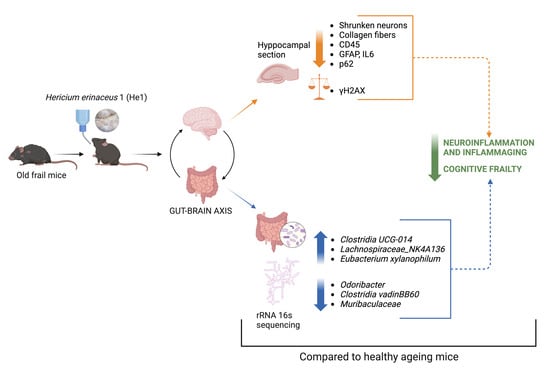
Hericium erinaceus Extract Exerts Beneficial Effects on Gut–Neuroinflammaging–Cognitive Axis in Elderly Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
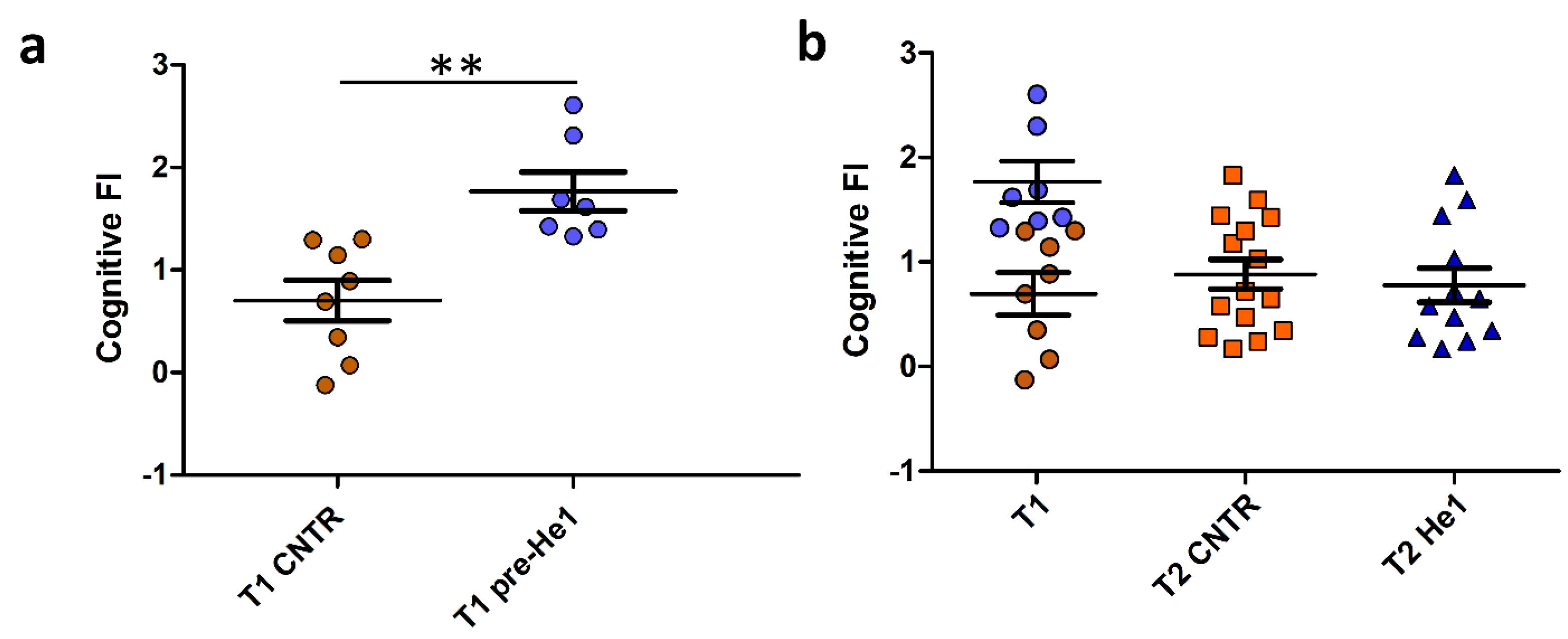
2.2. Experimental Plan, Behavioural Tests, and Cognitive Frailty Index Measurement
2.3. Hericium erinaceus Extracts: Content and Metabolites
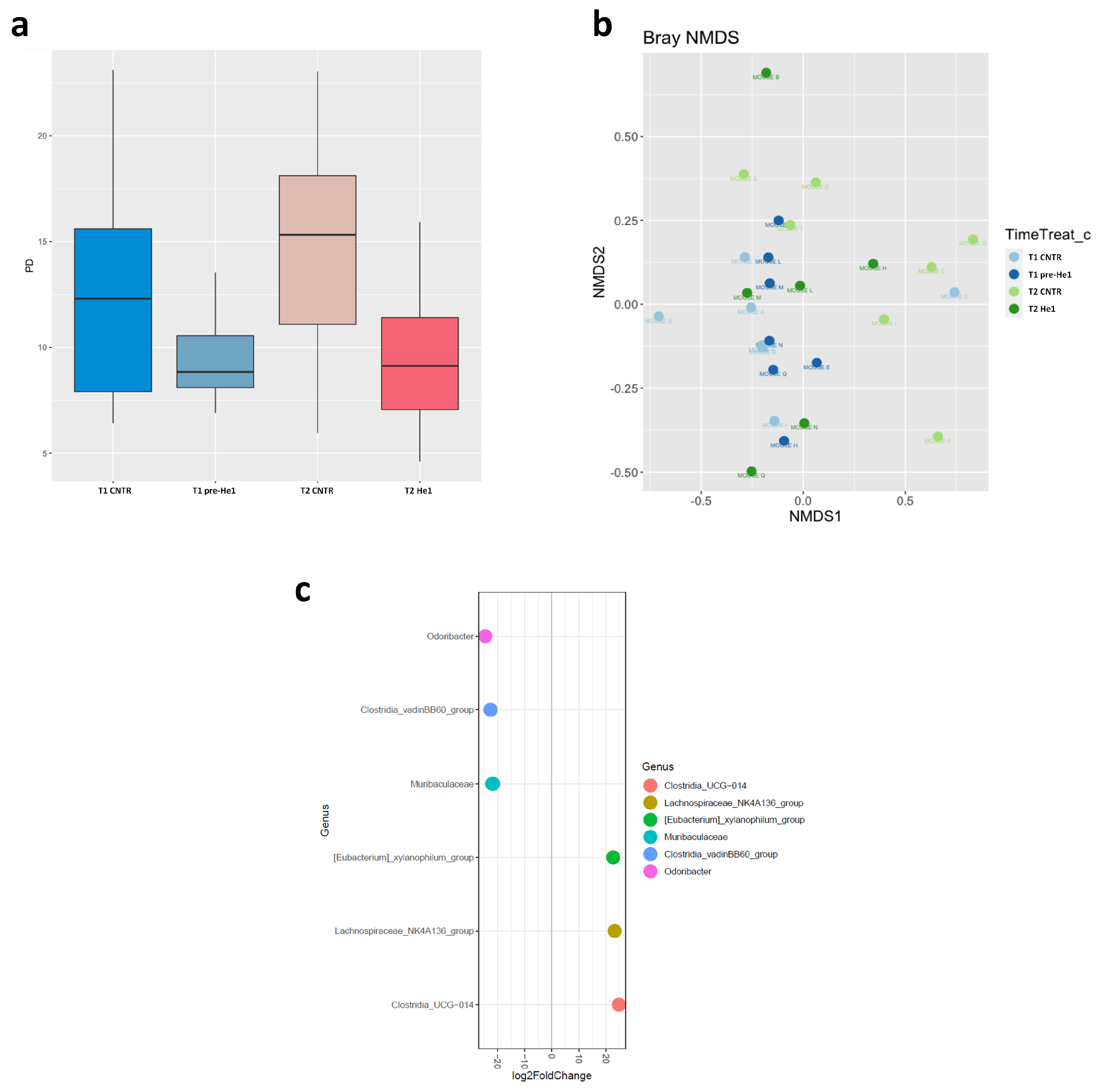
2.4. Bacterial DNA Extraction, 16s rRNA Sequencing, Illumina Data Processing, and Gut Microbiome Characterisation
2.5. Necropsy and Brain Specimen Preparation
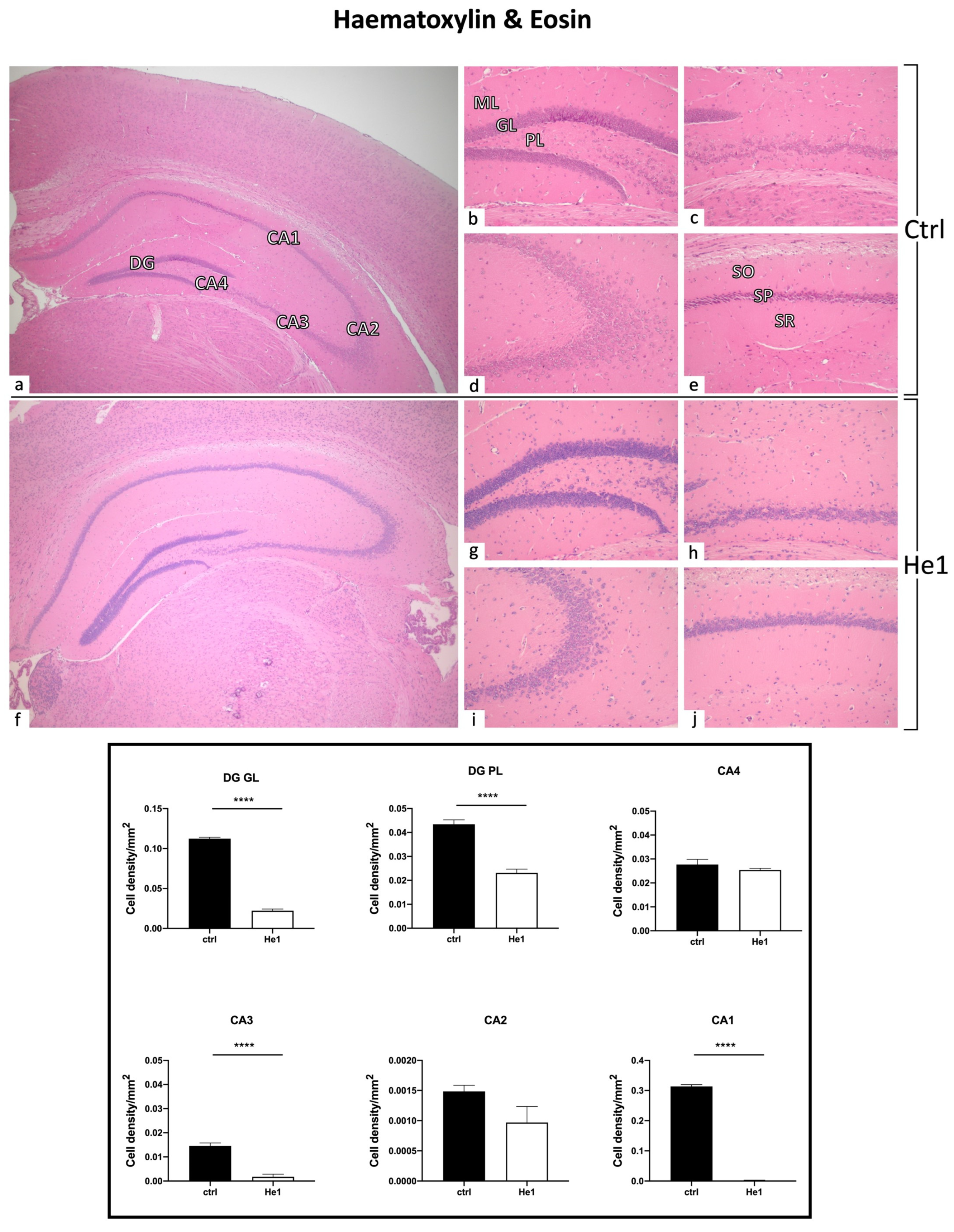
2.6. Haematoxylin and Eosin (H&E) Staining
2.7. Picrosirius Red (PSR) Staining
2.8. Immunohistochemical and Immunofluorescence Assessment and Quantitative Evaluations
2.9. Statistics
3. Results
3.1. Metabolites in Hericium erinaceus Extract (He1)
3.2. Cognitive Frailty Index as Selection Criterion for Mice Recruitment
3.3. The Effect of He1 Treatment on the Gut Microbiome Composition during Ageing
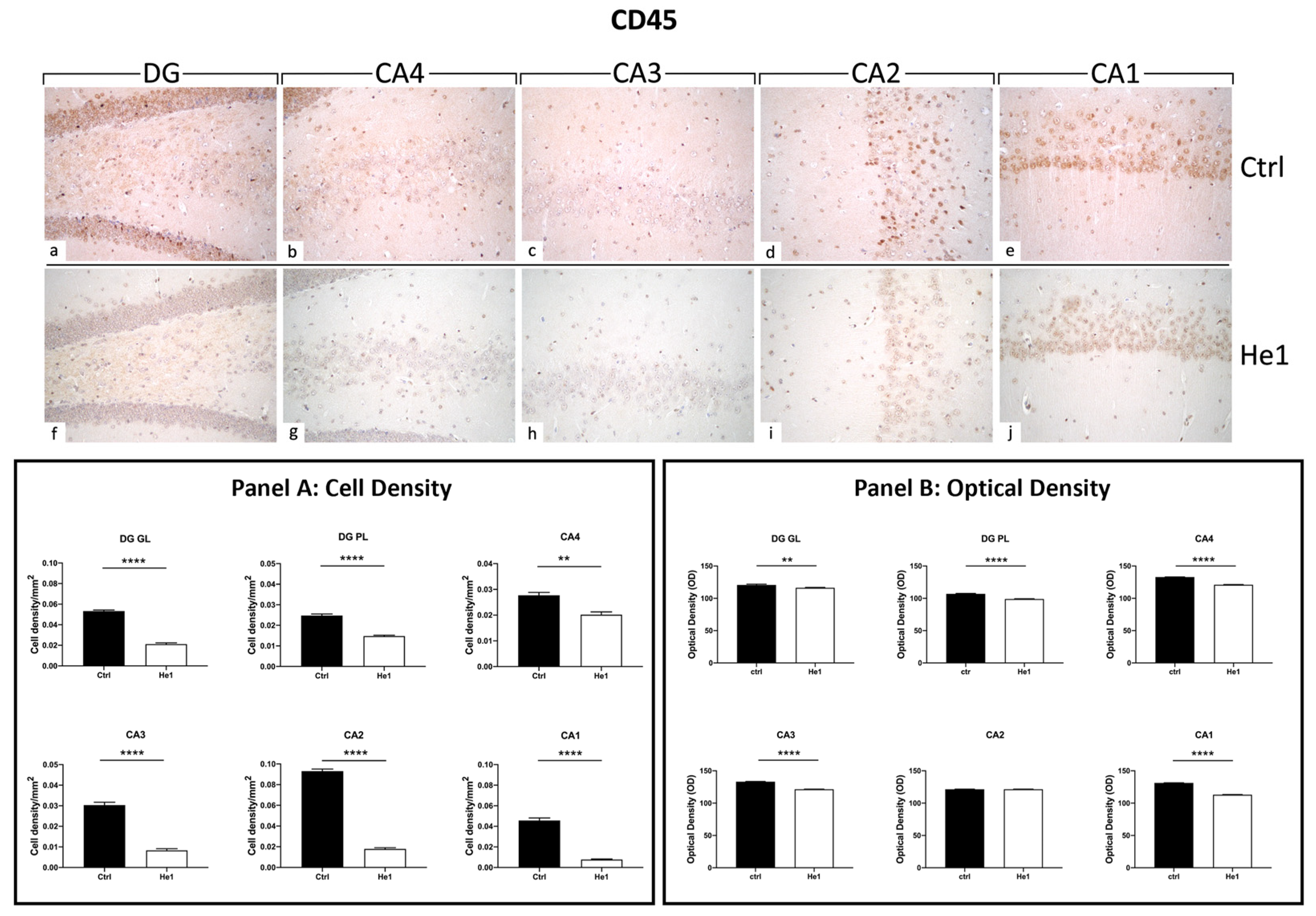
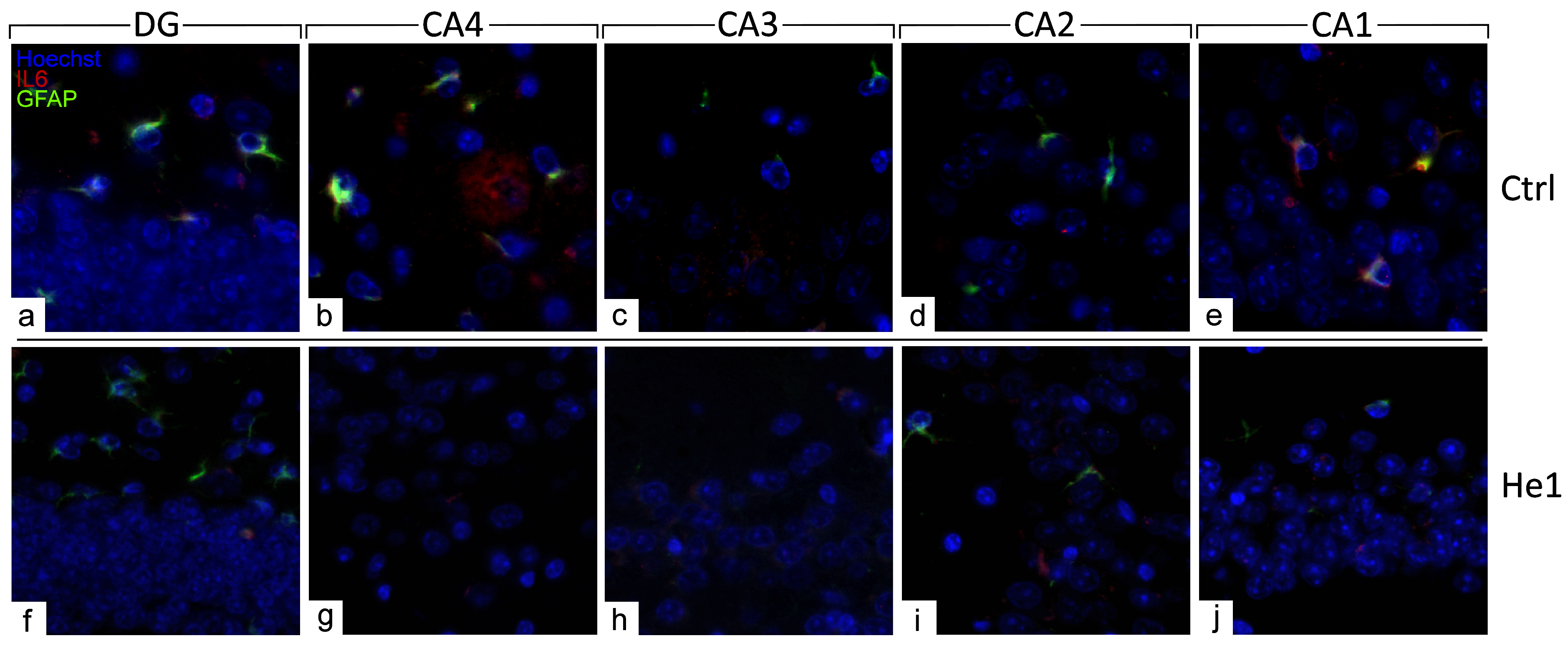
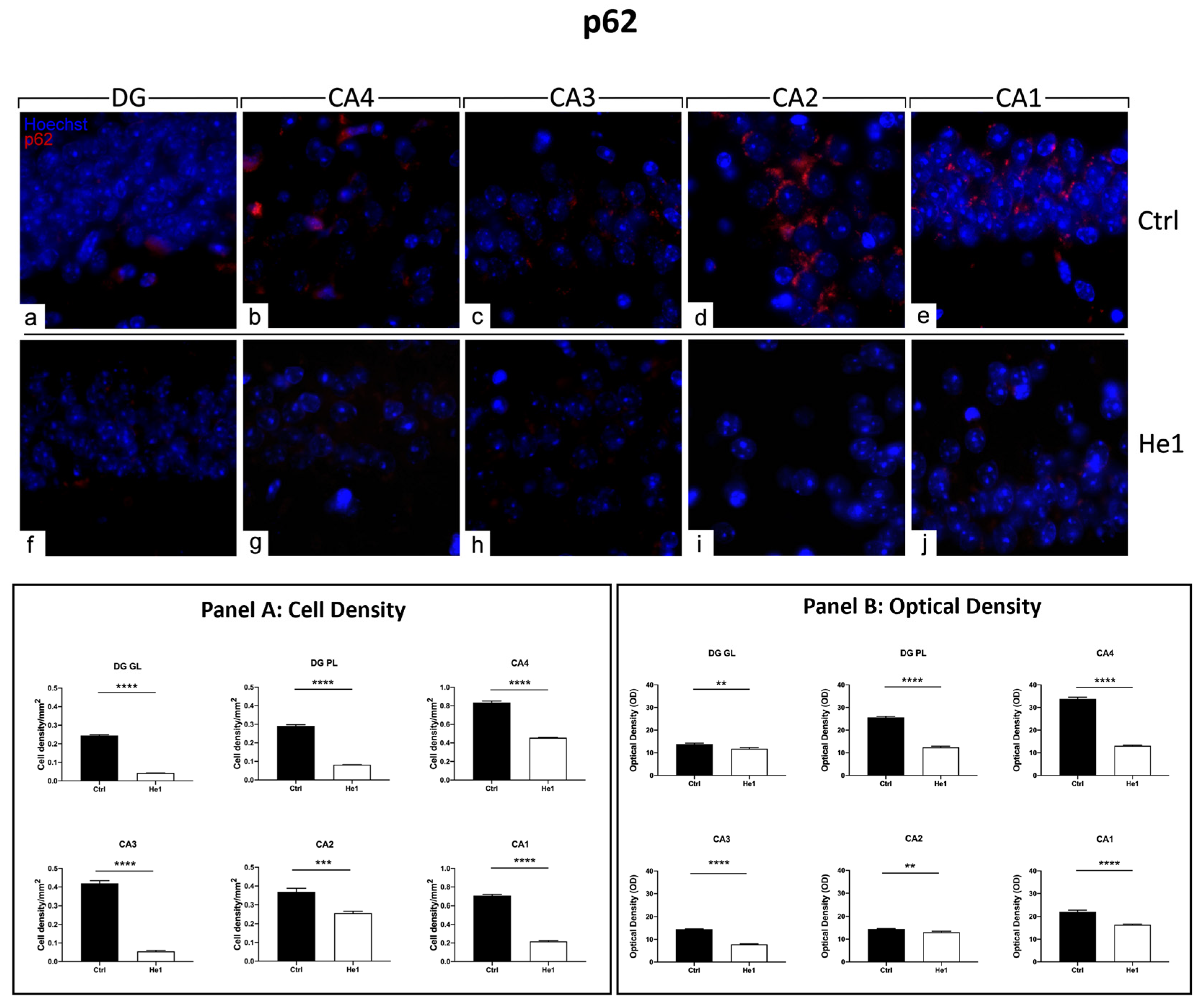
3.4. Light Microscopy Evaluation and Immunohistochemical Study
3.4.1. He1 Supplementation Preserves Healthy Hippocampus Cytoarchitecture
3.4.2. Picrosirius Red Staining: Fibrillar Collagen Network Evaluation
3.4.3. He1 Supplementation Decreases Microglia Activation
3.4.4. He1 Supplement Reduces Neuroinflammaging
3.4.5. He1 Supplement Counteracts Autophagy Pathway Activation
3.4.6. He1 Supplement Reduces Cellular Senescence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seals, D.R.; Justice, J.N.; LaRocca, T.J. Physiological Geroscience: Targeting Function to Increase Healthspan and Achieve Optimal Longevity. J. Physiol. 2016, 594, 2001–2024. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) Approach to Healthy Ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The Aging Process and Potential Interventions to Extend Life Expectancy. Clin. Interv. Aging 2007, 2, 401–412. [Google Scholar] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in Elderly People. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Kelaiditi, E.; Cesari, M.; Canevelli, M.; van Kan, G.A.; Ousset, P.-J.; Gillette-Guyonnet, S.; Ritz, P.; Duveau, F.; Soto, M.E.; Provencher, V.; et al. Cognitive Frailty: Rational and Definition from an (I.A.N.A./I.A.G.G.) International Consensus Group. J. Nutr. Health Aging 2013, 17, 726–734. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Eichenbaum, H. The Episodic Memory System: Neurocircuitry and Disorders. Neuropsychopharmacology 2010, 35, 86–104. [Google Scholar] [CrossRef]
- Geinisman, Y.; Detoledo-Morrell, L.; Morrell, F.; Heller, R.E. Hippocampal Markers of Age-Related Memory Dysfunction: Behavioral, Electrophysiological and Morphological Perspectives. Prog. Neurobiol. 1995, 45, 223–252. [Google Scholar] [CrossRef]
- Weerasinghe-Mudiyanselage, P.D.E.; Ang, M.J.; Kang, S.; Kim, J.-S.; Moon, C. Structural Plasticity of the Hippocampus in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 3349. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Thacker, J.S.; Rodgers, S.P.; Brocardo, P.S.; Christie, B.R.; Gil-Mohapel, J. Interplay between Hormones and Exercise on Hippocampal Plasticity across the Lifespan. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165821. [Google Scholar] [CrossRef]
- Driscoll, I.; Davatzikos, C.; An, Y.; Wu, X.; Shen, D.; Kraut, M.; Resnick, S.M. Longitudinal Pattern of Regional Brain Volume Change Differentiates Normal Aging from MCI. Neurology 2009, 72, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; Erickson, K.I.; Rodrigue, K.M.; Voss, M.W.; Colcombe, S.J.; Kramer, A.F.; Acker, J.D.; Raz, N. Age-Related Differences in Regional Brain Volumes: A Comparison of Optimized Voxel-Based Morphometry to Manual Volumetry. Neurobiol. Aging 2009, 30, 1657–1676. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.D.; Liu, X.-B.; King, A.N.; Luu, J.D.; Cheng, H.-J. Age-Related Changes in Synaptic Plasticity Associated with Mossy Fiber Terminal Integration during Adult Neurogenesis. eNeuro 2020, 7, ENEURO.0030-20.2020. [Google Scholar] [CrossRef]
