Measurement of New Biomarkers of Immunity and Welfare in Colostrum and Milk of Pigs: Analytical Validation and Changes During Lactation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing and General Management
2.2. Sampling Procedure
2.3. Biochemical Analysis of Colostrum/Milk Samples
2.3.1. Biomarkers of the Immune System
2.3.2. Biomarkers of Stress
2.4. Analytical Validation of the Methods for the Measurement of the Analytes of This Study
2.5. Changes in the Analytes in Lactation
2.6. Statistical Study
3. Results
3.1. Analytical Validation
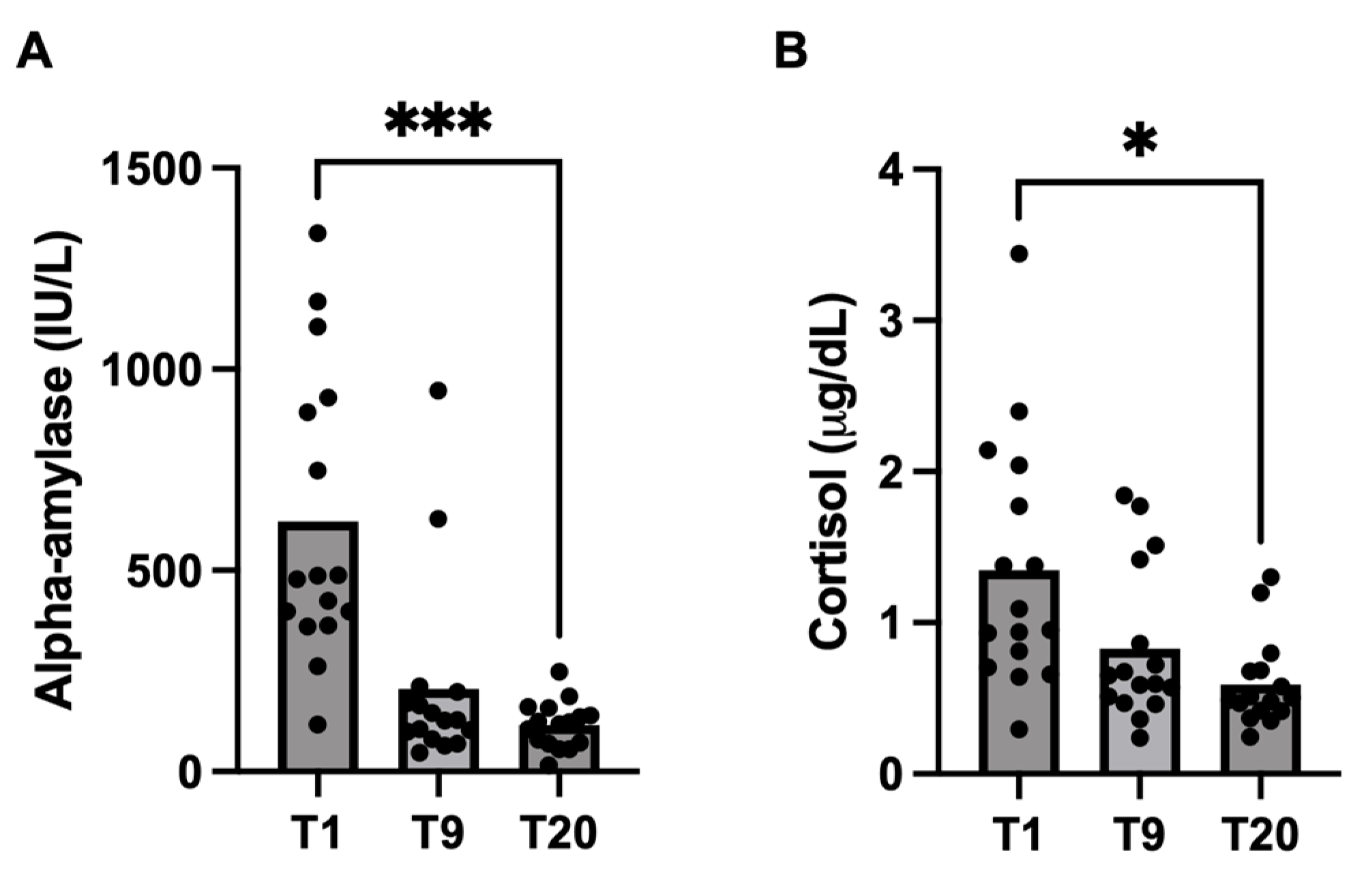
3.2. Changes in Lactation
3.3. Correlation between Analytes and IgG and IgA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inoue, R.; Tsukahara, T. Composition and physiological functions of the porcine colostrum. Anim. Sci. J. 2021, 92, e13618. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Mainau, E.; de Miguel, R.; Temple, D.; Salas, M.; Manteca, X. Oral Meloxicam Administration in Sows at Farrowing and Its Effects on Piglet Immunity Transfer and Growth. Front. Vet. Sci. 2021, 8, 574250. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.; Bourne, F.J. Immunoglobulin quantitation in sow serum, colostrum and milk and the serum of young pigs. Biochim. Biophys. Acta (BBA)—Protein Struct. 1971, 236, 319–332. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum intake: Influence on piglet performance and factors of variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef]
- Sato, M.; Imanishi, A.; Okada, K.; Yasuda, J. Clinical evaluation of bovine adenosine deaminase activities in the colostrums. Jpn. J. Large Anim. Clin. 2010, 1, 197–202. [Google Scholar] [CrossRef]
- Cooray, R. Use of bovine myeloperoxidase as an indicator of mastitis in dairy cattle. Vet. Microbiol. 1994, 42, 317–326. [Google Scholar] [CrossRef]
- Cerón, J.J.; Ortín-Bustillo, A.; López-Martínez, M.J.; Martínez-Subiela, S.; Eckersall, P.D.; Tecles, F.; Tvarijonaviciute, A.; Muñoz-Prieto, A. S-100 Proteins: Basics and Applications as Biomarkers in Animals with Special Focus on Calgranulins (S100A8, A9, and A12). Biology 2023, 12, 881. [Google Scholar] [CrossRef]
- Zhong, K.; Zhang, C.Y.; Zha, G.M.; Wang, X.J.; Jiao, X.Q.; Zhu, H.S.; Wang, Y.Y. S100 calcium-binding protein A12 as a diagnostic index for subclinical mastitis in cows. Reprod. Domest. Anim. 2018, 53, 1442–1447. [Google Scholar] [CrossRef]
- Lalles, J.P.; Formal, M.; Fagerhol, M.K. Changes in calprotectin concentration in sow’s milk throughout lactation. In Proceedings of the XIII International Congress in Animal Hygiene Isah, Tartu, Estonia, 17–21 June 2007. [Google Scholar]
