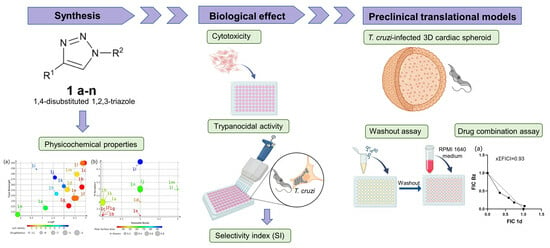
Antitrypanosomal Activity of 1,2,3-Triazole-Based Hybrids Evaluated Using In Vitro Preclinical Translational Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
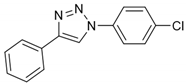
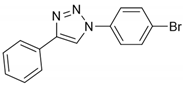
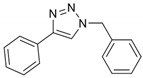
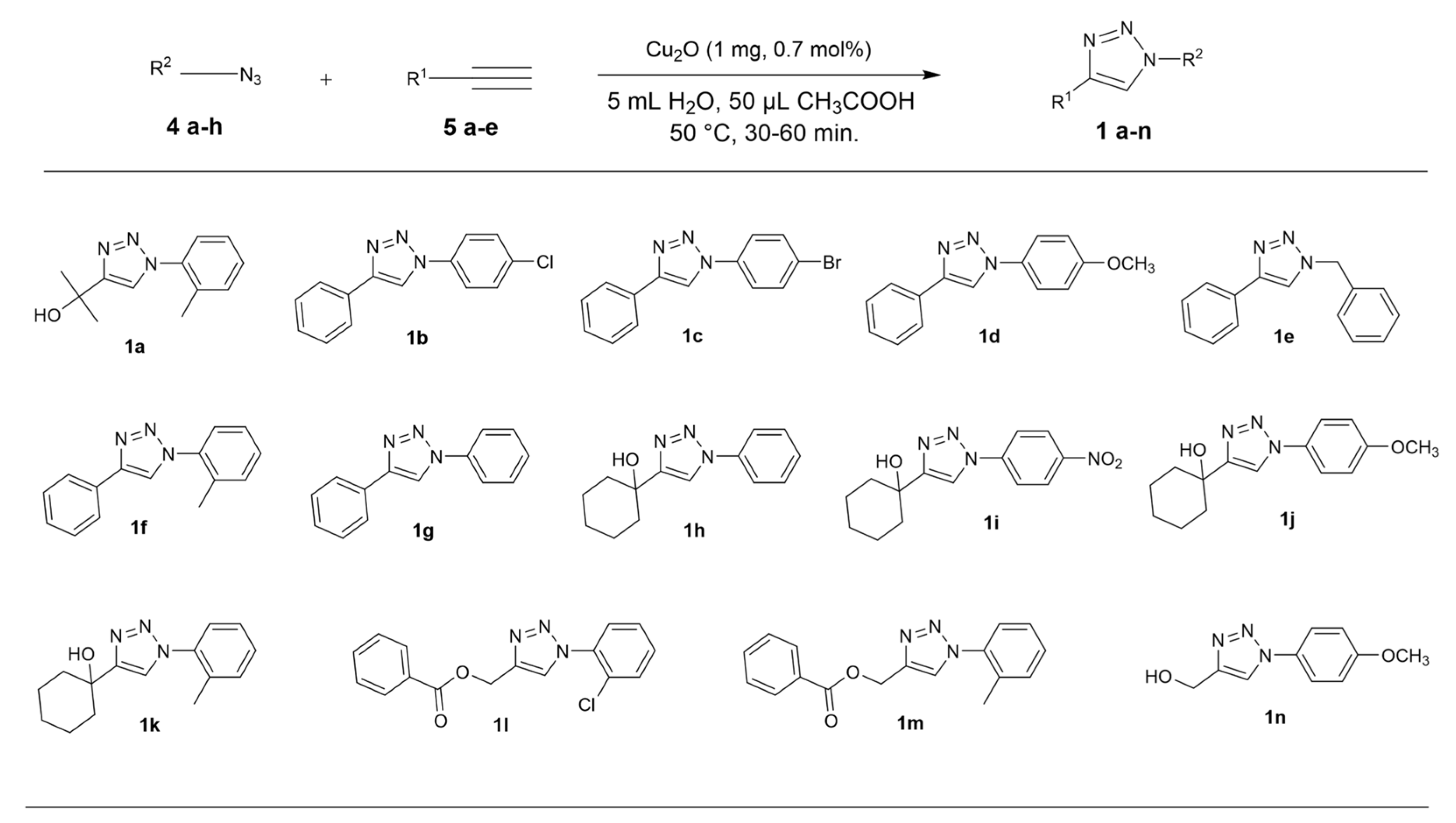
2.1. Chemistry
2.2. General Procedure for CuAAC of Organic Azides with Alkynes to 1,4-Disubstituted 1,2,3-Triazoles (1a–n)


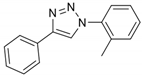
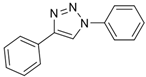
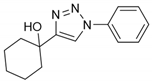
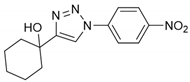
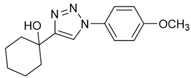
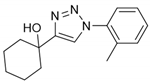
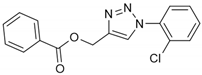
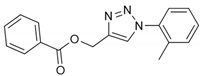