- Babcock, K.R.; Page, J.S.; Fallon, J.R.; Webb, A.E. Adult Hippocampal Neurogenesis in Aging and Alzheimer’s Disease. Stem Cell Rep. 2021, 16, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.G.; Walker, R.; McGeer, E.G. Patterns of Gliosis in Alzheimer’s Disease and Aging Cerebrum. Glia 1989, 2, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Capilla-Gonzalez, V.; Herranz-Pérez, V.; García-Verdugo, J.M. The Aged Brain: Genesis and Fate of Residual Progenitor Cells in the Subventricular Zone. Front. Cell. Neurosci. 2015, 9, 365. [Google Scholar] [CrossRef]
- Roda, E.; De Luca, F.; Ratto, D.; Priori, E.C.; Savino, E.; Bottone, M.G.; Rossi, P. Cognitive Healthy Aging in Mice: Boosting Memory by an Ergothioneine-Rich Hericium erinaceus Primordium Extract. Biology 2023, 12, 196. [Google Scholar] [CrossRef]
- Unger, J.W. Glial Reaction in Aging and Alzheimer’s Disease. Microsc. Res. Tech. 1998, 43, 24–28. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune-Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Bird, C.M. The Role of the Hippocampus in Recognition Memory. Cortex 2017, 93, 155–165. [Google Scholar] [CrossRef]
- Gasiorowska, A.; Wydrych, M.; Drapich, P.; Zadrozny, M.; Steczkowska, M.; Niewiadomski, W.; Niewiadomska, G. The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front. Aging Neurosci. 2021, 13, 654931. [Google Scholar] [CrossRef]
- Roda, E.; Ratto, D.; De Luca, F.; Desiderio, A.; Ramieri, M.; Goppa, L.; Savino, E.; Bottone, M.G.; Locatelli, C.A.; Rossi, P. Searching for a Longevity Food, We Bump into Hericium erinaceus Primordium Rich in Ergothioneine: The “Longevity Vitamin” Improves Locomotor Performances during Aging. Nutrients 2022, 14, 1177. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Bana, B.; Cabreiro, F. The Microbiome and Aging. Annu. Rev. Genet. 2019, 53, 239–261. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jackson, M.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of Early Frailty in the Gut Microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut Microbiome and Aging: Physiological and Mechanistic Insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef]
- Ratto, D.; Roda, E.; Romeo, M.; Venuti, M.T.; Desiderio, A.; Lupo, G.; Capelli, E.; Sandionigi, A.; Rossi, P. The Many Ages of Microbiome–Gut–Brain Axis. Nutrients 2022, 14, 2937. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Wasén, C.; Cox, L.M. The Gut Microbiota Is an Emerging Target for Improving Brain Health during Ageing. Gut Microbiome 2023, 4, E2. [Google Scholar] [CrossRef]
- Tooley, K.L. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium erinaceus: An Edible Mushroom with Medicinal Values. J. Complement. Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef]
- Cesaroni, V.; Brusoni, M.; Cusaro, C.M.; Girometta, C.; Perini, C.; Picco, A.M.; Rossi, P.; Salerni, E.; Savino, E. Phylogenetic Comparison between Italian and Worldwide Hericium Species (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 943–954. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E.; et al. Hericium erinaceus in Neurodegenerative Diseases: From Bench to Bedside and Beyond, How Far from the Shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus Improves Recognition Memory and Induces Hippocampal and Cerebellar Neurogenesis in Frail Mice during Aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective Metabolites of Hericium erinaceus Promote Neuro-Healthy Aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef]
- Brandalise, F.; Cesaroni, V.; Gregori, A.; Repetti, M.; Romano, C.; Orrù, G.; Botta, L.; Girometta, C.; Guglielminetti, M.L.; Savino, E.; et al. Dietary Supplementation of Hericium erinaceus Increases Mossy Fiber-CA3 Hippocampal Neurotransmission and Recognition Memory in Wild-Type Mice. Evid. Based Complement. Alternat Med. 2017, 2017, 3864340. [Google Scholar] [CrossRef]
- Corana, F.; Cesaroni, V.; Mannucci, B.; Baiguera, R.M.; Picco, A.M.; Savino, E.; Ratto, D.; Perini, C.; Kawagishi, H.; Girometta, C.E.; et al. Array of Metabolites in Italian Hericium erinaceus Mycelium, Primordium, and Sporophore. Molecules 2019, 24, 3511. [Google Scholar] [CrossRef]
- Roda, E.; Bottone, M.G.; Biggiogera, M.; Milanesi, G.; Coccini, T. Pulmonary and Hepatic Effects after Low Dose Exposure to Nanosilver: Early and Long-Lasting Histological and Ultrastructural Alterations in Rat. Toxicol. Rep. 2019, 6, 1047–1060. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Pang, S.; Lu, Z.; Jiang, J.; Zhao, L.; Lin, L.; Li, X.; Lian, T.; Huang, M.; Yang, W.; Feng, Q. Hippocampus Segmentation Based on Iterative Local Linear Mapping with Representative and Local Structure-Preserved Feature Embedding. IEEE Trans. Med. Imaging 2019, 38, 2271–2280. [Google Scholar] [CrossRef]
