Simple Summary
This is the first study to investigate the spatial distribution and population structure of the tub gurnard (Chelidonichthys lucerna) in different regions of the northeast Atlantic. To analyze fish body shape, a morphometry-based method, which helps identify variations in fish body shape that may exist due to genetic factors or environmental adaptability, was used. This study relied on C. lucerna individuals captured in the following three fishing areas: Conwy Bay (United Kingdom), Biscay Bay (Spain) and Matosinhos (Portugal). The findings indicate the existence of significant regional differences in fish bodies, thus highlighting the existence of distinct fish populations in the three regions. Results also suggest that the Spanish and British populations may inhabit similar habitats, as some similarities in body shape were found. To confirm these findings, we recommend future research using a holistic approach with alternative and complimentary stock assessment tools.
Abstract
The study of geometric morphometrics among stocks has proven to be a valuable tool in delineating fish spatial distributions and discriminating distinct population units. Variations in fish body morphology can be linked to genetic factors or to phenotypic adaptability in response to environmental variables. The tub gurnard (Chelidonichthys lucerna) is a demersal species that usually lives in the bottom of the continental shelf, being widely distributed along the northeast Atlantic, Mediterranean and Black seas. Worldwide interest in the species has increased since 2006, when ICES recognized its potential for commercial exploitation. However, despite its broad geographic occurrence, to date, research on C. lucerna population structure at large spatial scales is still lacking. In this paper, body geometric morphometrics, using a landmark-based truss network, was applied in order to discriminate C. lucerna populations caught in three different fishery grounds areas along the northeast Atlantic: Conwy Bay (United Kingdom), Biscay Bay (Spain) and Matosinhos (Portugal). The results obtained in this study revealed a high overall relocation success (95%) of samples to their original locations, thus demonstrating the existence of significant regional differences and indicating that we are dealing with different fish population units. Moreover, the data revealed a partial overlap between individuals from Spain and United Kingdom, suggesting that in geographically distant areas these populations may inhabit similar environments. However, to corroborate these findings, future works using a holistic approach with alternative and complimentary stock assessment tools (e.g., genetic and phenotypic natural tags) are highly recommended.
1. Introduction
Fish populations can be distributed over extensive geographical areas characterized by distinctive environmental characteristics (e.g., temperature, salinity, depth, habitat and currents), which together with the challenges related to food availability and predators can exert influence over significant demographic aspects, thereby affecting the dynamics of the populations including reproductive patterns, fecundity and lifespan [1,2,3]. In addition, other factors, such as fishing pressure, anthropic pollution, habitat destruction and climate change, can also affect the abundance and distribution of fish species and consequently fish stocks dynamics [4,5,6]. Understanding population structure is, therefore, an important component of fisheries management as it allows to effectively estimate stock-wise population abundance, determine how each stock responds to fisheries exploitation or environmental changes and the potential impacts on related and dependent species, thereby ensuring species sustainability [7,8,9].
Fish morphometric variation among stocks has been shown to be a useful tool to describe fish spatial distributions and identify different population units, as fish body morphological differences (e.g., length, width and depth) can be associated with genetic background [10,11,12] or processes of phenotypic plasticity as a response to different environmental conditions [13,14,15]. Exposure to variations in factors, such as temperature, salinity and food availability, can result in different behavioral patterns (e.g., aggregation, migration and others) and the adoption of different adaptation strategies, which could be reflected in fish morphometric features and contribute to the definition of different phenotypic stocks [16,17,18]. The truss network system is a geometric morphometrics method commonly used for stock discrimination purposes that provides information on phenotypic traits [9,19,20]. This approach is a powerful tool for the analysis of the fish contour shape and consists of covering all or most of the animal’s body with a landmark-based uniform network that allows for the measurement of a series of distances across the body form [21].
Chelidonichthys lucerna [22], commonly named tub gurnard, can be found in the northeast Atlantic, from Norway and the southern North Sea extending along the Atlantic shoreline of Europe around to the British Isles, and also in the Mediterranean and Black seas and the northwest coast of Africa [23]. This species is demersal and usually inhabits sand, muddy or gravel substrates of the continental shelf in depths ranging from 20 to 318 m, but it is more abundant in inshore waters up to 150 m [24,25,26]. Following a larval pelagic phase [27,28], C. lucerna exhibits a particular pattern of seasonal migratory movements during the juvenile and adult stages within its depth ranges throughout the year, showing a more pronounced concentration of individuals in shallower depths during spring and summer, moving progressively into deeper waters in the winter period [29,30,31]. Recently, it has been shown that C. lucerne, although mainly a marine fish, can occupy and migrate among habitats with diverse salinity degrees, thereby showing high environmental plasticity and adaptation [32]. In 2006, ICES classified C. lucerna as a potential species for commercial exploitation and has recommended that monitoring programs should be conducted to acquire information on biological parameters for stock assessment purposes [33]. Since 2010, although with a slight decrease between 2017 and 2020, worldwide fisheries landings have shown an increasing trend, reaching 4759 tons in 2021 [34]. At present, there is no minimum landing size, allowed quotas, fishing closure seasons, or other fishery regulations. According to the International Union for Conservation of Nature (IUCN), C. lucerna is listed as Least Concern, but it would be helpful to quantify the population trend of this species throughout the Atlantic Ocean and Mediterranean Sea [35].
Due to its broad spatial distribution and the presence of several physical and oceanographic barriers within its wide distribution range, this species could potentially consist of various distinct population units. However, to date, information about the stock structure of this species is scarce. A study, conducted using genetic and morphological analyses of fish caught in the Black Sea, Marmara, Aegean and northeastern Mediterranean coasts of Turkey, showed that only the Black Sea population is differentiated from other populations [36]. More recently, a study conducted in the Portuguese Atlantic waters using otolith shape and microchemistry fingerprints suggested that along the mainland coast the species is, although not homogeneous, apparently a single-population unit [37]. However, its population structure at the larger northeast Atlantic spatial scale is unknown.
Therefore, the present study aimed at investigating the spatial morphological variability of C. lucerna among three fishery grounds (British, Cantabrian and Portuguese waters) in the northeast Atlantic using a truss network approach.
