The Response of Endogenous ABA and Soluble Sugars of Platycladus orientalis to Drought and Post-Drought Rehydration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Material Selection
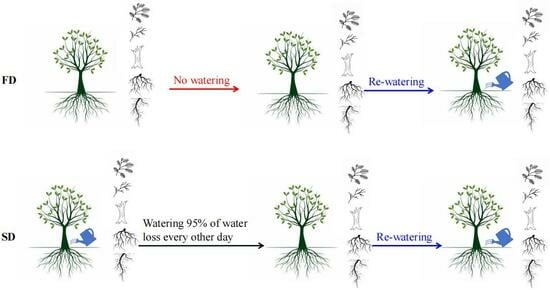
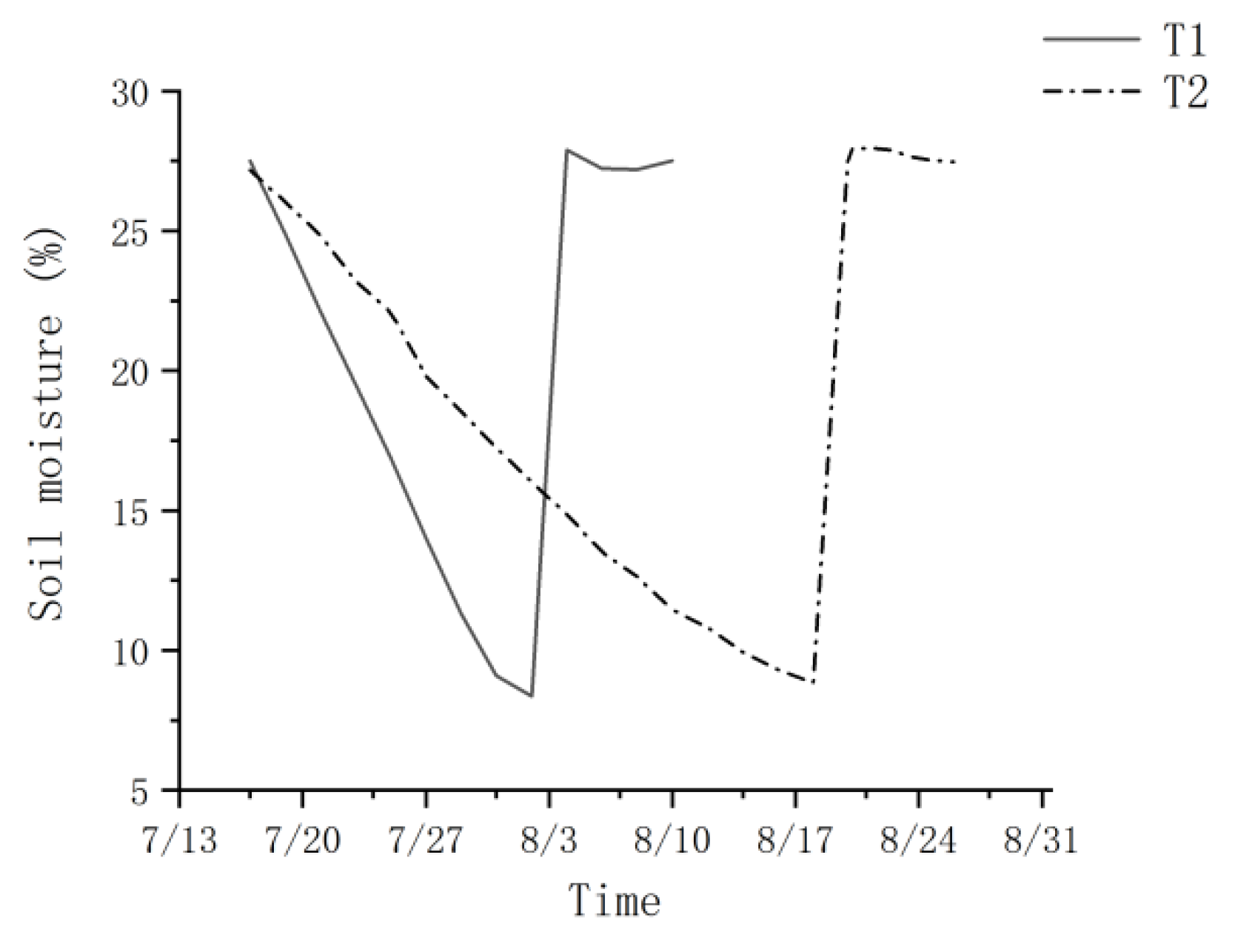
2.2. Experimental Design
2.3. Photosynthetic Parameter Assay
2.4. Endogenous ABA Content Assay
2.5. Soluble Sugar Content Assay
2.6. Data Analysis
3. Results
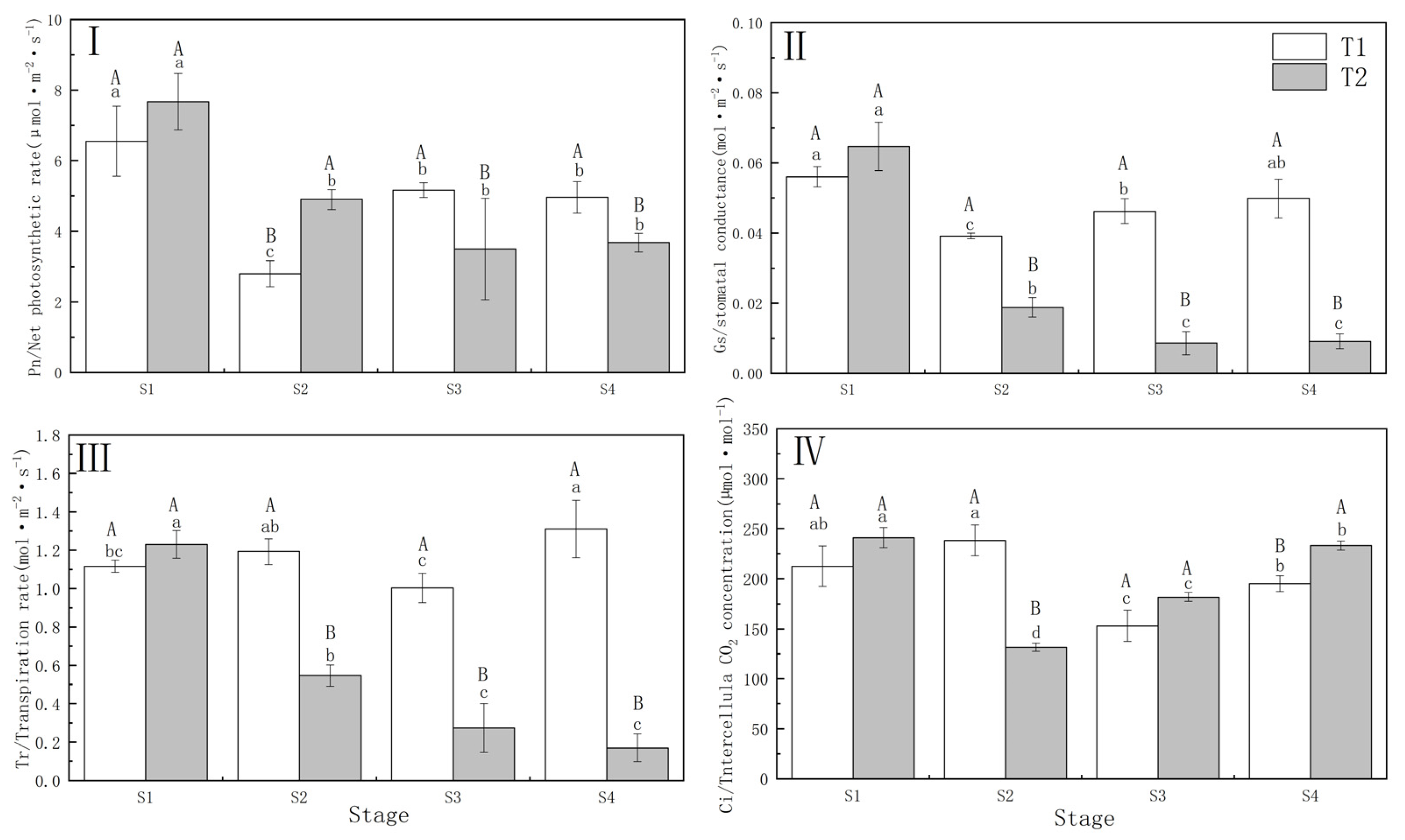
3.1. Photosynthetic Performance under Different Drought–Rehydration Treatments
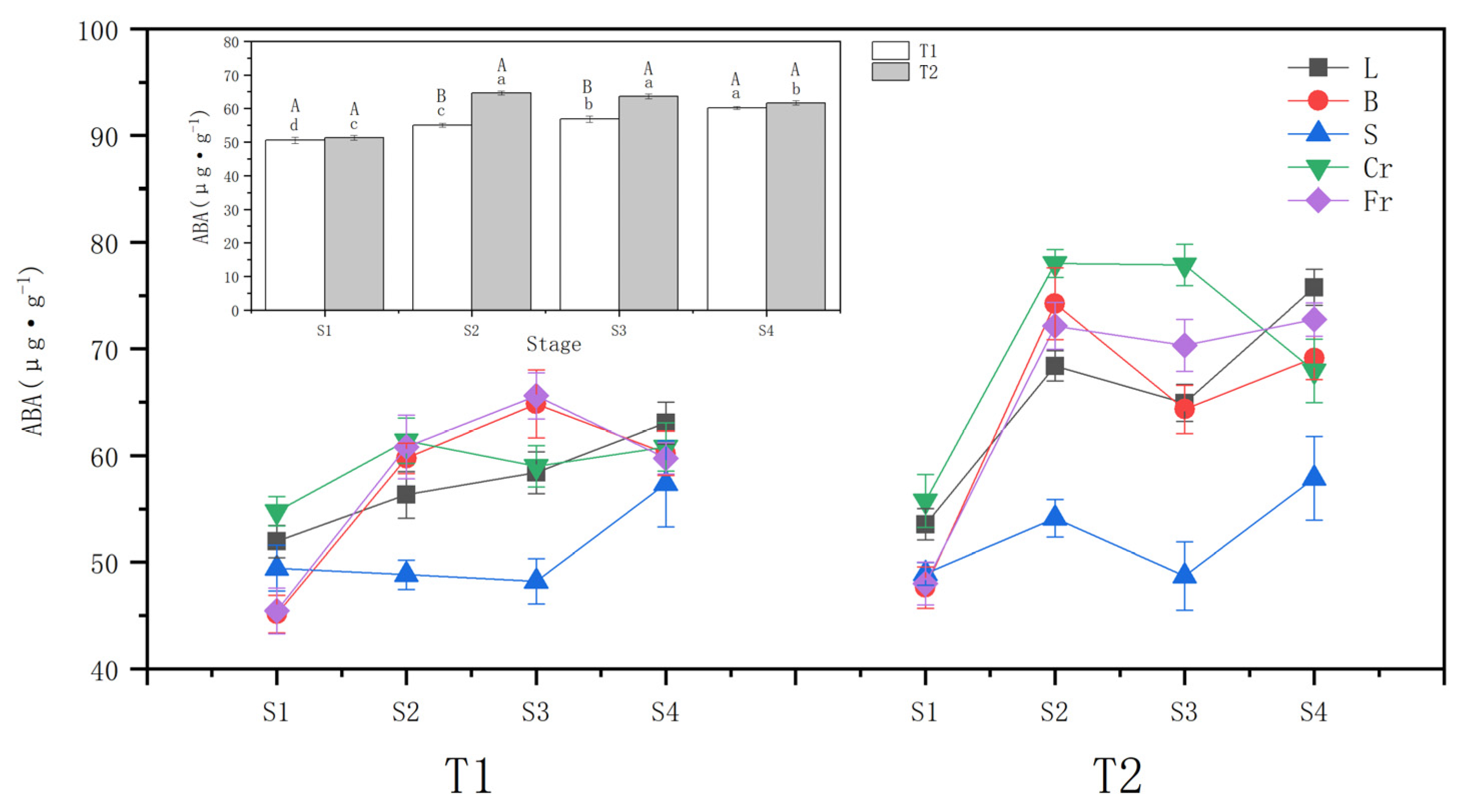
3.2. Effects of Different Drought Treatments on Endogenous Hormone Abscisic Acid Content
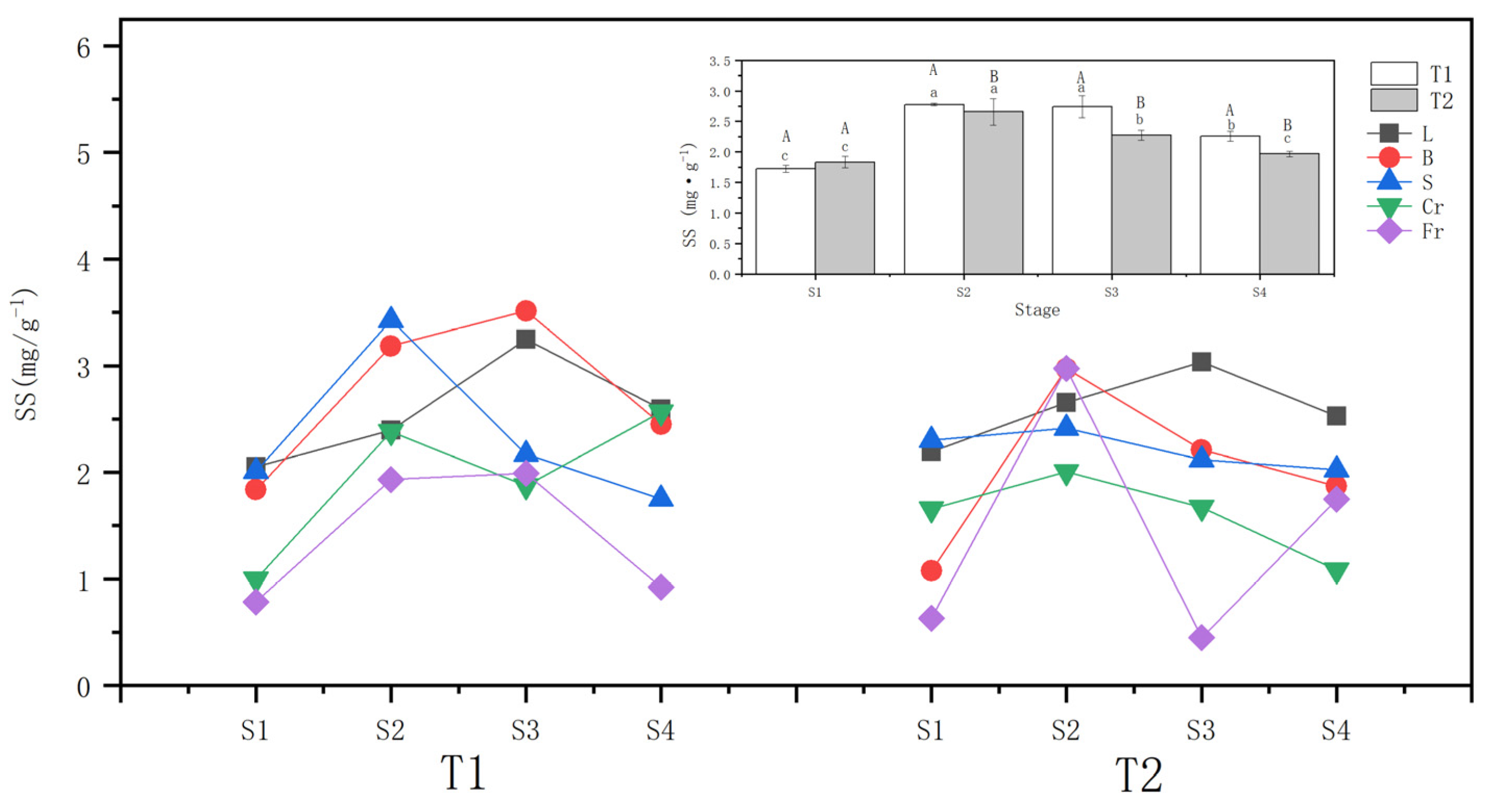
3.3. Effects of Different Drought Treatments on the Soluble Sugar Content
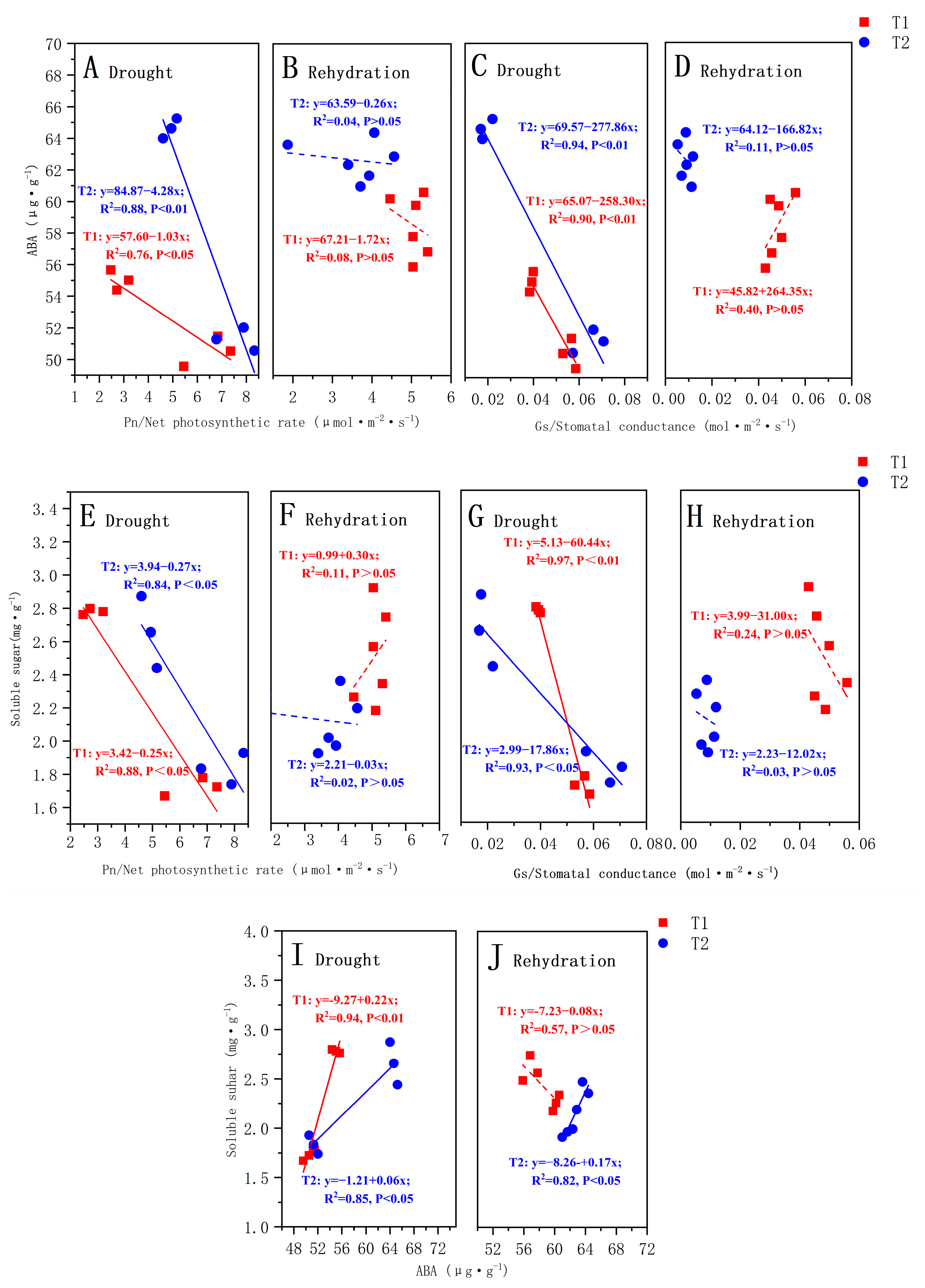
3.4. Correlation between Soluble Sugar and Abscisic Acid Content
4. Discussion
4.1. Response of Photosynthetic Characteristics to Different Drought Treatments
4.2. Response of Endogenous Hormone ABA Content to Different Drought Treatments
4.3. Response of Soluble Sugar Content to Different Drought Treatments