- Chao, O.Y.; de Souza Silva, M.A.; Yang, Y.-M.; Huston, J.P. The Medial Prefrontal Cortex-Hippocampus Circuit That Integrates Information of Object, Place and Time to Construct Episodic Memory in Rodents: Behavioral, Anatomical and Neurochemical Properties. Neurosci. Biobehav. Rev. 2020, 113, 373–407. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Kitt, M.M.; Watkins, L.R.; Maier, S.F. Neuroinflammation in the Normal Aging Hippocampus. Neuroscience 2015, 309, 84–99. [Google Scholar] [CrossRef]
- Luca, F.D.; Roda, E.; Ratto, D.; Desiderio, A.; Venuti, M.T.; Ramieri, M.; Bottone, M.G.; Savino, E.; Rossi, P. Fighting Secondary Triple-Negative Breast Cancer in Cerebellum: A Powerful Aid from a Medicinal Mushrooms Blend. Biomed. Pharmacother. 2023, 159, 114262. [Google Scholar] [CrossRef]
- Benmamar-Badel, A.; Owens, T.; Wlodarczyk, A. Protective Microglial Subset in Development, Aging, and Disease: Lessons From Transcriptomic Studies. Front. Immunol. 2020, 11, 430. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312. [Google Scholar] [CrossRef]
- Islam, M.A.; Sooro, M.A.; Zhang, P. Autophagic Regulation of P62 Is Critical for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 1405. [Google Scholar] [CrossRef]
- Valente, D.; Gentileschi, M.P.; Guerrisi, A.; Bruzzaniti, V.; Morrone, A.; Soddu, S.; Verdina, A. Factors to Consider for the Correct Use of ΓH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation. Cancers 2022, 14, 6204. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Chen, L.-H.; Wang, M.-F.; Chang, C.-C.; Huang, S.-Y.; Pan, C.-H.; Yeh, Y.-T.; Huang, C.-H.; Chan, C.-H.; Huang, H.-Y. Lacticaseibacillus Paracasei PS23 Effectively Modulates Gut Microbiota Composition and Improves Gastrointestinal Function in Aged SAMP8 Mice. Nutrients 2021, 13, 1116. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Solanki, R.; Karande, A.; Ranganathan, P. Emerging Role of Gut Microbiota Dysbiosis in Neuroinflammation and Neurodegeneration. Front. Neurol. 2023, 14, 1149618. [Google Scholar] [CrossRef]
- Costea, L.; Mészáros, Á.; Bauer, H.; Bauer, H.-C.; Traweger, A.; Wilhelm, I.; Farkas, A.E.; Krizbai, I.A. The Blood–Brain Barrier and Its Intercellular Junctions in Age-Related Brain Disorders. Int. J. Mol. Sci. 2019, 20, 5472. [Google Scholar] [CrossRef]
- Liu, P.; Gao, M.; Liu, Z.; Zhang, Y.; Tu, H.; Lei, L.; Wu, P.; Zhang, A.; Yang, C.; Li, G.; et al. Gut Microbiome Composition Linked to Inflammatory Factors and Cognitive Functions in First-Episode, Drug-Naive Major Depressive Disorder Patients. Front. Neurosci. 2021, 15, 800764. [Google Scholar] [CrossRef] [PubMed]
- Chesnokova, V.; Pechnick, R.N.; Wawrowsky, K. Chronic Peripheral Inflammation, Hippocampal Neurogenesis, and Behavior. Brain Behav. Immun. 2016, 58, 1–8. [Google Scholar] [CrossRef]
- Umu, Ö.C.O.; Rudi, K.; Diep, D.B. Modulation of the Gut Microbiota by Prebiotic Fibres and Bacteriocins. Microb. Ecol. Health Dis. 2017, 28, 1348886. [Google Scholar] [CrossRef]
- Fernandes, A.; Nair, A.; Kulkarni, N.; Todewale, N.; Jobby, R. Exploring Mushroom Polysaccharides for the Development of Novel Prebiotics: A Review. Int. J. Med. Mushrooms 2023, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, E3759. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, E.; Kloosterhuis, N.J.; Koster, M.; Dekker, D.C.; Gijbels, M.J.J.; van der Velden, S.; Ríos-Morales, M.; van Faassen, M.J.R.; Loreti, M.G.; de Bruin, A.; et al. A Proinflammatory Gut Microbiota Increases Systemic Inflammation and Accelerates Atherosclerosis. Circ. Res. 2019, 124, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Quan, M.; Zhao, H.; Jia, J. Gut Microbiota Changes and Their Correlation with Cognitive and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 81, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Van Averbeke, V.; Berkell, M.; Mysara, M.; Rodriguez-Ruiz, J.P.; Xavier, B.B.; De Winter, F.H.R.; Jongers, B.S.; Jairam, R.K.; Hotterbeekx, A.; Goossens, H.; et al. Host Immunity Influences the Composition of Murine Gut Microbiota. Front. Immunol. 2022, 13, 828016. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Lu, J.; Lin, W.; Wei, X.; Li, H.; Zhao, X.; Jiang, A.; Yuan, J. Effects of a Galacto-Oligosaccharide-Rich Diet on Fecal Microbiota and Metabolite Profiles in Mice. Food Funct. 2018, 9, 1612–1620. [Google Scholar] [CrossRef]