- Hall, S.A.; Farish, M.; Coe, J.; Baker, E.; Camerlink, I.; Lawrence, A.B.; Baxter, E.M. Minimally invasive biomarkers to detect maternal physiological status in sow saliva and milk. Animal 2021, 15, 100369. [Google Scholar] [CrossRef]
- Dewit, O.; Dibba, B.; Prentice, A. Breast-Milk Amylase Activity in English and Gambian Mothers: Effects of Prolonged Lactation, Maternal Parity, and Individual Variations. Pediatr. Res. 1990, 28, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Tvarijonaviciute, A.; Monkeviciene, I.; Martín-Cuervo, M.; González-Arostegui, L.G.; Franco-Martínez, L.; Cerón, J.J.; Tecles, F.; Escribano, D. Characterization of total adenosine deaminase activity (ADA) and its isoenzymes in saliva and serum in health and inflammatory conditions in four different species: An analytical and clinical validation pilot study. BMC Vet. Res. 2020, 16, 384. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, T.; Katsuramaki, T.; Shiraishi, H.; Yokoyama, M.M. Automated enzymatic measurement of adenosine deaminase isoenzyme activities in serum. Anal. Biochem. 1990, 187, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A.J.; Yang, J.J.; Roy, T.A.; Robbins, D.J.; Mackerer, C.R. An automated myeloperoxidase assay. Clin. Chem. 1990, 36, 158. [Google Scholar] [CrossRef]
- López-Martínez, M.J.; Martínez-Subiela, S.; Cerón, J.J.; Ortín-Bustillo, A.; Ramis, G.; López-Arjona, M.; Martínez-Miró, S.; Manzanilla, E.G.; Eckersall, P.D.; Tecles, F.; et al. Measurement of Calprotectin (S100A8/A9) in the Saliva of Pigs: Validation Data of A Commercially Available Automated Assay and Changes in Sepsis, Inflammation, and Stress. Animals 2023, 13, 1190. [Google Scholar] [CrossRef]
- Ortín-Bustillo, A.; Botía, M.; López-Martínez, M.J.; Martínez-Subiela, S.; Cerón, J.J.; González-Bulnes, A.; Manzanilla, E.G.; Goyena, E.; Tecles, F.; Muñoz-Prieto, A. Changes in S100A8/A9 and S100A12 and Their Comparison with Other Analytes in the Saliva of Pigs with Diarrhea Due to E. coli. Animals 2023, 13, 2556. [Google Scholar] [CrossRef]
- Fuentes, M.; Tecles, F.; Gutiérrez, A.; Otal, J.; Martínez-Subiela, S.; Cerón, J.J. Validation of an automated method for salivary alpha-amylase measurements in pigs (Sus scrofa domesticus) and its application as a stress biomarker. J. Vet. Diagn. Invest. 2011, 23, 282–287. [Google Scholar] [CrossRef]
- Escribano, D.; Fuentes-Rubio, M.; Cerón, J.J. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J. Vet. Diagn. Invest. 2012, 24, 918–923. [Google Scholar] [CrossRef]