2.3. In Silico Prediction
2.4. Two- and Three-Dimensional Cell Cultures
2.5. Parasites
2.6. Cytotoxicity Analysis
2.7. Trypanocidal Activity
2.8. Drug Efficacy in 3D Microtissue
2.9. Washout Assay
2.10. Drug Combination Assay
2.11. Statistical Analysis
3. Results and Discussion
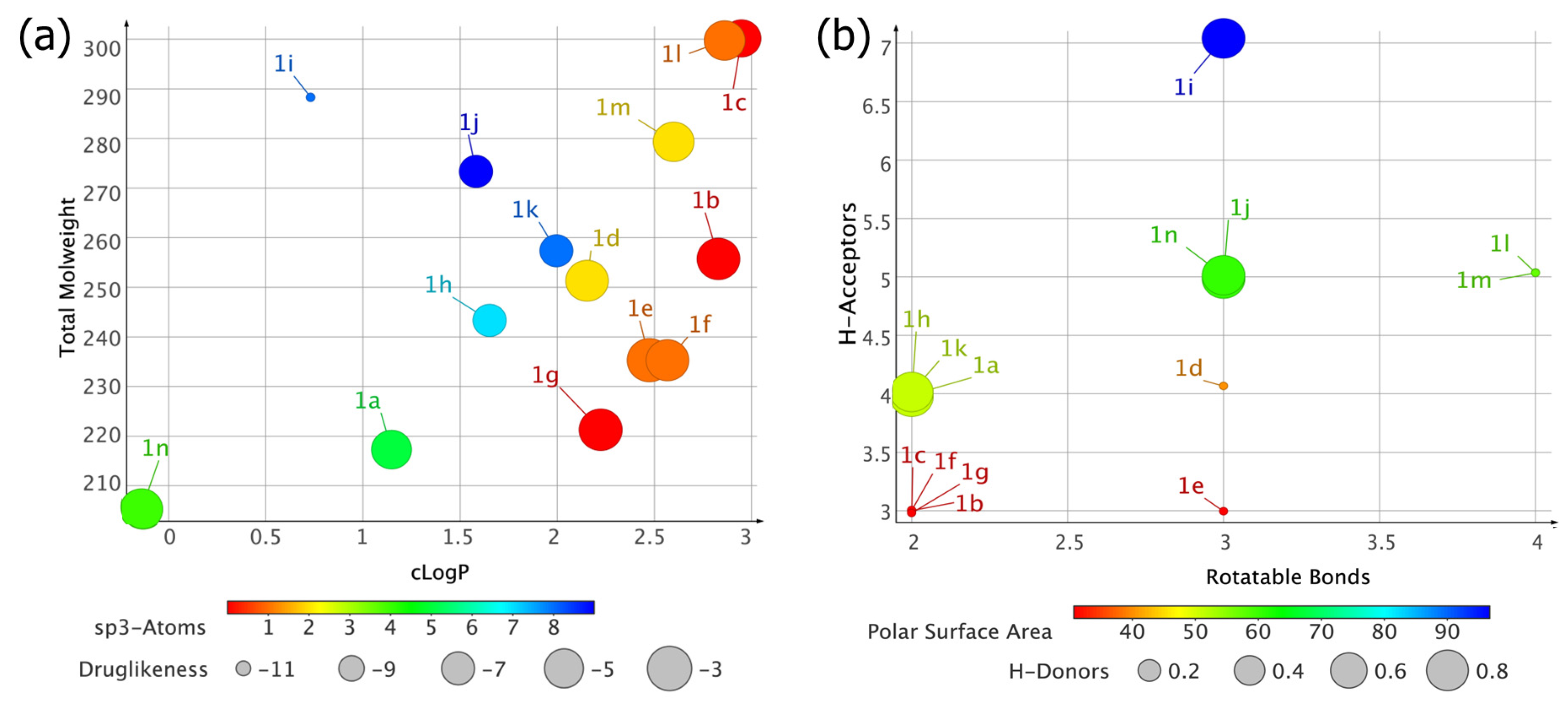
3.1. In Silico Characterization of 1,2,3-Triazole Derivatives
3.2. Cytotoxicity and Biological Activity
3.3. Cardiotoxic Effect of 1,2,3-Triazole Candidates
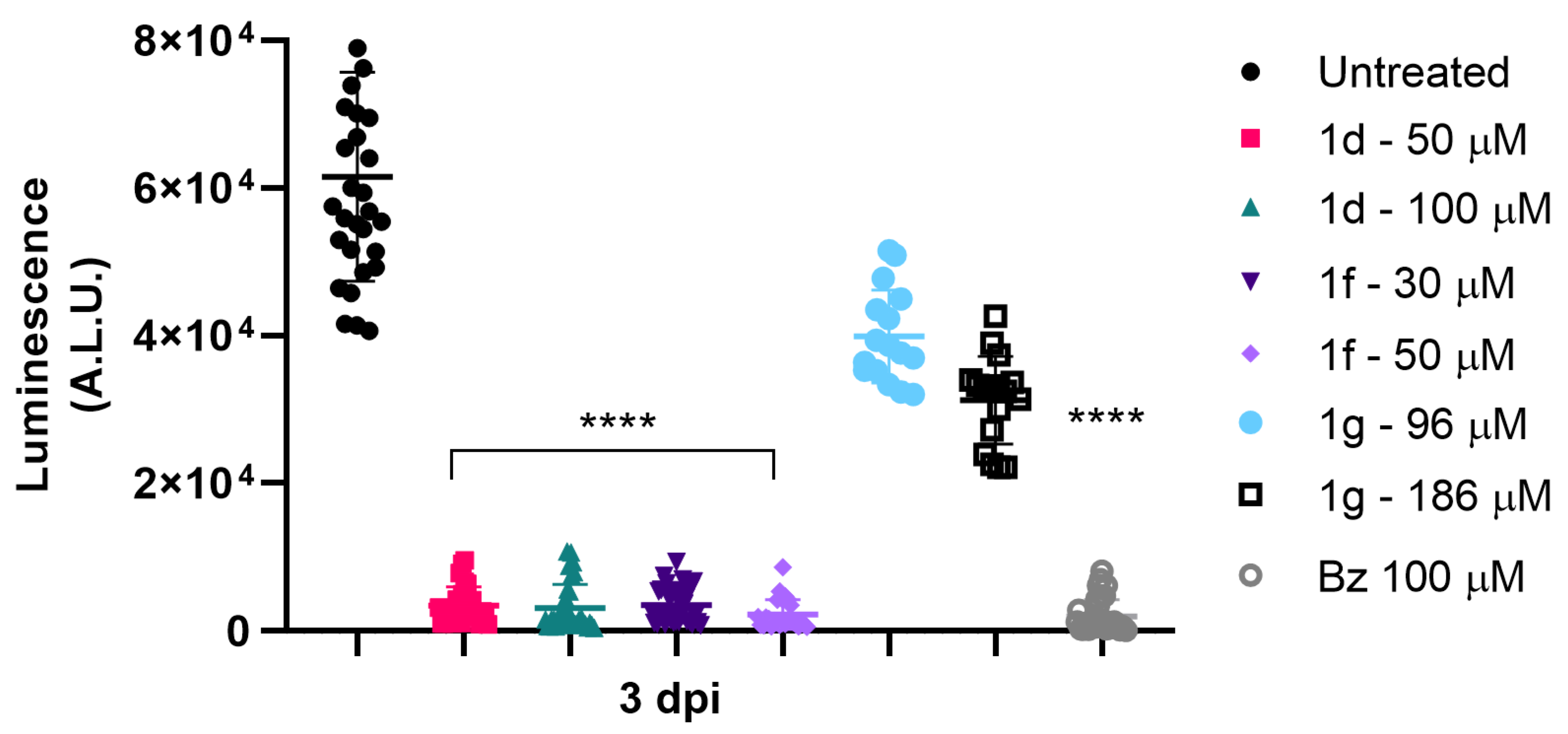
3.4. Drug Efficacy in T. cruzi-Infected 3D Cardiac Spheroid
3.5. Drug Potential to Inhibit Parasite Resurgence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Neglected Tropical Diseases. 2023. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_3 (accessed on 20 April 2023).