- Chen, H.; Ye, C.; Cai, B.; Zhang, F.; Wang, X.; Zhang, J.; Zhang, Z.; Guo, Y.; Yao, Q. Berberine Inhibits Intestinal Carcinogenesis by Suppressing Intestinal Pro-Inflammatory Genes and Oncogenic Factors through Modulating Gut Microbiota. BMC Cancer 2022, 22, 566. [Google Scholar] [CrossRef]
- Song, H.; Wang, W.; Shen, B.; Jia, H.; Hou, Z.; Chen, P.; Sun, Y. Pretreatment with Probiotic Bifico Ameliorates Colitis-Associated Cancer in Mice: Transcriptome and Gut Flora Profiling. Cancer Sci. 2018, 109, 666–677. [Google Scholar] [CrossRef]
- Hardham, J.M.; King, K.W.; Dreier, K.; Wong, J.; Strietzel, C.; Eversole, R.R.; Sfintescu, C.; Evans, R.T. Transfer of Bacteroides Splanchnicus to Odoribacter Gen. Nov. as Odoribacter Splanchnicus Comb. Nov., and Description of Odoribacter Denticanis Sp. Nov., Isolated from the Crevicular Spaces of Canine Periodontitis Patients. Int. J. Syst. Evol. Microbiol. 2008, 58, 103–109. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable Probiotics Modulate Inflammation and Gut Microbiota for Inflammatory Bowel Disease Treatment after Effective Oral Delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L.; Nie, K. Gut Microbiota Altered in Mild Cognitive Impairment Compared with Normal Cognition in Sporadic Parkinson’s Disease. Front. Neurol. 2020, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Borgo, F.; Macandog, A.D.; Diviccaro, S.; Falvo, E.; Giatti, S.; Cavaletti, G.; Melcangi, R.C. Alterations of Gut Microbiota Composition in Post-Finasteride Patients: A Pilot Study. J. Endocrinol. Investig. 2021, 44, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Peters, B.A.; Lee, S.; Fitzgerald, K.; Wang, Z.; Sollecito, C.C.; Grassi, E.; Wiek, F.; St Peter, L.; D’Souza, G.; et al. Gut Microbiota and Cognitive Function Among Women Living with HIV. J. Alzheimers Dis. 2023, 95, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Ida, M.; Peterson, V.L.; Prenderville, J.A.; Moloney, G.M.; Izumo, T.; Murphy, K.; Murphy, A.; Ross, R.P.; Stanton, C.; et al. Revisiting Metchnikoff: Age-Related Alterations in Microbiota-Gut-Brain Axis in the Mouse. Brain Behav. Immun. 2017, 65, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, L.; Han, L.; Wang, B.; Shi, R.; Ye, J.; Xia, B.; Zhao, Z.; Zhao, B.; Liu, X. Leucine-Restricted Diet Ameliorates Obesity-Linked Cognitive Deficits: Involvement of the Microbiota–Gut–Brain Axis. J. Agric. Food Chem. 2023, 71, 9404–9418. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fu, Y.; Cao, W.-T.; Wang, Z.; Zhang, K.; Jiang, Z.; Jia, X.; Liu, C.-Y.; Lin, H.-R.; Zhong, H.; et al. Gut Microbiome, Cognitive Function and Brain Structure: A Multi-Omics Integration Analysis. Transl. Neurodegener. 2022, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zong, C.; Yu, X.; Ding, Y.; Chang, B.; Wang, R.; Sang, L. Alanyl-Glutamine (Ala-Gln) Ameliorates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by Regulating the Gut Microbiota, PI3K-Akt/NF-ΚB/STAT3 Signaling, and Associated Pulmonary Injury. ACS Infect. Dis. 2023, 9, 979–992. [Google Scholar] [CrossRef]
- Deng, L.; Wojciech, L.; Png, C.W.; Koh, E.Y.; Aung, T.T.; Kioh, D.Y.Q.; Chan, E.C.Y.; Malleret, B.; Zhang, Y.; Peng, G.; et al. Experimental Colonization with Blastocystis ST4 Is Associated with Protective Immune Responses and Modulation of Gut Microbiome in a DSS-Induced Colitis Mouse Model. Cell. Mol. Life Sci. 2022, 79, 245. [Google Scholar] [CrossRef]
- Juckel, G.; Manitz, M.-P.; Freund, N.; Gatermann, S. Impact of Poly I:C Induced Maternal Immune Activation on Offspring’s Gut Microbiome Diversity—Implications for Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110306. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Liu, Z.; Zhang, W.; Zhang, L.; Jing, J.; Gao, A. Genus Unclassified_Muribaculaceae and Microbiota-Derived Butyrate and Indole-3-Propionic Acid Are Involved in Benzene-Induced Hematopoietic Injury in Mice. Chemosphere 2023, 313, 137499. [Google Scholar] [CrossRef]