4.4. Correlation between Endogenous Hormone ABA and Soluble Sugar Content under Different Drought Treatments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaufmann, I.; Schulze-Till, T.; Schneider, H.U.; Zimmermann, U.; Jakob, P.; Wegner, L.H. Functional repair of embolized vessels in maize roots after temporal drought stress, as demonstrated by magnetic resonance imaging. New Phytol. 2009, 184, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Rivest, D.; Paquette, A.; Shipley, B.; Reich, P.B.; Messier, C. Tree communities rapidly alter soil microbial resistance and resilience to drought. Funct. Ecol. 2015, 29, 570–578. [Google Scholar] [CrossRef]
- Baer, A.; Wheeler, J.K.; Pittermann, J. Not dead yet: The seasonal water relations of two perennial ferns during California’s exceptional drought. New Phytol. 2016, 210, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Adams, H.D.; Anderegg, W.R.L.; Jansen, S.; Zeppel, M.J.B. Research frontiers in drought-induced tree mortality: Crossing scales and disciplines. New Phytol. 2015, 205, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Nalt. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efciency for greater yield. Annu. Rev. Plant. Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- West, A.G.; Dawson, T.E.; February, E.C.; Midgley, G.F.; Bond, W.J.; Aston, T.L. Diverse functional responses to drought in a Mediterranean-type shrubland in South Africa. New Phytol. 2012, 195, 396–407. [Google Scholar] [CrossRef]