- World Health Organization (WHO). Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/338565 (accessed on 20 April 2023).
- Bocchi, E.A. Heart failure in South America. Curr. Cardiol. 2013, 9, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet Infect. Dis. 2010, 375, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet Infect. Dis. 2018, 6, 82–94. [Google Scholar] [CrossRef]
- Echavarría, N.G.; Echeverría, L.E.; Stewart, M.; Gallego, C.; Saldarriaga, C. Chagas disease: Chronic Chagas cardiomyopathy. Curr. Probl. Cardiol. 2021, 46, 100507. [Google Scholar] [CrossRef]
- Andrade, J.P.; Marin-Neto, J.A.; de Paola, A.A.V.; Vilas-Boas, F.; Oliveira, G.M.M.; Sociedade Brasileira de Cardiologia. I diretriz Latino-Americana para o diagnóstico e tratamento da cardiopatia Chagásica. Arq. Bras. Cardiol. 2011, 97, 1–48. [Google Scholar] [CrossRef]
- Keegan, R.; Yeung, C.; Baranchuk, A. Sudden cardiac death risk stratification and prevention in Chagas disease: A non-systematic review of the literature. Arrhythm. Electrophysiol. Rev. 2020, 9, 175–181. [Google Scholar] [CrossRef]
- Heal, C.; Viles-Gonzalez, J.F.; Sáenz, L.C.; Soto, M.; Ramírez, J.D.; d’Avila, A. Arrhythmias in chagasic cardiomyopathy. Card. Electroph. Clin. 2015, 7, 251–268. [Google Scholar] [CrossRef]
- Miranda-Arboleda, A.F.; González-Barrera, L.G.; Liblik, K.; Farina, J.; Zaidel, E.J.; Saldarriaga, C.; Zhou, Z.; Al-Rawi, R.; López-López, J.P.; Juarez-Lloclla, J.P.; et al. Neglected tropical diseases and sudden cardiac death: The NET-heart project. Rev. Cardiovasc. Med. 2022, 23, 254. [Google Scholar] [CrossRef]
- Dias, J.C.P. Facing Chagas disease. Rev. Soc. Bras. Med. Trop. 2017, 50, 285–286. [Google Scholar] [CrossRef]
- Petravicius, P.O.; Costa-Martins, A.G.; Silva, M.N.; Reis-Cunha, J.L.; Bartholomeu, D.C.; Teixeira, M.M.; Zingales, B. Mapping benznidazole resistance in trypanosomatids and exploring evolutionary histories of nitroreductases and ABCG transporter protein sequences. Acta Trop. 2019, 200, 105–161. [Google Scholar] [CrossRef]
- Morillo, C.A.; Marin-Neto, J.A.; Avezum, A.; Sosa-Estani, S.; Rassi, A., Jr.; Rosas, F.; Villena, E.; Quiroz, R.; Bonilla, R.; Britto, C.; et al. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N. Engl. J. Med. 2015, 373, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Molina, I.; Prat, J.G.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I.; et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.A.; Waskin, H.; Sosa-Estani, S.; Del Carmen Bangher, M.; Cuneo, C.; Milesi, R.; Mallagray, M.; Apt, W.; Beloscar, J.; Gascon, J.; et al. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. cruzi carriers: The STOP-CHAGAS Trial. J. Am. Coll. Cardiol. 2017, 69, 939–947. [Google Scholar] [CrossRef]
- Torrico, F.; Gascón, J.; Barreira, F.; Blum, B.; Almeida, I.C.; Alonso-Vega, C.; Barboza, T.; Bilbe, G.; Correia, E.; Garcia, W.; et al. New regimens of benznidazole monotherapy and in combination with fosravuconazole for treatment of Chagas disease (BENDITA): A phase 2, double-blind, randomised trial. Lancet Infect. Dis. 2021, 21, 1129–1140. [Google Scholar] [CrossRef]