2. Materials and Methods
2.1. Sampling
A total of 129 fish were collected between October 2020 and December 2021 in three different fishery grounds in the northeast Atlantic: Conwy Bay, United Kingdom (Irish Sea), Bay of Biscay, Spain (Cantabrian Sea), and Matosinhos, Portugal (northwest Portuguese waters) (Figure 1 and Table 1). All individuals were captured using bottom trawl fishing. Upon collection, or immediately after landing, fish were transported to the laboratory in isothermal containers preserved with ice for biological processing.
Figure 1.
Sampling locations of Chelidonichthys lucerna individuals collected between October 2020 and December 2021 in the northeast Atlantic (the blue, red and green solid circles represent the eastern Irish Sea, the Cantabria Sea and the northwest Portuguese waters, respectively).

Table 1.
Sampling locations, date, sample size (N) and standard length (SL: mean ± standard deviation) of Chelidonichthys lucerna used in this study.
2.2. Body Morphometric Analysis
The body morphometrics of individual C. lucerna were analyzed using a truss network system standard protocol [21]. All individuals were measured for standard length (SL, 1 mm) (Table 1), and their left (lateral) and dorsal (upper) sides were photographed from a fixed distance using a precise scale (10 mm) with a high-quality digital camera for body morphometric analysis, following recommendations, to minimize the effects of distortion [38]. Instructions of landmark criteria and a reference image of where to place each landmark were shared among researchers to avoid image-based bias [39].
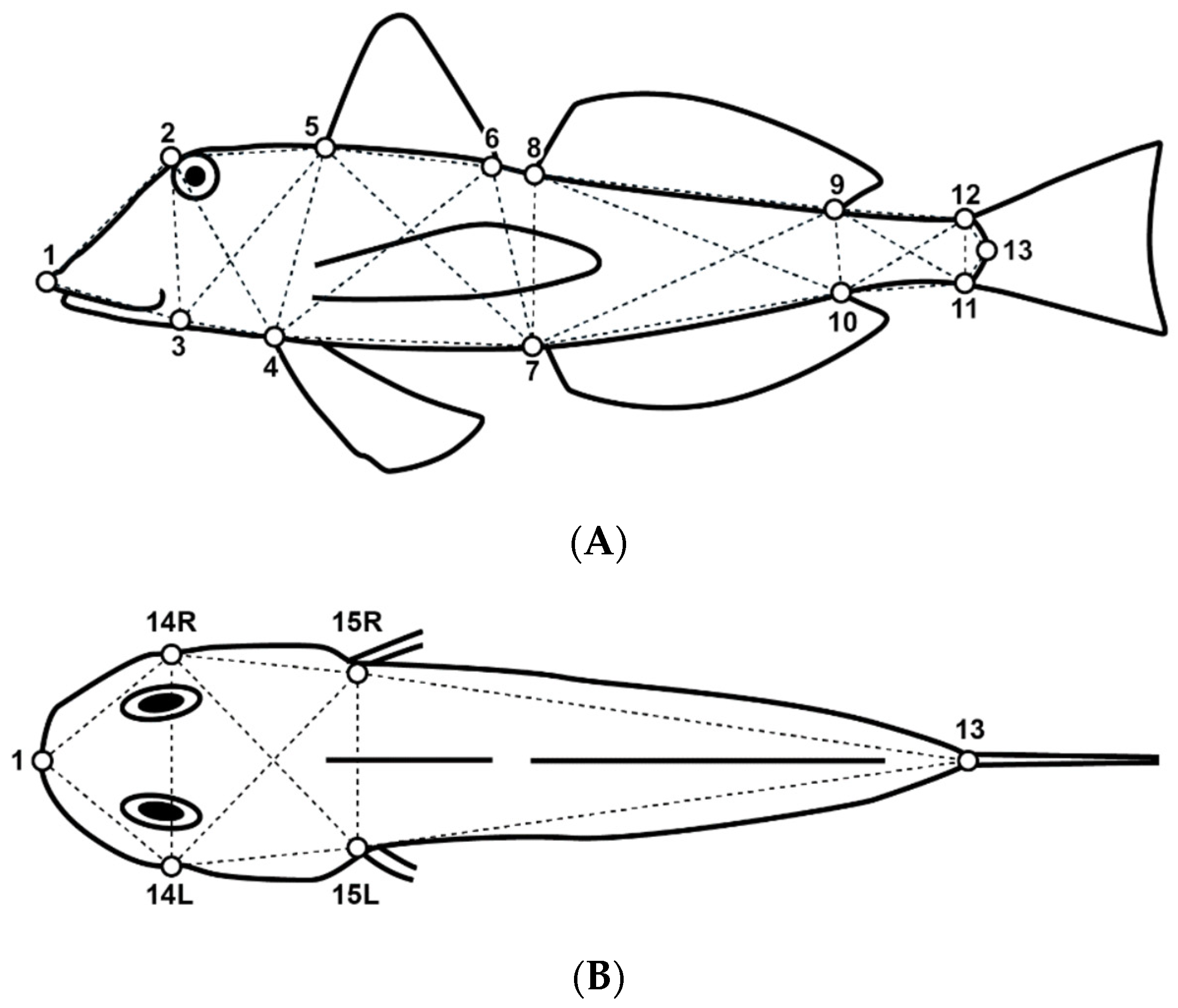
A total of 13 and 6 landmarks were defined along the body contour on the fish’s lateral and dorsal views, respectively (Figure 2). Location coordinates of homologous landmarks were processed and digitized using tpsUtil Version 1.83 [40], and tpsDig Version 2.32 [41] software and used to determine 37 linear distances (D) of the box–truss network (Table 2).
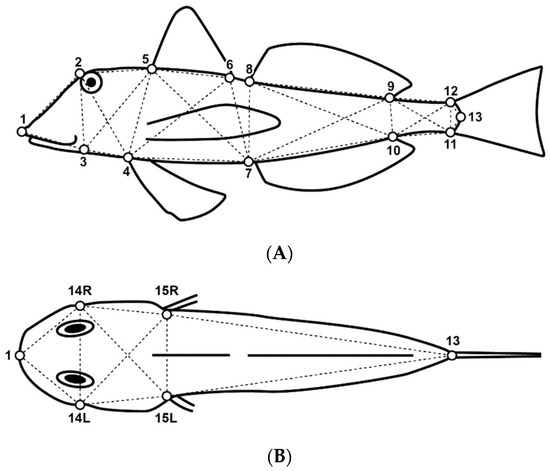
Figure 2.