- Sibai, M.; Altuntaş, E.; Yıldırım, B.; Öztürk, G.; Yıldırım, S.; Demircan, T. Microbiome and Longevity: High Abundance of Longevity-Linked Muribaculaceae in the Gut of the Long-Living Rodent Spalax Leucodon. OMICS 2020, 24, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, M.; Kou, G.; Li, Y. The Relationship between Gut Microbiota and Inflammatory Response, Learning and Memory in Mice by Sleep Deprivation. Front. Cell. Infect. Microbiol. 2023, 13, 1159771. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-T.; Chen, J.-W. Direct CCL4 Inhibition Modulates Gut Microbiota, Reduces Circulating Trimethylamine N-Oxide, and Improves Glucose and Lipid Metabolism in High-Fat-Diet-Induced Diabetes Mellitus. J. Inflamm. Res. 2021, 14, 6237–6250. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, Z.; Jiang, X.; Qu, L.; Zhou, W.; Sun, X.; Hou, J.; Xu, H.; Cheng, M. Gut Microbiota Taxon-Dependent Transformation of Microglial M1/M2 Phenotypes Underlying Mechanisms of Spatial Learning and Memory Impairment after Chronic Methamphetamine Exposure. Microbiol. Spectr. 2023, 11, e0030223. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, X.; Ma, W.; Li, M.; Wen, J.; Chen, C.; Liu, L.; Nie, S. Bioactive Polysaccharides Promote Gut Immunity via Different Ways. Food Funct. 2023, 14, 1387–1400. [Google Scholar] [CrossRef]
- He, Z.; Ma, Y.; Yang, S.; Zhang, S.; Liu, S.; Xiao, J.; Wang, Y.; Wang, W.; Yang, H.; Li, S.; et al. Gut Microbiota-Derived Ursodeoxycholic Acid from Neonatal Dairy Calves Improves Intestinal Homeostasis and Colitis to Attenuate Extended-Spectrum β-Lactamase-Producing Enteroaggregative Escherichia Coli Infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, Y.; Xie, Q.; Lv, X.; Zhou, L.; Smith, E.E.; Cao, T.; Zhang, Y.; Li, B.; Huo, G.; et al. Bifidobacterium Bifidum E3 Combined with Bifidobacterium Longum Subsp. Infantis E4 Improves LPS-Induced Intestinal Injury by Inhibiting the TLR4/NF-ΚB and MAPK Signaling Pathways In Vivo. J. Agric. Food Chem. 2023, 71, 8915–8930. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, R.; Su, K.; Gu, Y.; Fang, L.; Fan, Y.; Gao, J.; Ruan, X.; Feng, X. Acupuncture Ameliorates Breast Cancer-Related Fatigue by Regulating the Gut Microbiota-Gut-Brain Axis. Front. Endocrinol. 2022, 13, 921119. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.-H.; Xue, M.; Raygoza Garay, J.A.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered Gut Microbiome Composition and Function Are Associated with Gut Barrier Dysfunction in Healthy Relatives of Patients with Crohn’s Disease. Gastroenterology 2022, 163, 1364–1376.e10. [Google Scholar] [CrossRef]
- Guo, M.; Xing, D.; Wang, J.; Zhang, Y.; Li, Z.; Jiao, X. Potent Intestinal Mucosal Barrier Enhancement of Nostoc Commune Vaucher Polysaccharide Supplementation Ameliorates Acute Ulcerative Colitis in Mice Mediated by Gut Microbiota. Nutrients 2023, 15, 3054. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L.; et al. Dietary Supplementation of Inulin Ameliorates Subclinical Mastitis via Regulation of Rumen Microbial Community and Metabolites in Dairy Cows. Microbiol. Spectr. 2021, 9, e0010521. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ma, X.; Yang, X.; Chen, Q.; Wen, Z.; Yang, M.; Fu, J.; Yin, T.; Lu, G.; Qi, J.; et al. Natural Shikonin and Acetyl-Shikonin Improve Intestinal Microbial and Protein Composition to Alleviate Colitis-Associated Colorectal Cancer. Int. Immunopharmacol. 2022, 111, 109097. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yin, R.; Yang, Z.; Wei, F.; Hu, J. The Effects of Rhein on D-GalN/LPS-Induced Acute Liver Injury in Mice: Results from Gut Microbiome-Metabolomics and Host Transcriptome Analysis. Front. Immunol. 2022, 13, 971409. [Google Scholar] [CrossRef] [PubMed]
- Marizzoni, M.; Mirabelli, P.; Mombelli, E.; Coppola, L.; Festari, C.; Lopizzo, N.; Luongo, D.; Mazzelli, M.; Naviglio, D.; Blouin, J.-L.; et al. A Peripheral Signature of Alzheimer’s Disease Featuring Microbiota-Gut-Brain Axis Markers. Alzheimers Res. Ther. 2023, 15, 101. [Google Scholar] [CrossRef]
- Zhang, Z.; Cao, H.; Song, N.; Zhang, L.; Cao, Y.; Tai, J. Long-Term Hexavalent Chromium Exposure Facilitates Colorectal Cancer in Mice Associated with Changes in Gut Microbiota Composition. Food Chem. Toxicol. 2020, 138, 111237. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine Improves Gut Barrier Integrity and Gut Microbiota Function in Diet-Induced Obese Mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-Induced Gut Microbiota Reduces Obesity in High-Fat Diet-Fed Mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef]