- Deeks, E.D. Fexinidazole: First global approval. Drugs 2019, 79, 215–220. [Google Scholar] [CrossRef]
- Torrico, F.; Gascón, J.; Ortiz, L.; Pinto, J.; Rojas, G.; Palacios, A.; Barreira, F.; Blum, B.; Schijman, A.G.; Vaillant, M.; et al. A phase-2, randomized, multicenter, placebo-controlled, proof-of-concept trial of oral fexinidazole in adults with chronic indeterminate Chagas disease. Clin. Infect. Dis. 2022, 4, 579. [Google Scholar] [CrossRef]
- Guo, H.Y.; Chen, Z.A.; Shen, Q.K.; Quan, Z.S. Application of triazoles in the structural modification of natural products. J. Enzyme Inhib. Med. Chem. 2021, 36, 1115–1144. [Google Scholar] [CrossRef]
- Mantoani, S.P.; Andrade, P.; Chierrito, T.P.C.; Figueredo, A.S.; Carvalho, I. Potential triazole-based molecules for the treatment of neglected diseases. Curr. Med. Chem. 2019, 26, 4403–4434. [Google Scholar] [CrossRef]
- França, R.R.F.; Menozzi, C.A.C.; Castelo-Branco, F.S.; Hoelz, L.V.B.; Boechat, N. The medicinal chemistry of 3-nitro-1,2,4-triazoles: Focus on infectious diseases. Curr. Top. Med. Chem. 2021, 21, 2072–2100. [Google Scholar] [CrossRef]
- Assunção, E.L.F.; Carvalho, D.B.; das Neves, A.R.; Shiguemotto, C.Y.K.; Portapilla, G.B.; Albuquerque, S.; Baroni, A.C.M. Synthesis and antitrypanosomal activity of 1,4-disubstituted triazole compounds based on a 2-Nitroimidazole scaffold: A structure-activity relationship study. Chem. Med. Chem. 2020, 15, 2019–2028. [Google Scholar] [CrossRef]
- Shao, C.; Zhu, R.; Luo, S.; Zhang, Q.; Wang, X.; Hu, Y. Copper(I) oxide and benzoic acid ‘on water’: A highly practical and efficient catalytic system for copper(I)-catalyzed azide–alkyne cycloaddition. Tetrahedron Lett. 2021, 52, 3782–3785. [Google Scholar] [CrossRef]
- Pauli, G.F.; Chen, S.-N.; Simmler, C.; Lankin, D.C.; Gödecke, T.; Jaki, B.U.; Friesen, B.; McAlpine, J.B.; Napolitano, J.G. Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay. J. Med. Chem. 2014, 57, 9220–9231. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Fang, Z.; Yang, Z.; Li, X.; Zhu, N.; Wan, L.; Wei, P.; Guo, K. A two-step continuous flow synthesis of 1,4-disubstituted 1,2,3-triazoles under metal- and azide-free conditions. RSC Adv. 2016, 6, 89073–89079. [Google Scholar] [CrossRef]
- Meng, X.; Xu, X.; Gao, T.; Chen, B. Zn/C-catalyzed cycloaddition of azides and aryl alkynes. Eur. J. Org. Chem. 2010, 28, 5409–5414. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Unsupported copper nanoparticles in the 1,3-dipolar cycloaddition of terminal alkynes and azides. Eur. J. Org. Chem. 2010, 10, 1875–1884. [Google Scholar] [CrossRef]
- Sarkar, A.; Mukherjee, T.; Kapoor, S. PVP-stabilized copper nanoparticles: A reusable catalyst for “click” reaction between terminal alkynes and azides in nonaqueous solvents. J. Phys. Chem. 2008, 112, 3334–3340. [Google Scholar] [CrossRef]