Illustration of a Chelidonichthys lucerna specimen showing the selected landmarks for the lateral (A) and dorsal (B) body views. See Table 2 for further details.

Table 2.
Body landmarks defined along the body contour of Chelidonichthys lucerna and morphometric distances used for the body shape analysis. For more details, please see Figure 2.
2.3. Statistical Analysis
The relationship between the thirty-seven morphometric distances (D1 to D37) and fish standard length (SL) was verified using One-Way Analysis of Covariance (ANCOVA). All of them presented a significant positive correlation (p < 0.05). Each distance was corrected to remove the size effect, and the positive allometric relationship between variables was corrected using the following transformation [42]: DT = 10^[log(D) − β [log(SL) − log(SLmean)], where DT is the transformed distance, D is the original distance, β is the slope of the regression of log(D) on log(SL), SL is the standard length of the individual, and SLmean is the overall mean of standard length for all locations.
For the univariate statistics, the DT dataset was checked for normality (Shapiro–Wilk test) and homogeneity of variances (Levene test), but only eight DTs (DT2, DT6, DT8, DT9, DT10, DT11, DT32 and DT35) fulfilled the parametric prerequisites. For this case, One-Way Analysis of Variance (ANOVA) was used to explore the statistical differences of each morphometric distance among the three sampling locations, followed by a Tukey post hoc test if significant differences exist (p < 0.05). However, the majority of DTs did not fulfil the above-mentioned prerequisites, even after being log (x + 1) transformed. For them, non-parametric statistics were then used. So, One-Way ANOVA On Ranks, followed by a Dunn’s test (p < 0.05), if needed, was performed.
Regarding the multivariate statistics, non-parametric tests were also performed. A permutational multivariate analysis of variance (main PERMANOVA) was used to compare the DTs among locations, and when statistically significant (p < 0.05), it was followed by a permutational pairwise comparisons (pseudo t-statistic).
Finally, a flexible discriminant analysis (FDA) followed by a Jackknifed re-classification matrix (leave-one-out cross-validation) was used to calculate the percentage of correctly re-classified individuals into the original location. FDA first randomly split the data into training (80%) and test (20%) sets. Predictors’ parameters were estimated by subtracting the mean of the predictor and scaling by its standard deviation. Estimated parameters were used to transform the train and test sets. The correct re-classification percentage of the discriminant functions was calculated using a Jackknifed matrix for the transformed training and test sets [43].
The univariate statistical analyses were performed using SigmaPlot 11.0. Multivariate tests were performed using R 4.3.0 [44]. A statistical level of significance (α) of 0.05 was considered. Morphometric data are presented as mean ± standard error deviation.
3. Results
Univariate tests showed significant differences in body morphology among the locations for 36 out of 37 DTs (Table 3). Nearly one-quarter of all measurements (DT15, DT23, DT24, DT26, DT27, DT31, DT33 and DT34) presented significant differences between all three sampling locations: 46% (DT1, DT3, DT7, DT8, DT9, DT12, DT13, DT16, DT17, DT18, DT19, DT21, DT28, DT29, DT30, DT35 and DT37) differentiated Spain from the other locations, 19% (DT4, DT5, DT6, DT11, DT20, DT22 and DT32) differentiated Portugal from the other locations, and only one measurement (DT10) differentiated the United Kingdom from other locations. Only 6% of the measurements showed no significant differences between Spain and the other two sampling locations (DT14) and between Portugal and the other two locations (DT25). DT2 was the only measurement that revealed no significant differences among the three locations.

Table 3.
Body morphometric transformed distances (DT: mean ± standard error) calculated for Chelidonichthys lucerna individuals. DTs, showing different letters, means that significant regional differences exist. For most DTs, One-Way ANOVA On Ranks, followed by a Dunn test (p < 0.05), if needed, was carried out. However, for DT2, DT6, DT8, DT9, DT10, DT11, DT32 and DT35 a One-Way ANOVA and a post hoc pairwise Tukey test were used. For more details, see M&M.
When analyzed together, the morphometric distances also presented significant differences among the three locations (PERMANOVA, p < 0.05; Table 3), and all pairwise tests also revealed significant differences between the locations (pseudo t-test, p < 0.05; Table 4).

Table 4.