- Maréchaux, I.; Bartlett, M.K.; Sack, L.; Baraloto, C.; Engel, J.; Joetzjer, E.; Chave, J. Drought tolerance as predicted by leaf water potential at turgor loss point varies strongly across species within an Amazonian forest. Funct. Ecol. 2015, 29, 1268–1277. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Manzi, D.; Masini, C.M.; Doni, S.; Mattii, G.B. Sustainable soil management: Effects of clinoptilolite and organic compost soil application on eco-physiology, quercitin, and hydroxylated, methoxylated anthocyanins on Vitis vinifera. Plants 2023, 12, 708. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheriro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2008, 103, 551–560. [Google Scholar] [CrossRef]
- Hipkins, M.F.; Wilkins, M.B. Photosynthesis. In Advanced Plant Physiology; Pitman Publishing. Pry, Ltd.: Melbourne, Australia, 1984. [Google Scholar]
- Diao, Z.L.; Chen, H.; Feng, J.L.; Liu, W.; Wang, Y. Effects of drought stress physiological and biochemical characteristics of Camellia oleifera seedlings. J. Anhui Agric. Univ. 2014, 41, 642–646. [Google Scholar]
- Zhang, S.Y.; Liu, Y.C.; Li, Y.T.; Huang, Y.R.; Zhang, J. Effects of drought stress on endogenous hormones in potted seedlings of ulmus pumila ‘Jinye’. J. West China For. Sci. 2021, 50, 40–45. [Google Scholar]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L.N. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant. Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Guo, X.Y.; Peng, C.H.; Li, T.; Huang, J.J.; Song, H.X.; Zhu, Q.; Wang, M. The Effects of Drought and Re-Watering on Non-Structural Carbohydrates of Pinus tabulaeformis Seedlings. Biology 2021, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Tung, S.A.; Smeeton, R.; White, C.A.; Black, C.R.; Taylor, I.B.; Hilton, H.W.; Thompson, A.J. Over-expression of LeNCED1 in tomato (Solanum lycopersicum L.) with the rbcS3C promoter allows recovery of lines that accumulate very high levels of abscisic acid and exhibit severe phenotypes. Plant. Cell Environ. 2008, 31, 968–981. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Korner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- McDowell, N.G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant. Physiol. 2011, 155, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G.; Richter, A.; Körner, C. Non-structural carbon compounds in temperate forest trees. Plant. Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Millard, P.; Sommerkorn, M.; Grelet, G.A. Environmental change and carbon limitation in trees: A biochemical, ecophysiological and ecosystem appraisal. New Phytol. 2007, 175, 11–28. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annual. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Kannenberg, S.A.; Novick, K.A.; Phillips, R.P. Coarse roots prevent declines in whole-tree non-structural carbohydrate pools during drought in an isohydric and an anisohydric species. Tree Physiol. 2018, 38, 582–590. [Google Scholar] [CrossRef]
- Reinhardt, K.; Germino, M.J.; Kueppers, L.M.; Domec, J.C.; Mitton, J. Linking carbon and water relations to drought-induced mortality in Pinus flexilis seedlings. Tree Physiol. 2015, 35, 771–782. [Google Scholar] [CrossRef]
- Anderegg, W.R.L. Complex aspen forest carbon and root dynamics during drought. Climatic Chang. 2012, 111, 983–991. [Google Scholar] [CrossRef]
- Nikinmaa, E.; Hölttä, T.; Hari, P.; Kolari, P.; Mäkelä, A.; Sevanto, S.; Vesala, T. Assimilate transport in phloem sets conditions for leaf gas exchange. Plant Cell Environ. 2013, 36, 655–669. [Google Scholar] [CrossRef]