- Bakherad, M.; Ghalenoei, A.K.; Keivanloo, A. Synthesis of 1,4-disubstituted 1,2,3-triazoles via 1,3-dipolar cycloaddition/C–N coupling of propargyl alcohols/amines and aryl azides. J. Heterocycl. Chem. 2018, 55, 2683–2692. [Google Scholar] [CrossRef]
- Boechat, N.; Ferreira, V.F.; Ferreira, S.B.; Ferreira, M.D.L.G.; da Silva, F.D.C.; Bastos, M.M.; dos Costa, M.S.; Lourenço, M.C.S.; Pinto, A.C.; Krettli, A.U.; et al. Novel 1,2,3-triazole derivatives for use against Mycobacterium tuberculosis H37Rv (ATCC 27294) strain. J. Med. Chem. 2011, 54, 5988–5999. [Google Scholar] [CrossRef]
- Gannarapu, M.R.; Vasamsetti, S.B.; Punna, N.; Kotamraju, S.; Banda, N. Synthesis of novel 1-substituted triazole linked 1,2-benzothiazine 1,1-dioxido propenone derivatives as potent anti-inflammatory agents and inhibitors of monocyte-to-macrophage differentiation. MedChemComm 2015, 6, 1494–1500. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; DataWarrior, R.C. An open-source program for chemistry aware data visualization and 1038 analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, M.N.; Juliano, L.; Carmona, E.; Silva, S.G.; Costa, E.M.; Murta, A.C.; Scharfstein, J. Inhibitors of the major cysteinyl proteinase (GP57/51) impair host cell invasion and arrest the intracellular development of Trypanosoma cruzi in vitro. Mol. Biochem. Parasitol. 1992, 52, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, L.R.; Adesse, D.; Soares, M.J.; Rossi, M.I.D.; Borojevic, R.; Mereirelles, M.d.N.L. Fibrosis and hypertrophy induced by Trypanosoma cruzi in a three-dimensional cardiomyocyte culture system. J. Infect. Dis. 2008, 197, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Henriques, C.; Henrique-Pons, A.; Meuser-Batista, M.; Ribeiro, A.S.; de Souza, W. In Vivo imaging of mice infected with bioluminescent Trypanosoma cruzi unveils novel sites of infection. Parasit. Vectors 2014, 7, 89. [Google Scholar] [CrossRef]
- Henriques, C.; Castro, D.P.; Gomes, L.H.; Garcia, E.S.; de Souza, W. Bioluminescent imaging of Trypanosoma cruzi infection in Rhodnius prolixus. Parasit. Vectors 2012, 5, 214. [Google Scholar] [CrossRef]
- Orlando, L.M.R.; Lechuga, G.C.; Lara, L.D.S.; Ferreira, B.S.; Pereira, C.N.; Silva, R.C.; dos Santos, M.S.; Pereira, M.C.S. Structural optimization, and biological activity of pyrazole derivatives: Virtual computational analysis, recovery assay and 3D culture model as potential predictive tools of effectiveness against Trypanosoma cruzi. Molecules 2021, 26, 6742. [Google Scholar] [CrossRef]
- Lara, L.S.; Lechuga, G.C.; Orlando, L.M.R.; Ferreira, B.S.; Souto, B.A.; Santos, M.S.; Pereira, M.C.S. Bioactivity of novel pyrazole-thiazolines scaffolds against Trypanosoma cruzi: Computational approaches and 3D spheroid model on drug discovery for Chagas disease. Pharmaceutics 2022, 14, 995. [Google Scholar] [CrossRef]