- Yan, C.; Kwek, E.; Ding, H.-F.; He, Z.; Ma, K.Y.; Zhu, H.; Chen, Z.-Y. Dietary Oxidized Cholesterol Aggravates Chemically Induced Murine Colon Inflammation and Alters Gut Microbial Ecology. J. Agric. Food Chem. 2022, 70, 13289–13301. [Google Scholar] [CrossRef]
- Huang, S.; Pang, D.; Li, X.; You, L.; Zhao, Z.; Cheung, P.C.-K.; Zhang, M.; Liu, D. A Sulfated Polysaccharide from Gracilaria Lemaneiformis Regulates Cholesterol and Bile Acid Metabolism in High-Fat Diet Mice. Food Funct. 2019, 10, 3224–3236. [Google Scholar] [CrossRef]
- Huang, J.-Q.; Wei, S.-Y.; Cheng, N.; Zhong, Y.-B.; Yu, F.-H.; Li, M.-D.; Liu, D.-Y.; Li, S.-S.; Zhao, H.-M. Chimonanthus Nitens Oliv. Leaf Granule Ameliorates DSS-Induced Acute Colitis Through Treg Cell Improvement, Oxidative Stress Reduction, and Gut Microflora Modulation. Front. Cell. Infect. Microbiol. 2022, 12, 907813. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Meng, X.-C.; Dong, Y.-F.; Zhao, X.-H.; Qian, J.-M.; Wang, H.-Y.; Li, J.-N. Effects of Probiotics and Prebiotics on Intestinal Microbiota in Mice with Acute Colitis Based on 16S RRNA Gene Sequencing. Chin. Med. J. 2019, 132, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Duan, W.; Zhang, Z.; Li, S.; Zhao, Y.; Geng, B.; Zheng, Y.; Yu, J.; Wang, M. Polyphenol-Rich Vinegar Extract Regulates Intestinal Microbiota and Immunity and Prevents Alcohol-Induced Inflammation in Mice. Food Res. Int. 2021, 140, 110064. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Yang, J.; Xu, Y.; Kong, X.; Wang, J.; Wang, Y. Alleviation Effects of Grape Seed Proanthocyanidin Extract on Inflammation and Oxidative Stress in a D-Galactose-Induced Aging Mouse Model by Modulating the Gut Microbiota. Food Funct. 2022, 13, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Engertsberger, L.; Komarova, I.; Feldbacher, N.; Leber, B.; Pichler, G.; Fink, N.; Scarpatetti, M.; Schippinger, W.; Schmidt, R.; et al. Dysbiosis, Gut Barrier Dysfunction and Inflammation in Dementia: A Pilot Study. BMC Geriatr. 2020, 20, 248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, H.; Zhou, J.; Wang, S. Chlorogenic Acid Relieves Lead-Induced Cognitive Impairments and Hepato-Renal Damage via Regulating the Dysbiosis of the Gut Microbiota in Mice. Food Funct. 2019, 10, 681–690. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, N.; Wu, Y.; Lu, M.; Wang, Q.; Xia, C.; Zhou, M.; Xu, Y. Urinary Metabolomic Changes and Microbiotic Alterations in Presenilin1/2 Conditional Double Knockout Mice. J. Transl. Med. 2021, 19, 351. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, N.; Lu, M.; Wang, Q.; Zhao, C.; Wang, J.; Zhou, M.; Xu, Y. Effects of Electroacupuncture on Urinary Metabolome and Microbiota in Presenilin1/2 Conditional Double Knockout Mice. Front. Microbiol. 2022, 13, 1047121. [Google Scholar] [CrossRef]
- Van Gylswyk, N.O.; Van der Toorn, J.J.T.K. Eubacterium uniforme Sp. Nov. and Eubacterium xylanophilum Sp. Nov., Fiber-Digesting Bacteria from the Rumina of Sheep Fed Corn Stover. Int. J. Syst. Evol. Microbiol. 1985, 35, 323–326. [Google Scholar] [CrossRef]
- Duncan, S.H.; Russell, W.R.; Quartieri, A.; Rossi, M.; Parkhill, J.; Walker, A.W.; Flint, H.J. Wheat Bran Promotes Enrichment within the Human Colonic Microbiota of Butyrate-Producing Bacteria That Release Ferulic Acid. Environ. Microbiol. 2016, 18, 2214–2225. [Google Scholar] [CrossRef]
- Zhuge, A.; Li, S.; Yuan, Y.; Li, B.; Li, L. The Synergy of Dietary Supplements Lactobacillus Salivarius LI01 and Bifidobacterium Longum TC01 in Alleviating Liver Failure in Rats Treated with D-Galactosamine. Food Funct. 2021, 12, 10239–10252. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut Microbes from the Phylogenetically Diverse Genus Eubacterium and Their Various Contributions to Gut Health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Diling, C.; Longkai, Q.; Yinrui, G.; Yadi, L.; Xiaocui, T.; Xiangxiang, Z.; Miao, Z.; Ran, L.; Ou, S.; Dongdong, W.; et al. CircNF1-419 Improves the Gut Microbiome Structure and Function in AD-Like Mice. Aging. 2020, 12, 260–287. Available online: https://pubmed.ncbi.nlm.nih.gov/31905172/ (accessed on 14 November 2023). [CrossRef] [PubMed]