- Aronhime, S.; Calcagno, C.; Jajamovich, G.H.; Dyvorne, H.A.; Robson, P.; Dieterich, D.; Fiel, M.I.; Martel-Laferriere, V.; Chatterji, M.; Rusinek, H.; et al. DCE-MRI of the liver: Effect of linear and non linear conversions on hepatic perfusion quantification and reproducibility. J. Magn. Reson. Imaging 2014, 40, 90. [Google Scholar] [CrossRef]
- Tecles, F.; Fuentes, P.; Martínez Subiela, S.; Parra, M.D.; Muñoz, A.; Cerón, J.J. Analytical validation of commercially available methods for acute phase proteins quantification in pigs. Res. Vet. Sci. 2007, 83, 133–139. [Google Scholar] [CrossRef]
- Ciardelli, L.; Garofoli, F.; Stronati, M.; Mazzucchelli, I.; Avanzini, M.A.; Figar, T.; Gasparoni, A.; De Silvestri, A.; Sabatino, G.; Chirico, G.; et al. Human colostrum T lymphocytes and their effector cytokines actively aid the development of the newborn immune system. Int. J. Immunopathol. Pharmacol. 2008, 21, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Dahl, J.; Jacobsen, S.; Jacobson, M.; Andersen, P.H.; Bækbo, P.; Escribano, D.; Cerón, J.J.; Tecles, F. Changes of adenosine deaminase activity in serum and saliva around parturition in sows with and without postpartum dysgalactia syndrome. BMC Vet. Res. 2021, 17, 352. [Google Scholar] [CrossRef] [PubMed]
- Botía, M.; Ortín-Bustillo, A.; López-Martínez, M.J.; Fuentes, P.; Escribano, D.; González-Bulnes, A.; Manzanilla, E.G.; Martínez-Subiela, S.; Tvarijonaviciute, A.; López-Arjona, M.; et al. Gaining knowledge about biomarkers of the immune system and inflammation in the saliva of pigs: The case of myeloperoxidase, S100A12, and ITIH4. Res. Vet. Sci. 2023, 164, 104997. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase. Proc. Assoc. Am. Physicians 1999, 111, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Buescher, E.S.; McIlheran, S.M. Antioxidant Properties of Human Colostrum. Pediatr. Res. 1988, 24, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, J.L.; Bannerman, D.D.; Shefcheck, K.; Ward, J.L. Proteomic analysis of differentially expressed proteins in bovine milk during experimentally induced Escherichia coli mastitis. J. Dairy Sci. 2008, 91, 4206–4218. [Google Scholar] [CrossRef]
- Prata, M.D.M.G.; Havt, A.; Bolick, D.T.; Pinkerton, R.; Lima, A.A.M.; Guerrant, R.L. Comparisons between myeloperoxidase, lactoferrin, calprotectin and lipocalin-2, as fecal biomarkers of intestinal inflammation in malnourished children. J. Transl. Sci. 2016, 2, 134. [Google Scholar] [CrossRef]