- Hoffer, L.; Muller, C.; Roche, P.; Moreli, X. Chemistry-driven hit-to-lead optimization guided by structure-based approaches. Mol. Inform. 2018, 37, 9–10. [Google Scholar] [CrossRef]
- Bunally, S.B.; Luscombe, C.N.; Young, R.J. Using physicochemical measurements to influence better compound design. SLAS Discov. 2019, 24, 791–801. [Google Scholar] [CrossRef]
- Shaker, B.; Ahmad, S.; Lee, J.; Jung, C.; Na, D. In silico methods and tools for drug discovery. Comput. Biol. Med. 2021, 137, 104851. [Google Scholar] [CrossRef]
- Chmiel, T.; Mieszkowska, A.; Kempinska-Kupczyk, D.; Kot-Wasik, A.; Namiesnik, J.; Mazerska, J. The impact of lipophilicity on environmental processes, drug delivery and bioavailability of food components. Microchem. J. 2019, 146, 393–406. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Chen, H.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Pone, K.B.; Dalhatou, S.; Paumo, H.K.; Katata-Seru, L.M.; Ferreira, E.I. Triazole-containing heterocycles: Privileged scaffolds in anti-Trypanosoma cruzi drug development. Curr. Drug Targets 2022, 23, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Ko, E.J.; Viayna, E.; Thompson, S.; Spinks, D.; Thomas, M.; Sandberg, L.; Francisco, A.F.; Jayawardhana, S.; Smith, V.C.; et al. Discovery and optimization of 5-Amino-1,2,3-triazole-4-carboxamide series against Trypanosoma cruzi. J. Med. Chem. 2017, 60, 7284–7299. [Google Scholar] [CrossRef]
- Chipoline, I.C.; Brasil, B.F.A.B.; Neto, J.S.S.; Valli, M.; Grogh, R.; Cenci, A.R.; Teixeira, K.F.; Zapp, E.; Brondani, D.; Ferreira, L.L.G.; et al. Synthesis and investigation of the trypanocidal potential of novel 1,2,3-triazole-selenide hybrids. Eur. J. Med. Chem. 2022, 243, 114687. [Google Scholar] [CrossRef]
- Souza, M.L.; Rezende Junior, C.O.; Ferreira, R.S.; Chávez, R.M.E.; Ferreira, L.L.G.; Slafer, B.W.; Magalhães, L.G.; Krogh, R.; Oliva, G.; Cruz, F.C.; et al. Discovery of potent, reversible, and competitive cruzain inhibitors with trypanocidal activity: A Structure-based drug design approach. J. Chem. Inf. Model. 2020, 24, 1028–1041. [Google Scholar] [CrossRef]
- Campo, V.L.; Sesti-Costa, R.; Carneiro, Z.A.; Silva, J.S.; Schenkman, S.; Carvalho, I. Design, synthesis and the effect of 1,2,3-triazole sialylmimetic neoglycoconjugates on Trypanosoma cruzi and its cell surface trans-sialidase. Bioorg. Med. Chem. 2012, 20, 145–156. [Google Scholar] [CrossRef]
- Zimmermann, L.A.; de Moraes, M.H.; da Rosa, R.; de Melo, E.B.; Paula, F.R.; Schenkel, E.P.; Bernardes, L.S.C. Synthesis and SAR of new isoxazole-triazole bis-heterocyclic compounds as analogues of natural lignans with antiparasitic activity. Bioorg. Med. Chem. 2018, 26, 4850–4862. [Google Scholar] [CrossRef]
- Rocha, D.A.; Silva, E.B.; Fortes, I.S.; Lopes, M.S.; Ferreira, R.S.; Andrade, S.F. Synthesis and structure-activity relationship studies of cruzain and rhodesain inhibitors. Eur. J. Med. Chem. 2018, 157, 1426–1459. [Google Scholar] [CrossRef]
