In Vitro Antioxidant and Antitrypanosomal Activities of Extract and Fractions of Terminalia catappa
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Obtaining Extract and Fractions
2.3. Chemical Characterization of T. catappa by HPLC-UV-ESI-IT/MS
2.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.5. Ferric Ion Reducing Antioxidant Power (FRAP) Assay
2.6. Cell Culture and Parasites
2.7. Cytotoxicity Assay
2.8. Antitrypanosomal Activity Assay
2.9. Transmission Electron Microscopy
2.10. Statistical Analysis
3. Results
3.1. Composition of Extract and Fractions of T. catappa
3.2. Antioxidant Activity of T. catappa
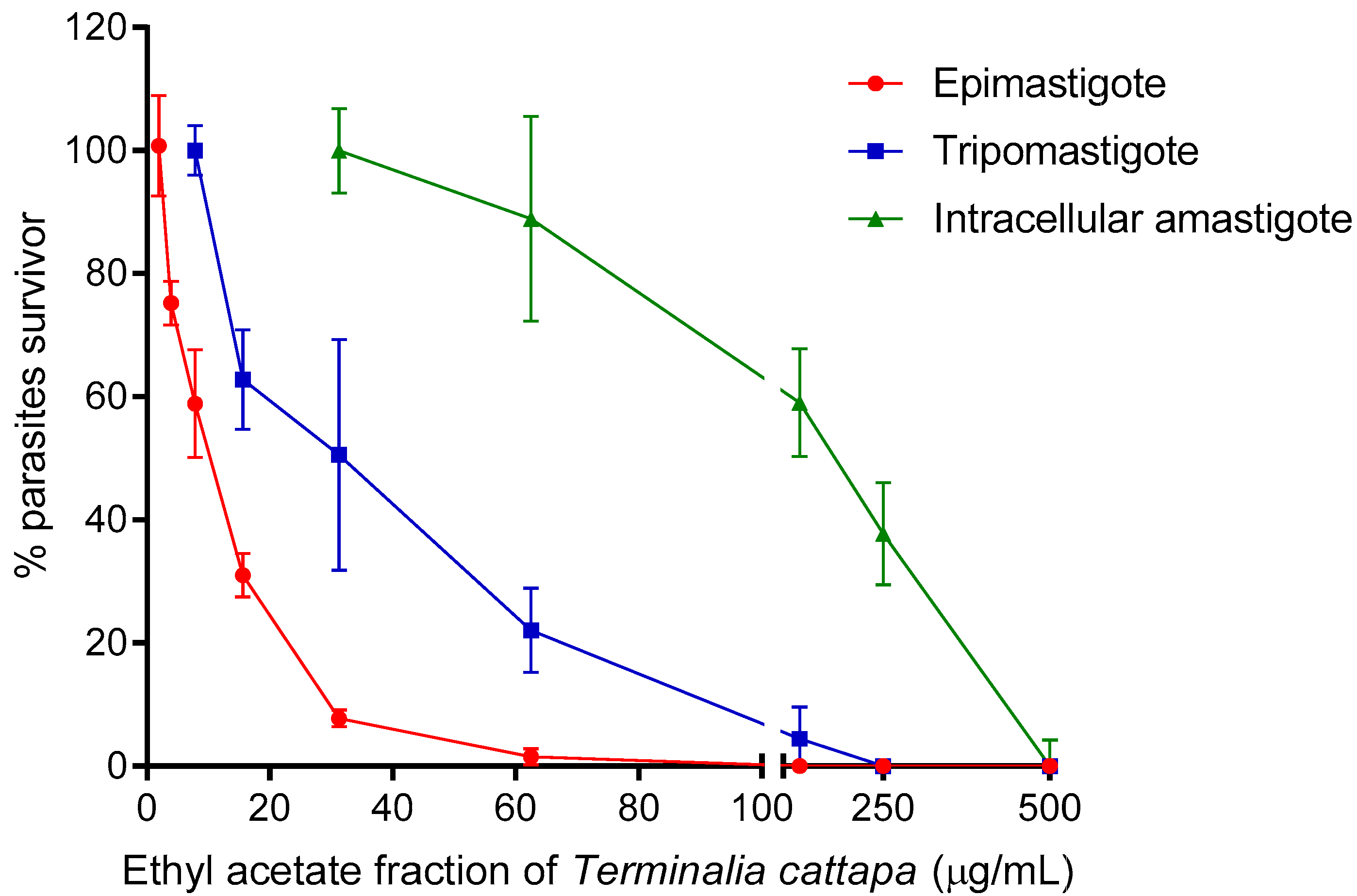
3.3. Antitrypanosomal Activity, Cytotoxicity and Selectivity Index of T. catappa
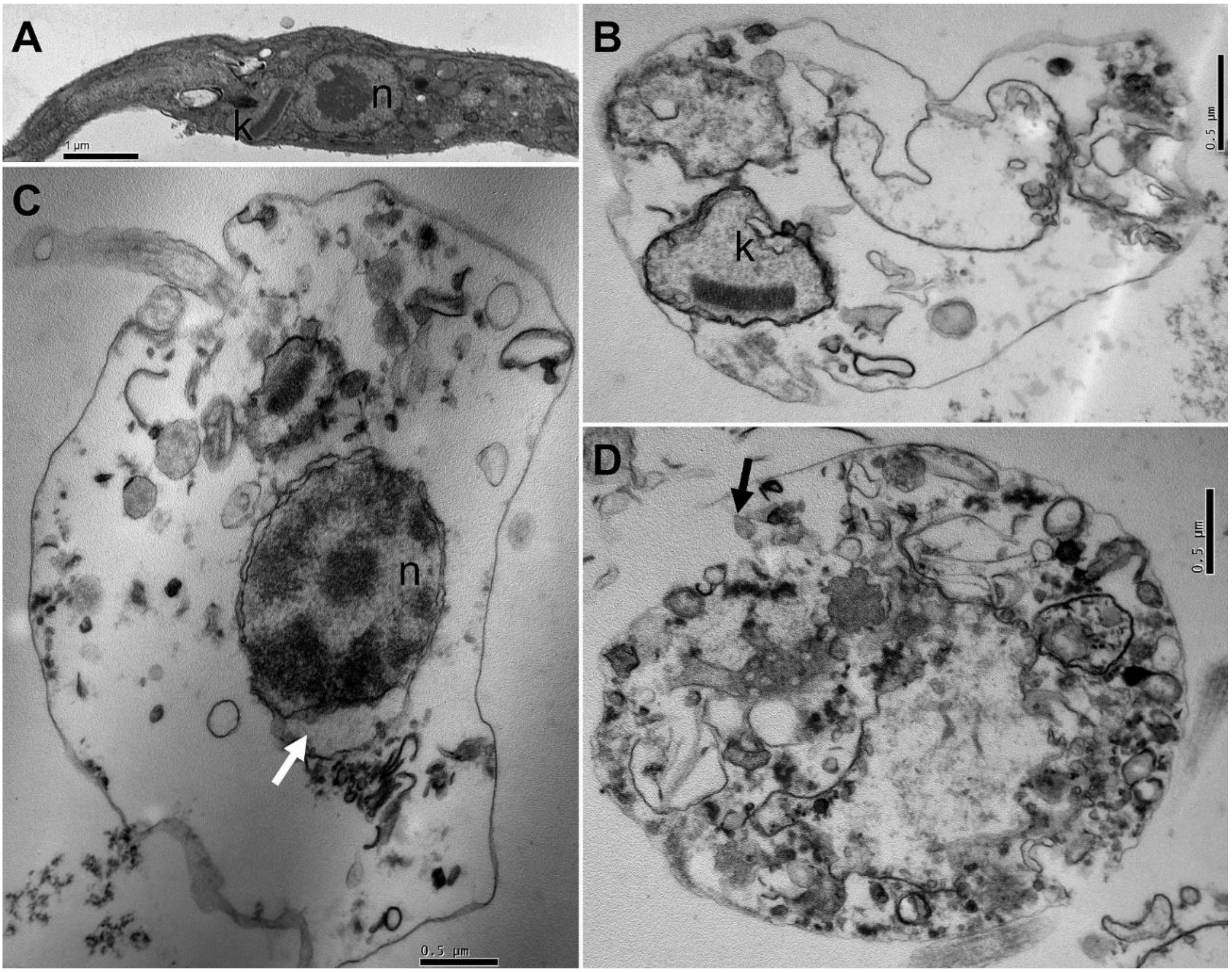
3.4. Effects of T. catappa on T. cruzi Morphology
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Londero, V.S.; Costa-Silva, T.A.; Tempone, A.G.; Namiyama, G.M.; Thevenard, F.; Antar, G.M.; Baitello, J.B.; Lago, J.H.G. Anti-Trypanosoma cruzi activity of costic acid isolated from Nectandra barbellata (Lauraceae) is associated with alterations in plasma membrane electric and mitochondrial membrane potentials. Bioorg. Chem. 2020, 95, 103510. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, J.S.; Suta-Velásquez, M.; Mateus, J.; Pardo-Rodriguez, D.; Puerta, C.J.; Cuéllar, A.; Robles, J.; Cuervo, C. Preliminary chemical characterization of ethanolic extracts from Colombian plants with promising anti-Trypanosoma cruzi activity. Exp. Parasitol. 2021, 223, 108079. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde. Boletim Epidemiológico Doença de Chagas; Secretaria de Vigilância em Saúde, Ministério da Saúde: Brasilia, Brazil, 2021; 38p.
- de Melo, A.R.B.; Higino, T.M.M.; da Rocha Oliveira, A.D.P.; Fontes, A.; da Silva, D.C.N.; de Castro, M.C.A.B.; Lopes, J.A.D.; de Figueiredo, R.C.B.Q. Lippia sidoides and Lippia origanoides essential oils affect the viability, motility and ultrastructure of Trypanosoma cruzi. Micron 2020, 29, 102781. [Google Scholar] [CrossRef] [PubMed]
- Santi, A.M.M.; Murta, S.M.F. Antioxidant defence system as a rational target for Chagas disease and Leishmaniasis chemotherapy. Mem. Inst. Oswaldo Cruz 2022, 117, e210401. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A. Specific chemotherapy of Chagas disease: Relevance, current limitations and new approaches. Acta Trop. 2010, 115, 55–68. [Google Scholar] [CrossRef]
- Zhang, X.R.; Kaunda, J.S.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. The genus Terminalia (Combretaceae): An ethnopharmacological, phytochemical and pharmacological review. Nat. Prod. Bioprospect. 2019, 9, 357–392. [Google Scholar] [CrossRef]