Mean and pairwise PERMANOVA comparisons for the 37 body morphometric transformed distances among the three Chelidonichthys lucerna sampling locations.
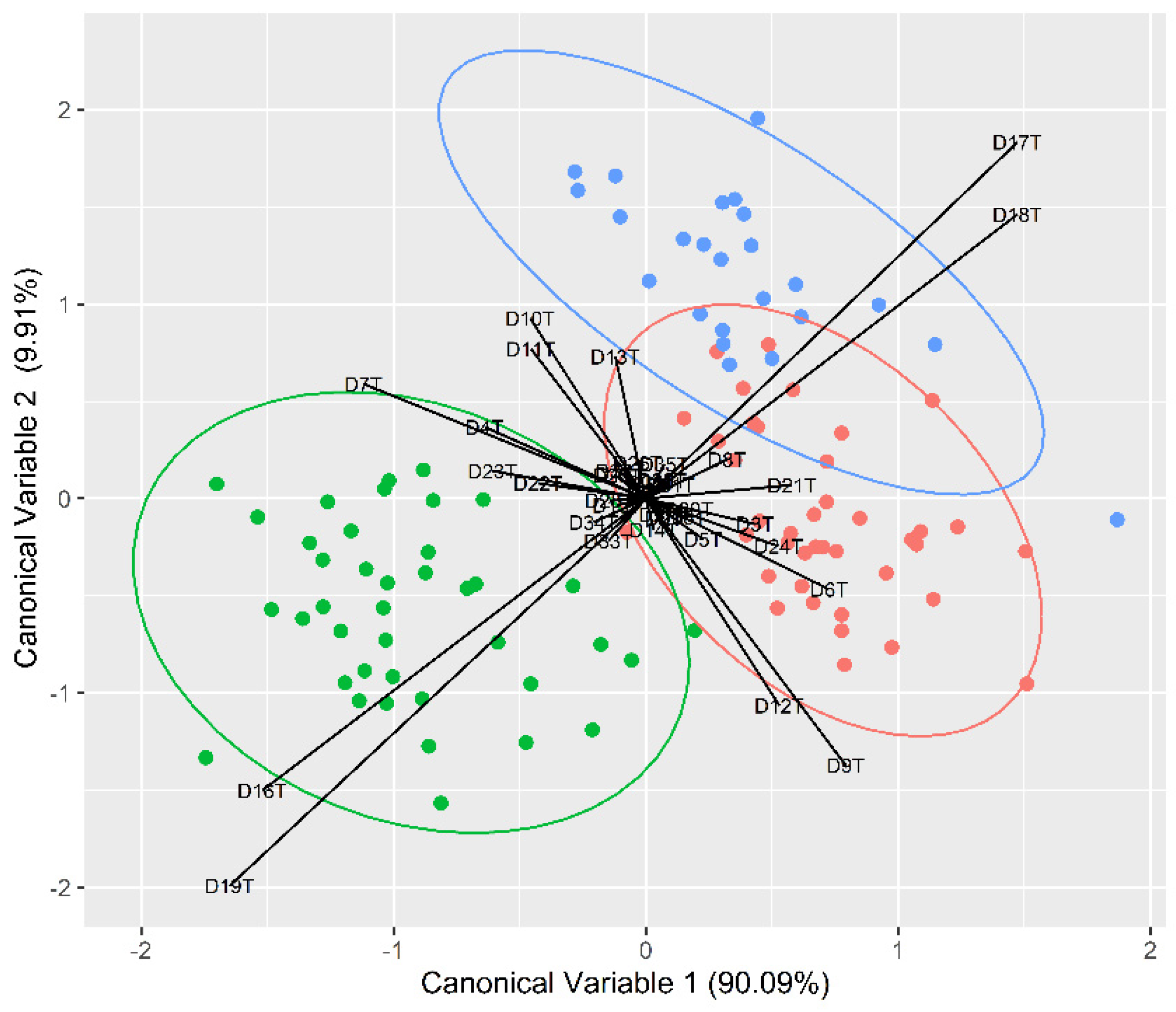
The FDA conducted showed a clear discrimination for all locations (Table 5; Figure 3), with an overall high classification accuracy reported for all three sampling locations (95%). Individuals from Portugal were reclassified correctly in 98% of the cases; it was 96% for the United Kingdom and 93% for Spain. The high discrimination pattern for Portugal was mainly driven by distances DT19 and DT16, which are related with fish posterior body height. DT7 and DT18, related with fish posterior body length, were mainly responsible for the discrimination of the United Kingdom individuals, while DT12 and DT9, related with fish anterior height, were responsible for the differences in the Spanish individuals.

Table 5.
Summary of the percentage of correct reclassification using the training base set following a flexible discriminant analysis (FDA) for the body morphometric transformed distances calculated for Chelidonichthys lucerna individuals.
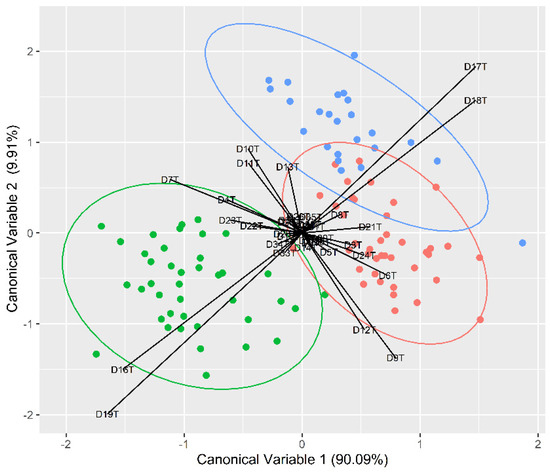
Figure 3.
Flexible discriminant function analysis plot obtained from body morphometric transformed distances (the blue, red and green solid circles represent the fish from the eastern Irish Sea, the Cantabria Sea and the northwest Portuguese waters, respectively).
4. Discussion
The present study aimed at investigating the differences in the body shape of C. lucerna along the northeast Atlantic using landmark-based truss network morphometrics.
Morphometric studies on C. lucerna are limited and, to date, only one study has been carried out in the Black Sea, Marmara, Aegean and eastern Mediterranean Sea [36] that combined genetics with body geometric morphometrics to analyze the existent population structure within Turkish marine waters. As in the former study, the sex of the animals was not considered in this study, since it was demonstrated that there are no morphometric differences between sexes of a related species (C. obscurus) [45], although the potential effect of the reproductive season on the sexual dimorphism should not be excluded.
Phenotypic variation in fish morphometric characteristics provides valuable information about population units and has long been used for stock identification as morphological differences are commonly explained as a response to dissimilar environmental conditions [7,11,46].
Advances in digital imaging systems and analytical methods in the past decades have facilitated progress and diversification of morphometric techniques, expanding the potential for using morphometric analysis as a stock identification tool [9,19,46]. In this context, landmark-based truss analysis has been successfully used alone by several authors for the discrimination of fish stocks [13,14,47] or combined with other discrimination methods such as genetics [48,49,50], otolith shape [15,17,51] and otolith elemental analyses [52,53,54].
Regarding body shape, the Spanish individuals, although bigger in SL (Table 1), after size allometric correction were found to have the smallest body measurements (75% of the total recorded distances), namely in terms of head length, heights and widths, the majority of fin lengths and distances and the anterior and posterior fish heights (DT1, DT3-DT13, DT15-DT19, DT25 and DT28 and DT37). The hydrology of the Spanish sampling location (Bay of Biscay) is particularly complex due to interactions between the general oceanic circulation, topography, highly energetic tidal currents, wind-induced currents and river inputs of freshwater, mainly located on the French coast [55,56]. Other authors have reported that body, head and fins in fish are highly affected by water velocity and fish with a streamlined morphology exhibit enhanced capability to counter hydrodynamic resistance within fast-flowing water [57,58,59]. In this context, the particular oceanographic characteristics of the Bay of Biscay could have induced some morphological adaptive variations in C. lucerna’s body shape and explain the smaller distances within fish shape, despite the larger size of the sampled individuals.
The United Kingdom individuals, that recorded a smaller SL (Table 1), have also recorded smaller mouth sizes and caudal peduncle areas (DT2 and DT20-DT24). Conwy Bay is an inlet of the Irish Sea, which is generally characterized by large tidal energy input from the Atlantic [60]. The Bay is recognized for its unusual and varied coastal and intertidal habitats and their associated reef communities [61], which are factors that can influence C. lucerna’s feeding regimes and fish habitats and explain the recorded phenotypic regional differences.