- Zhang, X.L.; Yu, S.N.; Qu, R.D.; Zhao, Q.Y.; Pan, W.Z.; Chen, X.S.; Zhang, Q.; Liu, Y.; Li, J.; Gao, Y.; et al. Mechanism of Learning and Memory Impairment in Rats Exposed to Arsenic and/or Fluoride Based on Microbiome and Metabolome. Biomed. Environ. Sci. 2023, 36, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Lie, D.C.; Song, H.; Colamarino, S.A.; Ming, G.; Gage, F.H. Neurogenesis in the Adult Brain: New Strategies for Central Nervous System Diseases. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 399–421. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Kim, H.G.; Kim, J.Y.; Kim, S.Y.; Cho, K.-O. Hericium erinaceus Extract Reduces Anxiety and Depressive Behaviors by Promoting Hippocampal Neurogenesis in the Adult Mouse Brain. J. Med. Food 2018, 21, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.L.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Age-Related Changes in Glutamate Release in the CA3 and Dentate Gyrus of the Rat Hippocampus. Neurobiol. Aging 2011, 32, 811–820. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, X.; Zhang, J.; Li, A.; Gong, H.; Luo, Q.; Zhang, H.; Gao, Z.; Jiang, H. High-Resolution Mapping of Brain Vasculature and Its Impairment in the Hippocampus of Alzheimer’s Disease Mice. Natl. Sci. Rev. 2019, 6, 1223–1238. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.; Graham, L.C.; Richter, H.J.; Simeone, S.N.; Radell, J.E.; Grabowska, W.; Funkhouser, W.K.; Howell, M.C.; Howell, G.R. APOE Stabilization by Exercise Prevents Aging Neurovascular Dysfunction and Complement Induction. PLoS Biol. 2015, 13, e1002279. [Google Scholar] [CrossRef]
- Enciu, A.-M.; Gherghiceanu, M.; Popescu, B.O. Triggers and Effectors of Oxidative Stress at Blood-Brain Barrier Level: Relevance for Brain Ageing and Neurodegeneration. Oxidative Med. Cell. Longev. 2013, 2013, e297512. [Google Scholar] [CrossRef]
- Kumar, A. Editorial: Neuroinflammation and Cognition. Front. Aging Neurosci. 2018, 10, 413. [Google Scholar] [CrossRef]
- Godbout, J.P.; Chen, J.; Abraham, J.; Richwine, A.F.; Berg, B.M.; Kelley, K.W.; Johnson, R.W. Exaggerated Neuroinflammation and Sickness Behavior in Aged Mice Following Activation of the Peripheral Innate Immune System. FASEB J. 2005, 19, 1329–1331. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Haage, V.; Semtner, M.; Vidal, R.O.; Hernandez, D.P.; Pong, W.W.; Chen, Z.; Hambardzumyan, D.; Magrini, V.; Ly, A.; Walker, J.; et al. Comprehensive Gene Expression Meta-Analysis Identifies Signature Genes That Distinguish Microglia from Peripheral Monocytes/Macrophages in Health and Glioma. Acta Neuropathol. Commun. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.L.; Ousman, S.S. Astrocytes and Aging. Front. Aging Neurosci. 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Le Guerroué, F.; Youle, R.J. Ubiquitin Signaling in Neurodegenerative Diseases: An Autophagy and Proteasome Perspective. Cell Death Differ. 2021, 28, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Bourdenx, M.; Fujimaki, M.; Karabiyik, C.; Krause, G.J.; Lopez, A.; Martín-Segura, A.; Puri, C.; Scrivo, A.; Skidmore, J.; et al. The Different Autophagy Degradation Pathways and Neurodegeneration. Neuron 2022, 110, 935–966. [Google Scholar] [CrossRef]
- Moscat, J.; Diaz-Meco, M.T. P62 at the Crossroads of Autophagy, Apoptosis, and Cancer. Cell 2009, 137, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.V.; Mills, J.; Lapierre, L.R. Selective Autophagy Receptor P62/SQSTM1, a Pivotal Player in Stress and Aging. Front. Cell Dev. Biol. 2022, 10, 793328. [Google Scholar] [CrossRef]
- Mahajan, U.V.; Varma, V.R.; Griswold, M.E.; Blackshear, C.T.; An, Y.; Oommen, A.M.; Varma, S.; Troncoso, J.C.; Pletnikova, O.; O’Brien, R.; et al. Dysregulation of Multiple Metabolic Networks Related to Brain Transmethylation and Polyamine Pathways in Alzheimer Disease: A Targeted Metabolomic and Transcriptomic Study. PLoS Med. 2020, 17, e1003012. [Google Scholar] [CrossRef]
- Mota, C.; Taipa, R.; das Neves, S.P.; Monteiro-Martins, S.; Monteiro, S.; Palha, J.A.; Sousa, N.; Sousa, J.C.; Cerqueira, J.J. Structural and Molecular Correlates of Cognitive Aging in the Rat. Sci. Rep. 2019, 9, 2005. [Google Scholar] [CrossRef]
- Barral, S.; Beltramo, R.; Salio, C.; Aimar, P.; Lossi, L.; Merighi, A. Phosphorylation of Histone H2AX in the Mouse Brain from Development to Senescence. Int. J. Mol. Sci. 2014, 15, 1554–1573. [Google Scholar] [CrossRef] [PubMed]



| Bioactive Metabolites | He1 | |