- Mamoshina, P.; Rodriguez, B.; Bueno-Orovio, A. Toward a broader view of mechanisms of drug cardiotoxicity. Cell. Rep. Med. 2021, 2, 100216. [Google Scholar] [CrossRef]
- Fung, M.; Thornton, A.; Mybeck, K.; Hsiao-Hiu, J.; Hornbuckle, K.; Muniz, E. Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets-1960–1999. Drug Inf. J. 2001, 35, 293–317. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Abdelsayed, G.; Ali, D.; Malone, A.; Saidi, J.; Myneni, M.; Rajagopal, K.; Faisal, H.; Cheema, F.H.; Hameed, A. 2D and 3D in vitro models for mimicking cardiac physiology. Appl. Eng. Sci. 2022, 12, 100115. [Google Scholar]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaité, V.; Vasconcelos, M.H. 3D Cell culture models as recapitulators of the tumor microenvironment for the screening of anti-cancer drugs. Cancers 2022, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, L.; Aghaei, B.; Law, A.M.K.; Dobrowolski, J.C.; Raftery, L.J.; Tjandra, A.D.; Yee, C.; Piloni, A.; Volkerling, A.; Ferris, J.F.; et al. Generation and analysis of 3D cell culture molds for drug discovery. Eur. J. Pharm. 2021, 163, 105876. [Google Scholar] [CrossRef]
- Varan, G.; Unal, S. Three-dimensional cell culture methods in infectious diseases and vaccine research. Future Pharmacol. 2023, 3, 4. [Google Scholar] [CrossRef]
- Alavi, M.; Tajvar, S.; Hajizadeh, A. Application of Cell Culture Models in Studying Viral Diseases (SARS, H1N1 Flu, MERS, COVID-19): A Review. J. Integr. Cardiol. Open Access 2021, 4, 2–7. [Google Scholar]
- Harimoto, T.; Singer, Z.S.; Velasquez, O.S.; Zhang, J.; Castro, S.; Hinchliffe, T.E.; Mather, W.; Danino, T. Rapid screening of engineered microbial therapies in a 3D multicellular model. Proc. Natl. Acad. Sci. USA 2019, 116, 9002–9007. [Google Scholar] [CrossRef]
- Fiuza, L.F.D.A.; Batista, D.G.J.; Girão, R.D.; Hulpia, F.; Finamore-Araújo, P.; Aldfer, M.M.; Elmahallawy, E.K.; De Koning, H.P.; Moreira, O.; Van Calenbergh, S.; et al. Phenotypic evaluation of nucleoside analogues against Trypanosoma cruzi infection: In Vitro and In Vivo approaches. Molecules 2022, 27, 8087. [Google Scholar] [CrossRef]
- Araújo-Lima, C.F.; Carvalho, R.C.C.; Peres, R.B.; Fiuza, L.F.A.; Galvão, B.V.D.; Castelo-Branco, F.; Bastos, M.M.; Boechat, N.; Felzenszwalb, I.; Soeiro, M.N. In silico and in vitro assessment of anti-Trypanosoma cruzi efficacy, genotoxicity, and pharmacokinetics of pentasubstituted pyrrolic Atorvastatin-aminoquinoline hybrid compounds. Acta Trop. 2023, 242, 106924. [Google Scholar] [CrossRef]
- Law, A.M.K.; Rodriguez de la Fuerte, L.; Grundy, T.J.; Fang, G.; Gallego-Ortega, D. Advancements in 3D cell culture systems for personalizing anti-cancer therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef] [PubMed]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Olliver, E.; Heymann, M.F.; Vallette, M.; Oliver, L.; Heymann, D. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- MacLean, L.M.; Thomas, J.; Lewis, M.D.; Cotillo, I.; Gray, D.W.; Rycker, M. Development of Trypanosoma cruzi in vitro assays to identify compounds suitable for progression in Chagas’ disease drug Discovery. PLoS Negl. Trop. Dis. 2018, 12, e0006612. [Google Scholar] [CrossRef] [PubMed]