The larger overall measurements (70% of the total recorded distances) were found for the Portuguese individuals, namely in terms of head length, mouth and anterior body size and peduncle area (DT1–DT9, DT11–DT13, DT15–DT17, DT20–DT24 and DT30–DT35). Fish head and mouth sizes may reflect differential habitat use, variations in feeding behaviors or the capacity to explore different ecological niches with different types of prey [62,63,64], while the lengthening of the caudal peduncle is usually associated with fish swimming ability in strong hydrodynamic environments (e.g., water currents) [65,66,67]. The Portuguese coast presents different hydrographical features influenced by the Canary and the Portuguese currents, both connected to the North Atlantic Subtropical Gyre [68] which could induce morphological variances in body shape, namely in the peduncle area. The largest distances between fins, larger 2nd dorsal fins, higher posterior body height, larger caudal fin areas and posterior body length (DT10, DT14, DT18–DT19, DT25–DT29 and DT36–DT37) were also recorded for the United Kingdom individuals. Fish body form, fin length and location are adaptations for movement that indicate differences in habitat exploitation [69], which is aligned with the distinctive and diverse environments found in the eastern Irish Sea [60,61].
Finally, the effect of the sampling period in the truss networking results cannot be disregarded in this study. At each site, samples were collected at different times of the year, which, in conjunction with the life cycle (e.g., spawning period), may have a significant impact on body shape. It is well known that for the Mediterranean Sea this species has a protracted spawning season but with peaks occurring at different sites [26,70]. However, data for the Atlantic Ocean is limited. Anyway, a previous study that was conducted in the NE Atlantic reported that females attained maturity at smaller sizes (27.7 cm vs. 29.1 cm) and younger ages (2.7 years vs. 2.8 years) compared to males [71]. This shows that basic data about the reproductive biology of C. lucerna are still needed in the Atlantic waters.
Another factor that can play a role in the differences observed among the three sampling locations could be attributed to distinct regional anthropogenic influences (e.g., pollution and habitat alteration) [4,6,72]. But further investigation is needed.
5. Conclusions
Regardless of the reason behind the regional morphological differences, our results concerning the geometric morphometrics analyses have shown significant differences among the three sampling locations, with a high overall reallocation success (95%) of individuals to the original locations. These data indicate that C. lucerna individuals caught in the three fishery grounds along the northeast Atlantic do not belong to a single and homogeneous population unit, despite a slight visual overlap in the FDA between the English and Spanish individuals, which suggests that fish from these locations may somewhat inhabit similar environments. Finally, the results suggest that these fisheries should be managed regionally as different population units.
However, since the regional differences found in this study regarding the sample number, size range, temporal collection window, sex ratio and age structure of the caught individuals could somewhat confound the ontogenic effects on phenotypic body variations [73], it is recommended to conduct future studies with a holistic approach using other natural tags, such as genetics, parasites fauna and otolith chemistry. In addition, studying individuals from a broader number of sampling locations would also allow for a better understanding of the northeast Atlantic population structure.
Author Contributions
I.F.: formal analysis; investigation; methodology; validation; visualization; data curation; writing—original draft; and writing—review and editing. R.S.: data curation and writing—review and editing. E.M.: data curation and writing—review and editing. I.O.: data curation and writing—review and editing. I.D.M.: data curation and writing—review and editing. A.T.C.: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; resources; supervision; validation; visualization; writing—original draft; and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study received support from international funds through FCT (Foundation for Science and Technology) as part of UIDB/04423/2020 and UIDP/04423/2020.
Institutional Review Board Statement
There is no legal European constraint since the fish samples used here were collected from fisheries.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Alberto Rocha e Diana Feijó for the Matosinhos fish acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Harvey, E.S.; Cappo, M.; Kendrick, G.A.; McLean, D.L. Coastal Fish Assemblages Reflect Geological and Oceanographic Gradients within an Australian Zootone. PLoS ONE 2013, 8, e80955. [Google Scholar] [CrossRef]
- Brooker, M.A.; de Lestang, S.; Fairclough, D.V.; McLean, D.; Slawinski, D.; Pember, M.B.; Langlois, T.J. Environmental and Anthropogenic Factors Affect Fish Abundance: Relationships Revealed by Automated Cameras Deployed by Fishers. Front. Mar. Sci. 2020, 7, 279. [Google Scholar] [CrossRef]
- Costa, E.F.S.; Teixeira, G.M.; Freire, F.A.M.; Dias, J.F.; Fransozo, A. Effects of Biological and Environmental Factors on the Variability of Paralonchurus Brasiliensis (Sciaenidae) Density: An GAMLSS Application. J. Sea Res. 2022, 183, 102203. [Google Scholar] [CrossRef]
- Franssen, N.R.; Harris, J.; Clark, S.R.; Schaefer, A.F.; Stewart, L.K. Shared and Unique Morphological Responses of Stream Fishes to Anthropogenic Habitat Alteration. Proc. R. Soc. 2013, 280, 20122715. [Google Scholar] [CrossRef]