- López-Arjona, M.; Tecles, F.; Mateo, S.V.; Contreras-Aguilar, M.D.; Martínez-Miró, S.; Cerón, J.J.; Martínez-Subiela, S. Measurement of cortisol, cortisone and 11β-hydroxysteroid dehydrogenase type 2 activity in hair of sows during different phases of the reproductive cycle. Vet. J. 2020, 259, 105458. [Google Scholar] [CrossRef]
- Botia, M.; Escribano, D.; Tecles, F.; Martínez-Subiela, S.; Cerón, J.J.; López-Arjona, M. Changes in cortisol, cortisone and 11β-hydroxysteroid dehydrogenase type II activity in saliva during pregnancy and lactation in sows. Domest. Anim. Endocrinol. 2024, 89, 106875. [Google Scholar] [CrossRef]
- Zielinska-Pukos, M.A.; Bryś, J.; Kucharz, N.; Chrobak, A.; Wesolowska, A.; Grabowicz-Chądrzyńska, I.; Hamulka, J. Factors Influencing Cortisol Concentrations in Breastmilk and Its Associations with Breastmilk Composition and Infant Development in the First Six Months of Lactation. Int. J. Environ. Res. Public Health 2022, 19, 14809. [Google Scholar] [CrossRef]
- Kaiser, M.; Jacobsen, S.; Andersen, P.H.; Bækbo, P.; Cerón, J.J.; Dahl, J.; Escribano, D.; Theil, P.K.; Jacobson, M. Hormonal and metabolic indicators before and after farrowing in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 2018, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Vacaru, S.V.; Brett, B.E.; Eckermann, H.; de Weerth, C. Determinants of maternal breast milk cortisol increase: Examining dispositional and situational factors. Psychoneuroendocrinology 2023, 158, 106385. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, A.M.; Murphy, A.M.; Guitarra, D.; Slonecker, E.; Suomi, S.J.; Rosenberg, K.L.; Novak, M.A.; Meyer, J.S.; Hinde, K. Cortisol in Neonatal Mother’s Milk Predicts Later Infant Social and Cognitive Functioning in Rhesus Monkeys. Child. Dev. 2018, 89, 525–538. [Google Scholar] [CrossRef]
- Hinde, K.; Skibiel, A.L.; Foster, A.B.; Rosso LDel Mendoza, S.P.; Capitanio, J.P. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav. Ecol. 2015, 26, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.B.; Mehta, N.R.; Hamosh, M. Alpha-Amylase in preterm human milk. J. Pediatr. Gastroenterol. Nutr. 1982, 1, 43–48. [Google Scholar] [CrossRef]
- Yang, M.; Yue, X.; Xu, X.; Wang, Y.; Wu, J.; Wu, R. Comparison of Milk Enzyme Activity in Different Lactation Periods. IERI Procedia 2014, 8, 46–51. [Google Scholar] [CrossRef][Green Version]
- Sureda, E.A.; Pierzynowska, K.; Weström, B.; Sangild, P.T.; Thymann, T. Exocrine Pancreatic Maturation in Pre-term and Term Piglets Supplemented with Bovine Colostrum. Front. Nutr. 2021, 8, 687056. [Google Scholar] [CrossRef]