- Li, W.B.; Hartmann, H.; Adams, H.D.; Zhang, H.X.; Jin, C.J.; Zhao, C.Y.; Guan, D.X.; Wang, A.Z.; Yuan, F.H.; Wu, J.B. The sweet side of global change-dynamic responses of non-structural carbohydrates to drought, elevated CO2 and nitrogen fertilization in tree species. Tree Physiol. 2018, 38, 1706–1723. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.T.; Pezeshki, S.R.; Shields, F.D. Responses of nonstructural carbohydrates to shoot removal and soil moisture treatments in Salix nigra. Trees-Struct. Funct. 2008, 22, 737–748. [Google Scholar] [CrossRef]
- Chen, Z.C.; Liu, C.; Li, X.; Li, S.; Wan, X.C.; Liu, S.R. The response of ABA and hydraulic indicator-mediated leaf gas exchange and nonstructural carbohydrate of Ginkgo biloba saplings to drought and rehydration. Acta Physiol. Plant. 2022, 44, 61. [Google Scholar] [CrossRef]
- Ghobadi, M.; Taherabadi, S.; Ghobadi, M.E.; Mohammadi, G.R.; Jalali-Honarmand, S. Antioxidant capacity, photosynthetic characteristics and water relations of sunflower (Helianthus annuus L.) cultivars in response to drought stress. Ind. Crops Prod. 2013, 50, 29–38. [Google Scholar] [CrossRef]
- Mitchell, P.J.; McAdam, S.A.M.; Pinkard, E.A.; Brodribb, T.J. Significant contribution from foliage-derived ABA in regulating gas exchange in Pinus radiata. Tree Physiol. 2017, 37, FEB2017. [Google Scholar]
- Beijing Water Resources Bulletin. 2022. Available online: https://swj.beijing.gov.cn/zwgk/szygb (accessed on 25 July 2023).