- Wright, P.J.; Pinnegar, J.K.; Fox, C. Impacts of Climate Change on Fish, Relevant to the Coastal and Marine Environment around the UK. MCCIP Sci. Rev. 2020, 354–381. [Google Scholar] [CrossRef]
- Santi, F.; Vella, E.; Jeffress, K.; Deacon, A.; Riesch, R. Phenotypic Responses to Oil Pollution in a Poeciliid Fish. Environ. Pollut. 2021, 290, 118023. [Google Scholar] [CrossRef]
- Begg, G.A.; Friedland, K.D.; Pearce, J.B. Stock Identification and Its Role in Stock Assessment and Fisheries Management: An Overview. Fish. Res. 1999, 43, 1–8. [Google Scholar] [CrossRef]
- Cadrin, S.X.; Kerr, L.A.; Mariani, S. Stock Identification Methods: Applications in Fishery Science; Cadrin, S.X., Kerr, L.A., Mariani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-397003-9. [Google Scholar]
- Rawat, S.; Benekappa, S.; Kumar, J.; Kumar Naik, A.S.; Pandey, G.; Pema, C.W. Identification of Fish Stocks Based on Truss Morphometric: A Review. J. Fish. Life Sci. 2017, 2, 9–14. [Google Scholar]
- Robinson, B.W.; Wilson, D.S. Genetic Variation and Phenotypic Plasticity in a Trophically Polymorphic Population of Pumpkinseed Sunfish (Lepomis gibbosus). Evol. Ecol. 1996, 10, 631–652. [Google Scholar] [CrossRef]
- Turan, C.; Oral, M.; Öztürk, B.; Düzgüneş, E. Morphometric and Meristic Variation between Stocks of Bluefish (Pomatomus saltatrix) in the Black, Marmara, Aegean and Northeastern Mediterranean Seas. Fish. Res. 2006, 79, 139–147. [Google Scholar] [CrossRef]
- Crispo, E. Modifying Effects of Phenotypic Plasticity on Interactions among Natural Selection, Adaptation and Gene Flow. J. Evol. Biol. 2008, 21, 1460–1469. [Google Scholar] [CrossRef]
- Hoff, N.T.; Dias, J.F.; de Lourdes Zani-Teixeira, M.; Soeth, M.; Correia, A.T. Population Structure of the Bigtooth Corvina Isopisthus parvipinnis from the Southwest Atlantic Ocean as Determined by Whole-Body Morphology. Reg. Stud. Mar. Sci. 2020, 39, 101379. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Triay-Portella, R.; Correia, A.T. Landmark-Based Geometric Morphometrics Analysis of Body Shape Variation among Populations of the Blue Jack Mackerel, Trachurus picturatus, from the North-East Atlantic. J. Sea Res. 2020, 163, 101926. [Google Scholar] [CrossRef]
- Muniz, A.A.; Moura, A.; Triay-Portella, R.; Moreira, C.; Santos, P.T.; Correia, A.T. Population Structure of the Chub Mackerel (Scomber colias) in the North-East Atlantic Inferred from Otolith Shape and Body Morphometrics. Mar. Freshw. Res. 2020, 72, 341–352. [Google Scholar] [CrossRef]
- Pulkkinen, K.; Ketola, T.; Laakso, J.; Mappes, J.; Sundberg, L.R. Rich Resource Environment of Fish Farms Facilitates Phenotypic Variation and Virulence in an Opportunistic Fish Pathogen. Evol. Appl. 2022, 15, 417–428. [Google Scholar] [CrossRef]
- Schroeder, R.; Schwingel, P.R.; Correia, A.T. Population Structure of the Brazilian Sardine (Sardinella brasiliensis) in the Southwest Atlantic Inferred from Body Morphology and Otolith Shape Signatures. Hydrobiologia 2022, 849, 1367–1381. [Google Scholar] [CrossRef]
- Quadroni, S.; De Santis, V.; Carosi, A.; Vanetti, I.; Zaccara, S.; Lorenzoni, M. Past and Present Environmental Factors Differentially Influence Genetic and Morphological Traits of Italian Barbels (Pisces: Cyprinidae). Water 2023, 15, 325. [Google Scholar] [CrossRef]
- Mallik, A.; Chakraborty, P.; Swain, S. Truss Networking: A Tool for Stock Structure Analysis of Fish. In Research Trends in Fisheries and Aquatic Sciences; Akinik Publications: New Delhi, India, 2020; pp. 96–108. [Google Scholar]
- Chakraborty, R.D. Truss Networking: A Tool for Stock Structure Analysis. In ICAR-CMFRI-Winter School on Recent Development in Taxonomic Techniques of Marine Fishes for Conservation and Sustainable Fisheries Management; ICAR-Central Marine Fisheries Research Institute: Kochi, India, 2022; pp. 84–94. [Google Scholar]
- Strauss, R.E.; Bookstein, F.L. The Truss: Body Form Reconstructions in Morphometrics. Syst. Biol. 1982, 31, 113–135. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis; Decima, Reformata; Laurentius Salvius: Holmiae, Turkey, 1758; Volume ii. [Google Scholar]
- FAO—Fisheries and Aquaculture Department. Species Fact. Sheets, Chelidonichthys lucerna (Linnaeus, 1758); FAO: Rome, Italy, 2023.
- Mytilineou, C.; Papaconstantinou, C.; Kavadas, S.; D’onghia, G.; Politou, C.-Y.; Papaconstantinou, C.; Sion, L. Deep-Water Fish Fauna in the Eastern Ionian Sea. Belg. J. Zool. 2005, 135, 229–233. [Google Scholar]
- ICES. Report. of the Working Group. on Assessment of New MoU Species (WGNEW), 11–15 October 2010; ICES: Copenhagen, Denmark, 2010. [Google Scholar]
- El-Serafy, S.S.; El-Gammal, F.I.; Mehanna, S.F.; Abdel-Hamid, N.-A.H.; Farrag, E.-S.F.E. Age, Growth and Reproduction of the Tub Gurnard, Chelidonichthys lucerna (Linnaeus, 1758) from the Egyptian Mediterranean Waters off, Alexandria. Int. J. Fish. Aquat. Sci. 2015, 4, 13–20. [Google Scholar] [CrossRef]
- Dulčić, J.; Grubišić, L.; Katavić, I.; Skakelja, N. Embryonic and Larval Development of the Tub Gurnard Trigla lucerna (Pisces: Triglidae). J. Mar. Biol. Assoc. United Kingd. 2001, 81, 313–316. [Google Scholar] [CrossRef]
- Vallisneri, M.; Montanini, S.; Stagioni, M. Size at Maturity of Triglid Fishes in the Adriatic Sea, Northeastern Mediterranean. J. Appl. Ichthyol. 2012, 28, 123–125. [Google Scholar] [CrossRef]
- Montanini, S.; Stagioni, M.; Benni, E.; Vallisneri, M. Feeding Strategy and Ontogenetic Changes in Diet of Gurnards (Teleostea: Scorpaeniformes: Triglidae) from the Adriatic Sea. Eur. Zool. J. 2017, 84, 356–367. [Google Scholar] [CrossRef]
- Carbonara, P.; Follesa, M.C. Handbook of Fish Age Determination: A Mediterranean Experience; FAO: Rome, Italy, 2019; ISBN 978-92-5-131176-9.