- Takashi, K.; Kayoko, S. Properties of amylase-linked immunoglobulins. Clin. Chim. Acta 1977, 76, 67–77. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Francisco, S. Macroamylasemia and Other Immunoglobulin-Complexed Enzyme Disorders. West. J. Med. 1980, 133, 392. [Google Scholar]

| Analyte | Mean | SD | CV (%) |
|---|---|---|---|
| tADA (IU/L) | |||
| Intra-assay | |||
| High concentration | 224.1 | 4.9 | 2.2 |
| Low concentration | 29.4 | 0.6 | 2 |
| Inter-assay | |||
| High concentration | 206.4 | 15.7 | 7.6 |
| Low concentration | 30.6 | 0.7 | 2.3 |
| ADA2 (IU/L) | |||
| Intra-assay | |||
| High concentration | 47 | 4.9 | 10.5 |
| Low concentration | 13.5 | 0.9 | 6.8 |
| Inter-assay | |||
| High concentration | 46.2 | 5 | 10.9 |
| Low concentration | 13 | 1.4 | 10.7 |
| Mpx (IU/L) | |||
| Intra-assay | |||
| High concentration | 5076 | 16.5 | 1.4 |
| Low concentration | 669.3 | 13.5 | 2 |
| Inter-assay | |||
| High concentration | 4629 | 754.2 | 16.2 |
| Low concentration | 606.4 | 82.4 | 13.6 |
| S100A8/A9 (mg/L) | |||
| Intra-assay | |||
| High concentration | 1.42 | 0.005 | 0.4 |
| Low concentration | 0.11 | 0.005 | 4.9 |
| Inter-assay | |||
| High concentration | 1.44 | 0.05 | 3.2 |
| Low concentration | 0.18 | 0.01 | 7.8 |
| S100A12 (mg/L) | |||
| Intra-assay | |||
| High concentration | 1155 | 38.8 | 3.4 |
| Low concentration | 157.9 | 10.3 | 6.5 |
| Inter-assay | |||
| High concentration | 1072 | 82.7 | 12.9 |
| Low concentration | 140.9 | 13.1 | 9.3 |
| Analyte | Mean | SD | CV (%) |
|---|---|---|---|
| Alpha-amylase (IU/L) | |||
| Intra-assay | |||
| High concentration | 1196 | 16.5 | 1.4 |
| Low concentration | 401.5 | 1.9 | 0.5 |
| Inter-assay | |||
| High concentration | 1181 | 11.3 | 1 |
| Low concentration | 401.7 | 6.4 | 1.6 |
| Cortisol (μg/dL) | |||
| Intra-assay | |||
| High concentration | 3.24 | 0.12 | 3.57 |
| Low concentration | 0.6 | 0.1 | 10.4 |
| Inter-assay | |||
| High concentration | 3.51 | 0.35 | 9.9 |
| Low concentration | 0.58 | 0.06 | 10.4 |
| Analyte | Value | Slope | Y-Intercept | R2 |
|---|---|---|---|---|
| tADA (IU/L) | ||||
| High concentration | 229.2 | 1 | 11.2 | 0.99 |
| Low concentration | 28.8 | 1.09 | 3.17 | 0.99 |
| ADA2 (IU/L) | ||||
| High concentration | 47.2 | 1 | 0.55 | 0.99 |
| Low concentration | 13.5 | 0.93 | 0.98 | 0.99 |
| Mpx (IU/L) | ||||
| High concentration | 4927 | 0.98 | 167.9 | 0.99 |
| Low concentration | 661.5 | 0.98 | 16.85 | 0.99 |
| S100A8/A9 (mg/L) | ||||
| High concentration | 1.42 | 0.97 | 0.06 | 0.95 |
| Low concentration | 0.23 | 0.84 | 0.02 | 0.99 |
| S100A12 (mg/L) | ||||
| High concentration | 1613 | 0.99 | 22.27 | 0.99 |
| Low concentration | 139.8 | 1 | 0.23 | 0.99 |
| Analyte | Value | Slope | Y-Intercept | R2 |