- Wu, S.R.; Chen, W.F.; Zhou, X. Enzyme linked immunosorbent assay for endogenous plant hormones. Plant Physiol. Commun. 1988, 5, 53–57. [Google Scholar]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T. Standardized protocols and procedures can precisely and accurately quantify non-structural carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Zhang, Q.A.; Li, X.S.; Cao, K.F. Gas exchange and hydraulics in seedlings of Hevea brasiliensis during water stress and recovery. Tree Physiol. 2010, 30, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ji, R.X.; Yu, X.; Bai, X.Q.; Chang, Y.; Liu, C. Changs of non-structural carbohydrates in Caryopteris mongolica seedlings during the process of drought-induced mortality. Chin. J. Appl. Ecol. 2019, 30, 2541–2548. [Google Scholar]
- Kirschbaum, M.U.F. Water-stress in Eucalyptus pauciflora comparison of effects on stomatal conductance with effects on the mesophyll capacity for photosynthesis, and investigation of a possible involvement of photoinhibition. Planta 1987, 171, 466–473. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. Recovery of photosynthesis from water stress in Eucalyptus pauciflora-a process in two stages. Plant Cell Environ. 1988, 11, 685–694. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, J.X.; Ma, R.; Lv, G.L.; Feng, S.L. Impacts of different drought stress durations and re-watering on chlorophyll fluorescence of Platycladus orientalis. Jiangsu Agric. Sci. 2021, 49, 127–132. [Google Scholar]
- Geiger, D.; Scherzer, S.; Mumm, P. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinasephosphatase pair. Proc. Natl. Acad. Sci. USA 2009, 106, 21425–21430. [Google Scholar] [CrossRef]
- Endo, A.; Sawada, Y.; Takahashi, H. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008, 147, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The coordination of responses to stress in plants. Plant Cell Environ. 2002, 25, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Will, R.E.; Wilson, S.M.; Zou, C.B.; Hennessey, T.C. Increased vapor pressure deficit due to higher temperature leads to greater transpiration and faster mortality during drought for tree seedlings common to the forest–grassland ecotone. New Phytol. 2013, 200, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.J.; Hacke, U.G. Solid mechanics of the torus-margo in conifer intertracheid bordered pits. New Phytol. 2021, 229, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, X.Q.; Zhao, W.; Shi, J.; Qi, L.; Zhang, Y.F.; Zhang, L.X. Study on the compensatory growth mechamism adjusted by ytokinin after corn seedling re-watering. Res. Soil Water Conserv. 2016, 23, 300–305+312. [Google Scholar]
- Fan, J.; Chen, C.; Brlansky, R.; Gmitter, F.G.; Li, Z.G. Changes in carbohydrate metabolism in Citrus sinensis infected with ‘Candidatus Liberibacter asiaticus’. Plant Pathol. 2010, 59, 1037–1043. [Google Scholar] [CrossRef]
- Inze, D.; Skirycz, A. More from less: Plant growth under limited water. Current. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar]
- Cernusak, L.A.; Arthur, D.J.; Pate, J.S.; Farquhar, G.D. Water relations link carbon and oxygen isotope discrimination to phloem sap sugar concentration in Eucalyptus globulus. Plant Physiol. 2003, 131, 1544–1554. [Google Scholar] [CrossRef]
- Keunen, E.L.S.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.I.M.; Cuypers, A.N.N. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Sala, A.; Woodruff, D.R.; Meinzer, F.C. Carbon dynamics in trees: Feast or famine? Tree Physiol. 2012, 32, 764–775. [Google Scholar] [CrossRef]
- Lei, H.; Wang, k.; Tian, H.; Gao, X.; Zhao, L.R. Responses of non-structural carbohydrates accumulation and distribution of Caragana microphylla seedlings to drought stress. Chin. J. Ecol. 2017, 36, 3168–3175. [Google Scholar]