- Campos, J.; Costa- Dias, S.; Bio, A.; Santos, P.T.; Jorge, I. Age and Growth of Tub Gurnard Chelidonichthys lucerna (Linnaeus, 1758) during Estuarine Occupation of a Temperate Atlantic Nursery. Int. J. Environ. Sci. Nat. Resour. 2022, 31. [Google Scholar] [CrossRef]
- Ferreira, I.; Daros, F.A.; Moreira, C.; Feijó, D.; Rocha, A.; Mendez-Vicente, A.; Pisonero, J.; Correia, A.T. Is Chelidonichthys lucerna (Linnaeus, 1758) a Marine Estuarine-Dependent Fish? Insights from Saccular Otolith Microchemistry. Fishes 2023, 8, 383. [Google Scholar] [CrossRef]
- ICES. Report. of the Working Group. on the Assessment of New MOU Species (WGNEW), 13–15 December 2005; ICES: Copenhagen, Denmark, 2006. [Google Scholar]
- FAO. Fishery and Aquaculture Statistics. Global Capture Production 1950–2021 (FishStatJ). Available online: https://www.fao.org/fishery/statistics-query/en/global_production/global_production_quantity (accessed on 15 September 2023).
- Nunoo, F.; Poss, S.; Bannermann, P.; Russell, B. Chelidonichthys lucerna. The IUCN Red List of Threatened Species 2015: E.T198752A15597014. 2015. Available online: https://dx.doi.org/10.2305/IUCN.UK.2015-4.RLTS.T198752A15597014.en (accessed on 17 December 2023).
- Uyan, A.; Turan, C. Genetic and Morphological Analyses of Tub Gurnard Chelidonichthys lucerna Populations in Turkish Marine Waters. Biochem. Syst. Ecol. 2017, 73, 35–40. [Google Scholar] [CrossRef]
- Ferreira, I.; Santos, D.; Moreira, C.; Feijó, D.; Rocha, A.; Correia, A.T. Population Structure of Chelidonichthys lucerna in Portugal Mainland Using Otolith Shape and Elemental Signatures. Mar. Biol. Res. 2019, 15, 500–512. [Google Scholar] [CrossRef]
- Muir, A.M.; Vecsei, P.; Krueger, C.C. A perspective on perspectives: Methods to reduce variation in shape analysis of digital images. Trans. Am. Fish. Soc. 2012, 141, 1161–1170. [Google Scholar] [CrossRef]
- O’Malley, B.P.; Schmitt, J.D.; Holden, J.P.; Weidel, B.C. Comparison of Specimen- and Image-Based Morphometrics for Cisco. J. ish Wildl. Manag. 2021, 12, 208–215. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsUtil—Version 1.83 Dated 04/03/2023. 2023. Available online: https://www.sbmorphometrics.org/soft-utility.html (accessed on 27 July 2023).
- Rohlf, F.J. TpsDig—Version 2.32 Dated 03/06/2021. 2021. Available online: https://www.sbmorphometrics.org/soft-dataacq.html (accessed on 27 July 2023).
- Reist, J.D. An Empirical Evaluation of Coefficients Used in Residual and Allometric Adjustment of Size Covariation. Can. J. Zool. 1986, 64, 1363–1368. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide To Cluster Analysis in R: Unsupervised Machine Learning (Multivariate Analysis I), 1st ed.; STHDA: Marseille, France, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Boudaya, L.; Feki, M.; Mosbahi, N.; Neifar, L. Stock discrimination of Chelidonichthys obscurus (Triglidae) in the central Mediterranean sea using morphometric analysis and parasite markers. J. Helminthol. 2020, 94, e74. [Google Scholar] [CrossRef] [PubMed]
- Cadrin, S.X.; Friedland, K.D. Morphometric Outlines. In Stock Identification Methods; Cadrin, S.X., Friedland, K.D., Waldman, J.R., Eds.; Academic Press: Cambridge, MA, USA, 2005; pp. 173–183. ISBN 9780080470436. [Google Scholar]
- Rasheeq, A.A.; Rajesh, M.; Kumar, T.T.A.; Rajesh, K.M.; Kathirvelpandian, A.; Kumar, S.; Singh, P.K. Stock Structure Analysis of the White-Spotted Spine Foot Fish (Siganus canaliculatus) along the Indian Coast Using Truss Morphometry. Reg. Stud. Mar. Sci. 2023, 65, 103072. [Google Scholar] [CrossRef]
- Kaouèche, M.; Bahri-Sfar, L.; Hammami, I.; Ben Hassine, O.K. Morphological and Genetic Variations of Diplodus vulgaris along the Tunisian Coasts. Cybium 2013, 37, 111–120. [Google Scholar]
- Hammami, I.; Ben Hassine, O.K.; Kaouèche, M.; Bahri-Sfar, L. Morphological and Genetic Characterization of the Sharpsnout Seabream Populations (Diplodus puntazzo, Sparidae) along a Boundary Area between the Two Mediterranean Basins. Mar. Biol. Res. 2016, 12, 842–853. [Google Scholar] [CrossRef]
- Zhang, C.P.; Chen, X.; Yuan, L.; Wu, Y.; Ma, Y.; Jie, W.; Jiang, Y.; Guo, J.; Qiang, L.; Han, C.; et al. Genetic Diversity and Population Structure of Chinese Gizzard Shad Clupanodon thrissa in South China Based on Morphological and Molecular Markers. Glob. Ecol. Conserv. 2023, 41, e02367. [Google Scholar] [CrossRef]
- Hari, M.S.; Kathrivelpandian, A.; Bhavan, S.G.; Sajina, A.M.; Gangan, S.S.; Abidi, Z.J. Deciphering the Stock Structure of Chanos chanos (Forsskål, 1775) in Indian Waters by Truss Network and Otolith Shape Analysis. Turk. J. Fish Aquat. Sci 2020, 20, 103–111. [Google Scholar] [CrossRef]
- Khan, M.A.; Miyan, K.; Khan, S.; Kumar Patel, D.; Ghazi Ansari, N. Studies on the Elemental Profile of Otoliths and Truss Network Analysis for Stock Discrimination of the Threatened Stinging Catfish Heteropneustes fossilis (Bloch 1794) from the Ganga River and Its Tributaries. Zool. Stud. 2012, 51, 1195–1206. [Google Scholar]