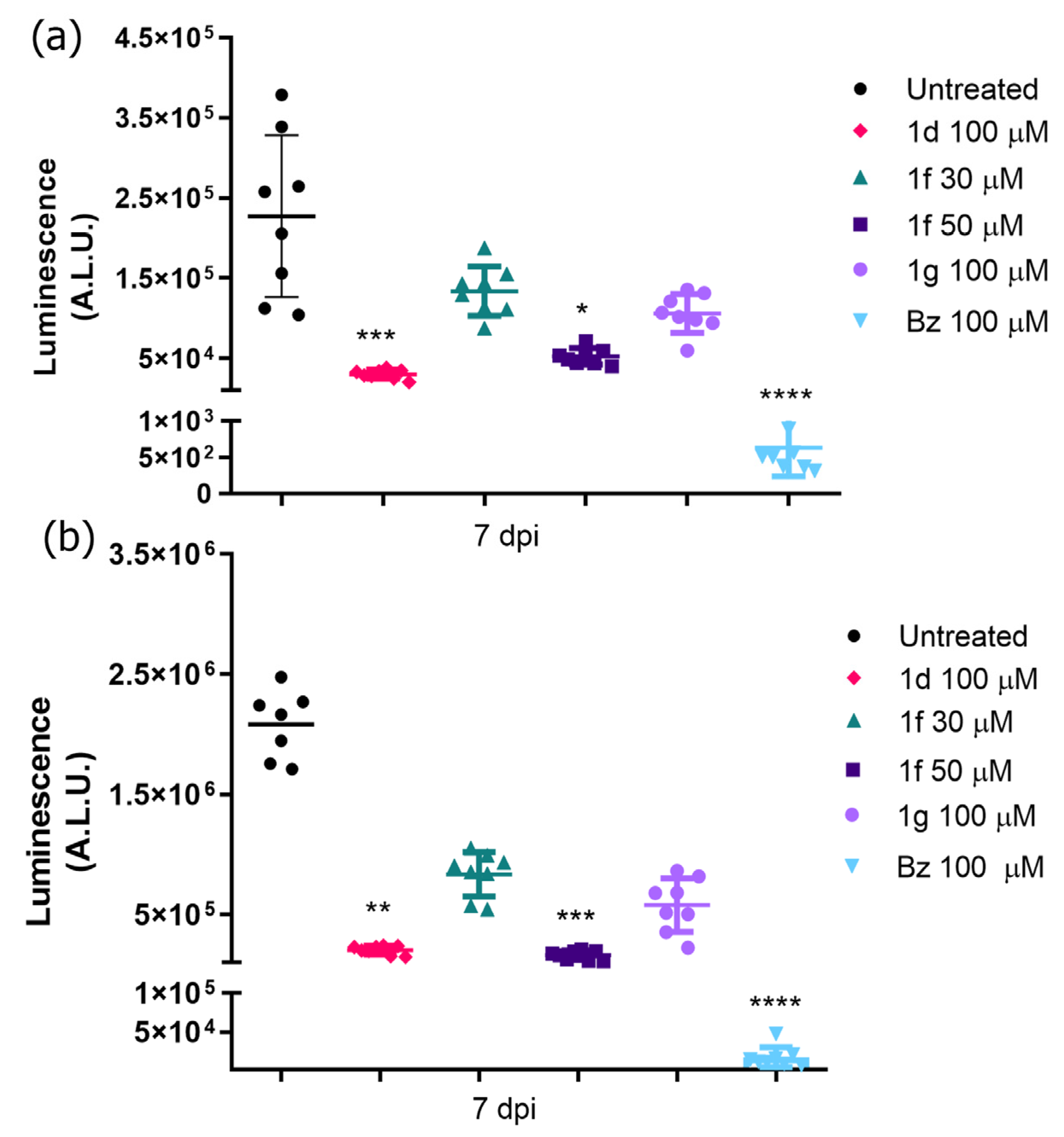
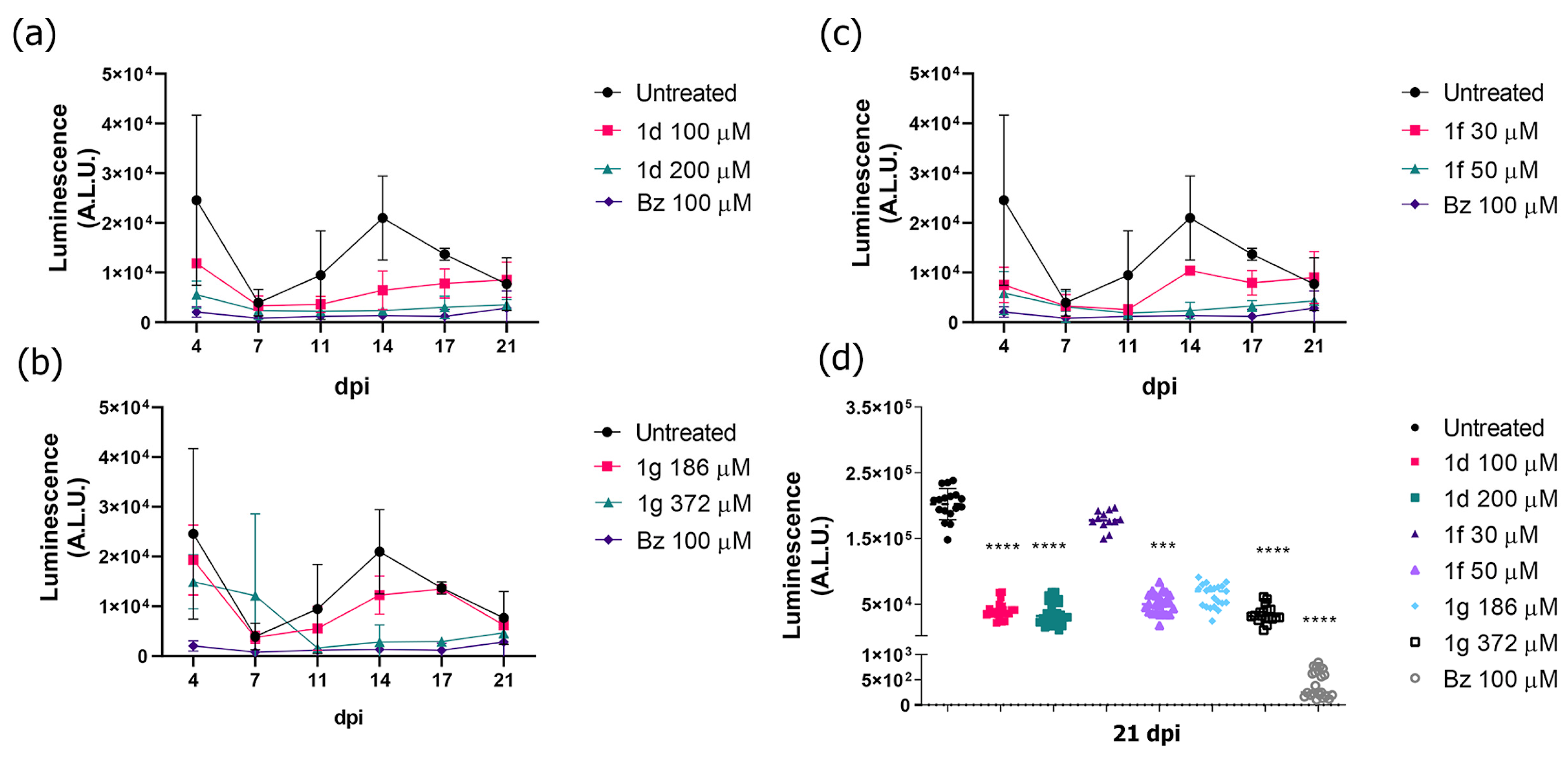
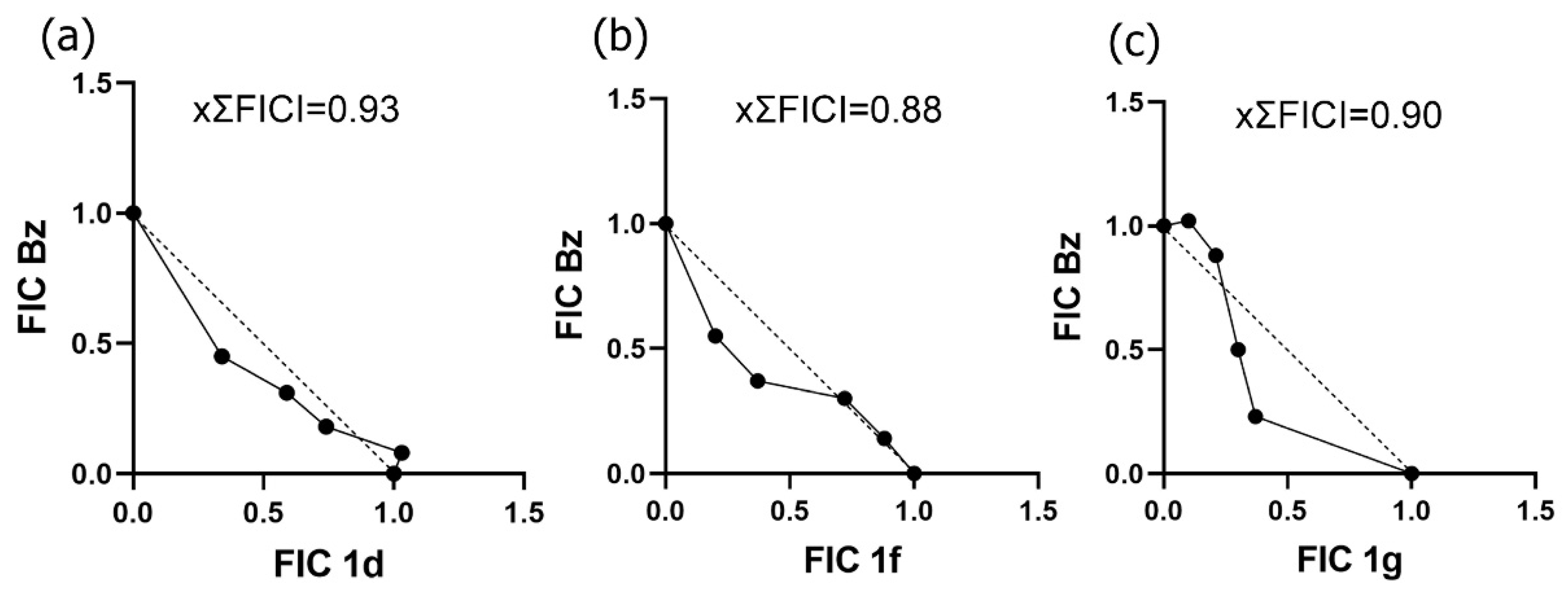
| Trypanocidal Activity Mean ± SD (µM) | Cytotoxicity | ||||||
|---|---|---|---|---|---|---|---|
| Compounds | Trypomastigotes | Intracellular Amastigotes | VERO Cells | ||||
| IC50 | IC90 | SI | IC50 | IC90 | SI | CC50 | |
| 1a | >100 | >100 | Nd | >100 | Nd | Nd | >500 |
| 1b | >100 | >100 | Nd | >100 | Nd | Nd | >500 |
| 1c | >100 | >100 | Nd | >100 | Nd | Nd | >500 |
| 1d | 0.21 ± 0.03 | 2.90 ± 0.16 | >2380 | 3.27 ± 0.90 | >100 | >152 | >500 |
| 1e | >100 | >100 | Nd | 83.24 ± 2.27 | Nd | >6 | >500 |
| 1f | 1.23 ± 0.24 | 3.68 ± 0.60 | 70.5 | 3.50 ± 0.39 | 30.42 ± 0.4 | 24.8 | 86.8 ± 2.73 |
| 1g | 2.28 ± 0.34 | >100 | >219 | 6.20 ± 1.06 | >100 | >80.6 | >500 |
| 1h | >100 | Nd | Nd | >100 | Nd | Nd | >500 |
| 1i | >100 | Nd | Nd | >100 | Nd | Nd | >500 |
| 1j | >100 | Nd | Nd | >100 | Nd | Nd | >500 |
| 1k | >100 | Nd | Nd | >100 | Nd | Nd | >500 |
| 1l | 80.42 ± 3.56 | >100 | 2.94 | 77.90 ± 2.00 | >100 | 3.03 | 236.41 ± 17.87 |
| 1m | >100 | Nd | Nd | >100 | Nd | Nd | >500 |
| 1n | >100 | Nd | Nd | >100 | Nd | Nd | >500 |
| Bz | 22.79 ± 4.12 | >100 | >100 | 4.67 ± 0.22 | 21.69 ± 2.37 | >107 | >500 |
| Compounds | Toxicity (Mean ± SD µM) | |
|---|---|---|
| 2D Culture | 3D Culture | |
| 1d | >500 | >500 |
| 1f | 111.33 ± 10.06 | >500 |
| 1g | >500 | >500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, L.M.R.; Lara, L.d.S.; Lechuga, G.C.; Rodrigues, G.C.; Pandoli, O.G.; de Sá, D.S.; Pereira, M.C.d.S. Antitrypanosomal Activity of 1,2,3-Triazole-Based Hybrids Evaluated Using In Vitro Preclinical Translational Models. Biology 2023, 12, 1222. https://doi.org/10.3390/biology12091222
Orlando LMR, Lara LdS, Lechuga GC, Rodrigues GC, Pandoli OG, de Sá DS, Pereira MCdS. Antitrypanosomal Activity of 1,2,3-Triazole-Based Hybrids Evaluated Using In Vitro Preclinical Translational Models. Biology. 2023; 12(9):1222. https://doi.org/10.3390/biology12091222
Chicago/Turabian StyleOrlando, Lorraine Martins Rocha, Leonardo da Silva Lara, Guilherme Curty Lechuga, Giseli Capaci Rodrigues, Omar Ginoble Pandoli, Druval Santos de Sá, and Mirian Claudia de Souza Pereira. 2023. "Antitrypanosomal Activity of 1,2,3-Triazole-Based Hybrids Evaluated Using In Vitro Preclinical Translational Models" Biology 12, no. 9: 1222. https://doi.org/10.3390/biology12091222
APA StyleOrlando, L. M. R., Lara, L. d. S., Lechuga, G. C., Rodrigues, G. C., Pandoli, O. G., de Sá, D. S., & Pereira, M. C. d. S. (2023). Antitrypanosomal Activity of 1,2,3-Triazole-Based Hybrids Evaluated Using In Vitro Preclinical Translational Models. Biology, 12(9), 1222. https://doi.org/10.3390/biology12091222