Angelo, E.; Casali, S. Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Front. Neural. Circuit 2013, 6, 116. [Google Scholar] [CrossRef]
- Wu, H.S.; Sugihara, I.; Shinoda, Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J. Comp. Neurol. 1999, 411, 97–118. [Google Scholar] [CrossRef]
- Jörntell, H.; Ekerot, C.F. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron 2002, 34, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, A.; Badura, A.; Deverett, B.; Najafi, F.; Pereira, T.D.; Gao, Z.; Ozden, I.; Kloth, A.D.; Pnevmatikakis, E.; Paninski, L.; et al. Cerebellar granule cells acquire a widespread predictive feedback signal during motor learning. Nat. Neurosci. 2017, 20, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Shimuta, M.; Häusser, M. Multimodal sensory integration in single cerebellar granule cells in vivo. eLife 2015, 4, e12916. [Google Scholar] [CrossRef]
- Sanger, T.D.; Yamashita, O.; Kawato, M. Expansion coding and computation in the cerebellum: 50 years after the Marr-Albus codon theory. J. Physiol. 2020, 598, 913–928. [Google Scholar] [CrossRef]
- Gao, Z.; van Beugen, B.J.; De Zeeuw, C.I. Distributed synergistic plasticity and cerebellar learning. Nat. Rev. Neurosci. 2012, 13, 619–635. [Google Scholar] [CrossRef]
- Sgritta, M.; Locatelli, F.; Soda, T.; Prestori, F.; D’Angelo, E.U. Hebbian Spike-timing dependent plasticity at the cerebellar input stage. J. Neurosci. 2017, 37, 2809–2823. [Google Scholar] [CrossRef]
- Ito, M.; Sakurai, M.; Tongroach, P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J. Physiol. 1982, 324, 113–134. [Google Scholar] [CrossRef]
- Ito, M.; Yamaguchi, K.; Nagao, S.; Yamazaki, T. Long-term depression as a model of cerebellar plasticity. Prog. Brain Res. 2014, 210, 1–30. [Google Scholar]
- Salin, P.A.; Robert, C.; Malenka, R.C.; Nicoll, R.A. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 1996, 16, 797–803. [Google Scholar] [CrossRef]
- Lonart, G.; Schoch, S.; Kaeser, P.S.; Larkin, C.J.; Südhof, T.C.; Linden, D.J. Phosphorylation of RIM1α by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell 2003, 115, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Kano, M.; Fukunaga, K.; Konnerth, A. Ca2+-induced rebound potentiation of γ-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc. Natl. Acad. Sci. USA 1996, 93, 13351–13356. [Google Scholar] [CrossRef] [PubMed]
- Rancillac, A.; Crépel, F. Synapses between parallel fibres and stellate cells express long-term changes in synaptic efficacy in rat cerebellum. J. Physiol. 2004, 554, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.F. In search of memory traces. Annu. Rev. Psychol. 2005, 56, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. The Cerebellum Brain for an Implicit Self; FT Press: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Blosa, M.; Bursch, C.; Weigel, S.; Holzer, M.; Jäger, C.; Janke, C.; Matthews, R.T.; Arendt, T.; Morawskin, M. Reorganization of synaptic connections and perineuronal nets in the deep cerebellar nuclei of Purkinje cell degeneration mutant mice. Neural Plast. 2016, 2016, 2828536. [Google Scholar] [CrossRef]
- Di Nuzzo, C.; Ruggiero, F.; Cortese, F.; Cova, I.; Priori, A.; Ferrucci, R. Non-invasive cerebellar stimulation in cerebellar disorders. CNS Neurol. Disord. Drug Targets 2018, 17, 193–198. [Google Scholar] [CrossRef]
- Wessel, M.J.; Hummel, F.C. Non-invasive cerebellar stimulation: A promising approach for stroke recovery? Cerebellum 2018, 17, 359–371. [Google Scholar] [CrossRef]
- Takahashi, M.; Shinoda, Y. Neural circuits of inputs and outputs of the cerebellar cortex and nuclei. Neuroscience 2021, 462, 70–88. [Google Scholar] [CrossRef]
- Strick, P.L.; Dum, R.P.; Fiez, J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009, 32, 413–434. [Google Scholar] [CrossRef]
- Choi, S.M. Movement disorders following cerebrovascular lesions in cerebellar circuits. J. Mov. Disord. 2016, 9, 80–88. [Google Scholar] [CrossRef]
- Handforth, A.; Lang, E.J. Increased Purkinje cell complex spike and deep cerebellar nucleus synchrony as a potential basis for syndromic essential tremor. A review and synthesis of the literature. Cerebellum 2021, 20, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Miyata, H.; Kawamura, M.; Harada, Y. Morphological and electrophysiological evidence for axonal regeneration of axotomized cerebellothalamic neuron in kittens. Neurosci. Lett. 1981, 25, 13–18. [Google Scholar] [CrossRef]
- Shutoh, F.; Ohki, M.; Kitazawa, H.; Itohara, S.; Nagao, S. Memory trace of motor learning shifts transynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience 2006, 139, 767–777. [Google Scholar] [CrossRef]
- Rabinowitch, I.; Bai, J. The foundations of cross-modal plasticity. Commun. Integr. Biol. 2016, 9, e1158378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beuriat, P.A.; Cristofori, I.; Richard, N.; Bardi, L.; Loriette, C.; Szathmari, A.; Di Rocco, F.; Leblond, P.; Frappaz, D.; Faure-Conter, C.; et al. Cerebellar lesions at a young age predict poorer long-term functional recovery. Brain Commun. 2020, 2, fcaa027. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitoma, H.; Kakei, S.; Tanaka, H.; Manto, M. Morphological and Functional Principles Governing the Plasticity Reserve in the Cerebellum: The Cortico-Deep Cerebellar Nuclei Loop Model. Biology 2023, 12, 1435. https://doi.org/10.3390/biology12111435
Mitoma H, Kakei S, Tanaka H, Manto M. Morphological and Functional Principles Governing the Plasticity Reserve in the Cerebellum: The Cortico-Deep Cerebellar Nuclei Loop Model. Biology. 2023; 12(11):1435. https://doi.org/10.3390/biology12111435
Chicago/Turabian StyleMitoma, Hiroshi, Shinji Kakei, Hirokazu Tanaka, and Mario Manto. 2023. "Morphological and Functional Principles Governing the Plasticity Reserve in the Cerebellum: The Cortico-Deep Cerebellar Nuclei Loop Model" Biology 12, no. 11: 1435. https://doi.org/10.3390/biology12111435
APA StyleMitoma, H., Kakei, S., Tanaka, H., & Manto, M. (2023). Morphological and Functional Principles Governing the Plasticity Reserve in the Cerebellum: The Cortico-Deep Cerebellar Nuclei Loop Model. Biology, 12(11), 1435. https://doi.org/10.3390/biology12111435