- Miyan, K.; Khan, M.A.; Patel, D.K.; Khan, S.; Ansari, N.G. Truss Morphometry and Otolith Microchemistry Reveal Stock Discrimination in Clarias batrachus (Linnaeus, 1758) Inhabiting the Gangetic River System. Fish. Res. 2016, 173, 294–302. [Google Scholar] [CrossRef]
- Schroeder, R.; Avigliano, E.; Volpedo, A.V.; Callicó Fortunato, R.; Barrulas, P.; Daros, F.A.; Schwingel, P.R.; Dias, M.C.; Correia, A.T. Lebranche Mullet Mugil liza Population Structure and Connectivity Patterns in the Southwest Altantic Ocean Using a Multidisciplinary Approach. Estuar. Coast. Shelf Sci. 2023, 288, 108368. [Google Scholar] [CrossRef]
- Druon, J.N.; Loyer, S.; Gohin, F. Scaling of Coastal Phytoplankton Features by Optical Remote Sensors: Comparison with a Regional Ecosystem Model. Int. J. Remote Sens. 2005, 26, 4421–4444. [Google Scholar] [CrossRef][Green Version]
- Karagiorgos, J.; Vervatis, V.; Sofianos, S. The Impact of Tides on the Bay of Biscay Dynamics. J. Mar. Sci. Eng. 2020, 8, 617. [Google Scholar] [CrossRef]
- Páez, D.J.; Hedger, R.; Bernatchez, L.; Dodson, J.J. The Morphological Plastic Response to Water Current Velocity Varies with Age and Sexual State in Juvenile Atlantic Salmon, Salmo salar. Freshw. Biol. 2008, 53, 1544–1554. [Google Scholar] [CrossRef]
- Akin, D.R.; Geheber, A.D. Conforming to the Status Flow: The Influence of Altered Habitat on Fish Body-Shape Characteristics. Freshw. Biol. 2020, 65, 1883–1893. [Google Scholar] [CrossRef]
- Sánchez-González, J.R.; Morcillo, F.; Ruiz-Legazpi, J.; Sanz-Ronda, F.J. Fish Morphology and Passage through Velocity Barriers. Experience with Northern Straight-Mouth Nase (Pseudochondrostoma duriense Coelho, 1985) in an Open Channel Flume. Hydrobiologia 2022, 849, 1351–1366. [Google Scholar] [CrossRef]
- Hadziabdic, P.; Rickards, L.J. Review of the Irish Sea (Area 6) Oceanography. Br. Oceanogr. Data Cent. 1999, 1–155. Available online: https://assets.publishing.service.gov.uk/media/5a75842540f0b6360e474b81/SEA6_Oceanography.pdf (accessed on 12 August 2023).
- Natural Resources Wales. Marine Character Areas: MCA 03 Red. Wharf & Conwy Bays; Natural Resources Wales: Cardiff, UK, 2015. Available online: https://cdn.cyfoethnaturiol.cymru/media/674481/mca-03-red-wharf-and-conwy-bays_final.pdf?mode=pad&rnd=131502218540000000 (accessed on 15 August 2023).
- Park, I.-S.; Im, J.H.; Ryu, D.K.; Nam, Y.K.; Kim, D.S. Effect of Starvation on Morphometric Changes in Rhynchocypris oxycephalus (Sauvage and Dabry). J. Appl. Ichthyol. 2001, 17, 277–281. [Google Scholar] [CrossRef]
- Kaouèche, M.; Bahri-Sfar, L.; Hammami, I.; Hassine, O.K. Ben Morphometric Variations in White Seabream Diplodus sargus (Linneus, 1758) Populations along the Tunisian Coast. Oceanologia 2017, 59, 129–138. [Google Scholar] [CrossRef]
- Baldasso, M.C.; Wolff, L.L.; Neves, M.P.; Delariva, R.L. Ecomorphological Variations and Food Supply Drive Trophic Relationships in the Fish Fauna of a Pristine Neotropical Stream. Env. Biol. Fishes 2019, 102, 783–800. [Google Scholar] [CrossRef]
- Hammami, I.; Bahri-Sfar, L.; Kaoueche, M.; Grenouillet, G.; Lek, S.; Kara, M.-H.; Ben Hassine, O.K. Morphological Characterization of Striped Seabream (Lithognathus mormyrus, Sparidae) in Some Mediterranean Lagoons. Cybium 2013, 37, 127–139. [Google Scholar] [CrossRef]
- de Barros, T.F.; Louvise, J.; Caramaschi, É.P. Flow Gradient Drives Morphological Divergence in an Amazon Pelagic Stream Fish. Hydrobiologia 2019, 833, 217–229. [Google Scholar] [CrossRef]
- Larouche, O.; Benton, B.; Corn, K.A.; Friedman, S.T.; Gross, D.; Iwan, M.; Kessler, B.; Martinez, C.M.; Rodriguez, S.; Whelpley, H.; et al. Reef-Associated Fishes Have More Maneuverable Body Shapes at a Macroevolutionary Scale. Coral Reefs 2020, 39, 1427–1439. [Google Scholar] [CrossRef]
- Barton, E.D. Canary and Portugal Currents. In Encyclopedia of Ocean Sciences; Academic Press: Cambridge, MA, USA, 2001; pp. 380–389. [Google Scholar]
- Webb, P.W. Body Form, Locomotion and Foraging in Aquatic Vertebrates. Am. Zool. 1984, 24, 107–120. [Google Scholar] [CrossRef]
- Boudaya, L.; Neifar, L.; Rizzo, P.; Badalucco, C.; Bouain, A.; Fiorentino, F. Growth and reproduction of Chelidonichthys lucerna (Linnaeus) (Pisces: Triglidae) in the Gulf of Gabès, Tunisia. J. Appl. Ichthyol. 2008, 24, 581–588. [Google Scholar] [CrossRef]
- McCarthy, I.D.; Marriott, A.L. Age, growth and maturity of tub gurnard (Chelidonichthys lucerna Linnaeus 1758; Triglidae) in the inshore coastal waters of Northwest Wales, UK. J. Appl. Ichthyol. 2018, 34, 581–589. [Google Scholar] [CrossRef]
- Kruitwagen, G.; Hecht, T.; Pratap, H.B.; Wendelaar Bonga, S.E. Changes in Morphology and Growth of the Mudskipper (Periophthalmus argentilineatus) Associated with Coastal Pollution. Mar. Biol. 2006, 149, 201–211. [Google Scholar] [CrossRef]
- Cadrin, S.X. Advances in Morphometric Identification of Fishery Stocks. Rev. Fish. Biol. Fish. 2000, 10, 91–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).