- Nardini, A.; Lo Gullo, M.A.; Salleo, S. Refilling embolized xylem conduits: Is it a matter of phloem unloading? Plant. Sci. 2011, 180, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Brodersen, C.R.; McElrone, A.J. Maintenance of xylem network transport capacity: A review of embolism repair in vascular plants. Front. Plant Sci. 2013, 4, 108. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.M.; Jordan, G.J.; Martins, S.C.V. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 14489–14493. [Google Scholar] [CrossRef]
- Ward, J.M.; Pei, Z.M.; Schroeder, J.I. Roles of ion channels in initiation in higher plants. Plant Cell 1995, 7, 833–844. [Google Scholar] [CrossRef]
- Huber, A.E.; Melcher, P.J.; Pineros, M.A.; Setter, T.L.; Bauerle, T.L. Signal coordination before, during and after stomatal closure in response to drought stress. New Phytol. 2019, 224, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, S.; Xiong, B.; Cao, B.; Cao, B.B.; Deng, X.P. Carbon/Nitrogen imbalance associated with drought-induced leaf senescence in Sorghum bicolor. PLoS ONE 2015, 10, e0137026. [Google Scholar] [CrossRef] [PubMed]
- Morita-Yamamuro, C.; Vernieri, P.; Yamaguchi, J. Low sugar status promotes endogenous ABA level and ABA sensitivity in Arabidopsis. Plant Biotechnol. 2004, 21, 9–14. [Google Scholar] [CrossRef]
- Brouwer, B.; Ziolkowska, A.; Bagard, M.; Keech, O.; Gardeström, P. The impact of light intensity on shade-induced leaf senescence. Plant Cell Environ. 2012, 35, 1084–1098. [Google Scholar] [CrossRef]
- Asad, M.A.U.; Wang, F.B.; Ye, Y.; Guan, X.Y.; Zhou, L.J.; Han, Z.Y.; Pan, G.; Cheng, F.M. Contribution of ABA metabolism and ROS generation to sugar starvation-induced senescence of rice leaves. Plant Growth Regul. 2021, 95, 241–257. [Google Scholar] [CrossRef]
- Li, Y.; Wan, L.Q.; Li, X.L.; Bi, S.Y.; Huang, H.Y.; Li, Z.Y.; Cao, J. Effects of water on the growth characteristics and root ABA content of alfalfa. J. China Agric. Univ. 2017, 22, 75–83. [Google Scholar]
- He, W.Q.; Liu, H.Y.; Qi, Y.; Liu, F.; Zhu, X.R. Patterns in nonstructural carbohydrate contents at the tree organ level in response to drought duration. Glob. Chang. Biol. 2022, 26, 3627–3638. [Google Scholar] [CrossRef] [PubMed]
| Forest Age (a) | Average Base Diameter (mm) | Average Tree Height (cm) | |
|---|---|---|---|
| Platycladus orientalis | 3 | 12.14 ± 1.71 | 123.63 ± 8.65 |
| Soil Bulk Density (g/cm3) | Soil Volumetric Moisture Content (%) | Total Nitrogen (g/kg) | Total Phosphorus (g/kg) | Total Potassium (g/kg) |
|---|---|---|---|---|
| 1.44 | 28 | 1.137 | 1.059 | 18.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Zhao, J.; Li, S.; Li, B.; Lv, J.; Gao, X.; Xu, X.; Lu, S. The Response of Endogenous ABA and Soluble Sugars of Platycladus orientalis to Drought and Post-Drought Rehydration. Biology 2024, 13, 194. https://doi.org/10.3390/biology13030194
Zhao N, Zhao J, Li S, Li B, Lv J, Gao X, Xu X, Lu S. The Response of Endogenous ABA and Soluble Sugars of Platycladus orientalis to Drought and Post-Drought Rehydration. Biology. 2024; 13(3):194. https://doi.org/10.3390/biology13030194
Chicago/Turabian StyleZhao, Na, Jiahui Zhao, Shaoning Li, Bin Li, Jiankui Lv, Xin Gao, Xiaotian Xu, and Shaowei Lu. 2024. "The Response of Endogenous ABA and Soluble Sugars of Platycladus orientalis to Drought and Post-Drought Rehydration" Biology 13, no. 3: 194. https://doi.org/10.3390/biology13030194
APA StyleZhao, N., Zhao, J., Li, S., Li, B., Lv, J., Gao, X., Xu, X., & Lu, S. (2024). The Response of Endogenous ABA and Soluble Sugars of Platycladus orientalis to Drought and Post-Drought Rehydration. Biology, 13(3), 194. https://doi.org/10.3390/biology13030194