|---|---|---|---|---|
| Alpha-amylase (IU/L) | ||||
| High concentration | 1215 | 1 | 31.9 | 0.99 |
| Low concentration | 400.8 | 0.99 | 5.53 | 0.99 |
| Cortisol (μg/dL) | ||||
| High concentration | 3.24 | 0.97 | 0.16 | 0.99 |
| Low concentration | 0.72 | 0.97 | 0.03 | 0.99 |
| Analyte/Time | Median | SD | SE | p-Value * |
|---|---|---|---|---|
| tADA (IU/L) | ||||
| T1 | 34.3 | 11.4 | 2.9 | |
| T9 | 31.5 | 35.8 | 8.9 | >0.99 |
| T20 | 21.3 | 27.7 | 6.3 | 0.02 a |
| ADA1 (IU/L) | ||||
| T1 | 21.5 | 10.4 | 2.6 | |
| T9 | 27.4 | 25.9 | 6.5 | >0.99 |
| T20 | 18.35 | 25.2 | 6.3 | 0.23 |
| ADA2 (IU/L) | ||||
| T1 | 6.7 | 5.9 | 1.5 | |
| T9 | 5.0 | 17.5 | 4.4 | 0.75 |
| T20 | 3.8 | 9.1 | 2.3 | 0.005 a |
| Mpx (IU/L) | ||||
| T1 | 901 | 824 | 212 | |
| T9 | 2171 | 7033 | 1816 | 0.03 b |
| T20 | 1749 | 1816 | 333 | 0.05 |
| S100A8/A9 (mg/L) | ||||
| T1 | 0.2 | 0.2 | 0.004 | |
| T9 | 0.3 | 5.6 | 1.4 | 0.1 |
| T20 | 0.4 | 3.6 | 0.9 | 0.2 |
| S100A12 (mg/L) | ||||
| T1 | 49.1 | 87.5 | 21.9 | |
| T9 | 38.2 | 76.4 | 19.1 | >0.99 |
| T20 | 78.6 | 84.2 | 21.1 | >0.99 |
| Analyte/Time | Median | SD | SE | p Value * |
|---|---|---|---|---|
| Alpha-amylase (IU/L) | ||||
| T1 | 482 | 360 | 90.0 | |
| T9 | 128 | 239 | 59.8 | 0.06 |
| T20 | 120 | 57.9 | 14.5 | 0.0001 a |
| Cortisol (μg/dL) | ||||
| T1 | 1.0 | 0.8 | 0.2 | |
| T9 | 0.6 | 0.5 | 0.1 | >0.99 |
| T20 | 0.5 | 0.3 | 0.1 | 0.04 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botía, M.; Escribano, D.; Mainau, E.; Muñoz-Prieto, A.; Cerón, J.J. Measurement of New Biomarkers of Immunity and Welfare in Colostrum and Milk of Pigs: Analytical Validation and Changes During Lactation. Biology 2024, 13, 829. https://doi.org/10.3390/biology13100829
Botía M, Escribano D, Mainau E, Muñoz-Prieto A, Cerón JJ. Measurement of New Biomarkers of Immunity and Welfare in Colostrum and Milk of Pigs: Analytical Validation and Changes During Lactation. Biology. 2024; 13(10):829. https://doi.org/10.3390/biology13100829
Chicago/Turabian StyleBotía, María, Damián Escribano, Eva Mainau, Alberto Muñoz-Prieto, and José J. Cerón. 2024. "Measurement of New Biomarkers of Immunity and Welfare in Colostrum and Milk of Pigs: Analytical Validation and Changes During Lactation" Biology 13, no. 10: 829. https://doi.org/10.3390/biology13100829
APA StyleBotía, M., Escribano, D., Mainau, E., Muñoz-Prieto, A., & Cerón, J. J. (2024). Measurement of New Biomarkers of Immunity and Welfare in Colostrum and Milk of Pigs: Analytical Validation and Changes During Lactation. Biology, 13(10), 829. https://doi.org/10.3390/biology13100829