|---|---|---|
| Sporophore | Mycelium | |
| Erinacine A (µg/g) | - | 150 |
| Hericenone C (µg/g) | 500 | - |
| Hericenone D (µg/g) | <20 | - |
| L-Ergothioneine (µg/g) | 340 | 580 |
| Bacterium Genera | Effects on Host Health | Ref. |
| Odoribacter | Dichotomous role: | |
| SCFAs producer. | [63,64,65,66] | |
| Opportunistic pathogen with a potential correlation with inflammation, increasing the levels of pro-inflammatory cytokines. | [67,68,69] | |
| Increased in (i) people with Alzheimer’s Disease, (ii) Parkinson’s disease patients with cognitive impairment, (iii) major depression patients with cognitive impairment, (iv) patients with post-finasteride treatment-associated cognitive and psychological disorders, (v) women with HIV and cognitive impairment, and (vi) during physiological ageing in wild-type mice with an impairment in spatial memory and anxiety-like behaviours. | [58,70,71,72,73,74] | |
| Odoribacter exhibits a protective association with cognitive impairment and hippocampal volume. | [75,76] | |
| Clostridia vadinBB60 | Dichotomous role: | |
| Commensal beneficial bacteria. | [77,78] | |
| Its increase is associated with neuroplasticity decrease in schizophrenic patients and elderly frail mice. | [29,79] | |
| Muribaculaceae | Dichotomous role: | |
| SCFAs producer. | [80,81] | |
| Its relative abundance is positively correlated with IL-1β in sleep-deprived mice. It could contribute to LPS production, and a reduction in Muribaculaceae relative abundance determined an anti-inflammatory effect, decreasing the expression of TNF-α and IL-6 in diabetic mice. | [82,83] | |
| It might disrupt the microglial M1/M2 phenotype ratio homeostasis after chronic methamphetamine exposure in mice. | [84] | |
| It could lead to learning and memory deficits after prolonged methamphetamine usage in mice. | [84] | |
| Bacterium Genera | Effects on Host Health | Ref. |
|---|---|---|
| Clostridia UCG-014 | Dichotomous role: | |
| SCFA producer, reducing gut and neuro-inflammation, communicating with the immune system, and strengthening the gut barrier in different preclinical and clinical models. | [85,86,87,88,89,90] | |
| Pro-inflammatory bacterium. | [91,92,93,94] | |
| Lachnospiraceae_NK4A136 | SCFA (butyrate) producer and possible role in bile acids metabolism and cholesterol homeostasis. Association among higher abundance of Lachnospiraceae NK4A136, enhancement of gut barrier function, and anti-inflammatory properties. | [95,96,97,98,99,100,101,102,103] |
| Reduced in patients with dementia or cognitive impairments.Its increase is correlated with improved memory performance. | [97,104,105] | |
| It is involved in the production of a few neurotransmitters, which play crucial roles in memory and cognitive function. | [106,107] | |
| Eubacterium xylanophilum | SCFA (formic, acetic, and butyric acids) producer. | [108,109,110,111] |
| An association between memory indicators and gut microbiota metabolites produced by Eubacterium xylanophilum was demonstrated in preclinical models. | [112,113] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priori, E.C.; Ratto, D.; De Luca, F.; Sandionigi, A.; Savino, E.; Giammello, F.; Romeo, M.; Brandalise, F.; Roda, E.; Rossi, P. Hericium erinaceus Extract Exerts Beneficial Effects on Gut–Neuroinflammaging–Cognitive Axis in Elderly Mice. Biology 2024, 13, 18. https://doi.org/10.3390/biology13010018
Priori EC, Ratto D, De Luca F, Sandionigi A, Savino E, Giammello F, Romeo M, Brandalise F, Roda E, Rossi P. Hericium erinaceus Extract Exerts Beneficial Effects on Gut–Neuroinflammaging–Cognitive Axis in Elderly Mice. Biology. 2024; 13(1):18. https://doi.org/10.3390/biology13010018
Chicago/Turabian StylePriori, Erica Cecilia, Daniela Ratto, Fabrizio De Luca, Anna Sandionigi, Elena Savino, Francesca Giammello, Marcello Romeo, Federico Brandalise, Elisa Roda, and Paola Rossi. 2024. "Hericium erinaceus Extract Exerts Beneficial Effects on Gut–Neuroinflammaging–Cognitive Axis in Elderly Mice" Biology 13, no. 1: 18. https://doi.org/10.3390/biology13010018
APA StylePriori, E. C., Ratto, D., De Luca, F., Sandionigi, A., Savino, E., Giammello, F., Romeo, M., Brandalise, F., Roda, E., & Rossi, P. (2024). Hericium erinaceus Extract Exerts Beneficial Effects on Gut–Neuroinflammaging–Cognitive Axis in Elderly Mice. Biology, 13(1), 18. https://doi.org/10.3390/biology13010018