- Das, G.; Kim, D.Y.; Fan, C.; Gutiérrez-Grijalva, E.P.; Heredia, J.B.; Nissapatorn, V.; Mitsuwan, W.; Pereira, M.L.; Nawaz, M.; Siyadatpanah, A.; et al. Plants of the genus Terminalia: An insight on its biological potentials, pre-clinical and clinical studies. Front. Pharmacol. 2020, 11, 561248. [Google Scholar] [CrossRef]
- Ribeiro, R.D.T.M.; Loiola, M.I.B.; Sales, M.F.D. Terminalia L. (Combretaceae) do Estado de Pernambuco, Brasil. Hoehnea 2018, 45, 307–313. [Google Scholar] [CrossRef]
- Abiodun, O.O.; Rodríguez-Nogales, A.; Algieri, F.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Antiinflammatory and immunomodulatory activity of an ethanolic extract from the stem bark of Terminalia catappa L. (Combretaceae): In vitro and in vivo evidences. J. Ethnopharmacol. 2016, 192, 309–319. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Proposed mechanisms of Terminalia catappa in hyperglycaemia and associated diabetic complications. J. Pharm. Pharmacol. 2017, 69, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Mbekou, M.I.K.; Dize, D.; Yimgang, V.L.; Djague, F.; Toghueo, R.M.K.; Sewald, N.; Lenta, B.N.; Boyom, F.F. Antibacterial and mode of action of extracts from endophytic fungi derived from Terminalia mantaly, Terminalia catappa, and Cananga odorata. BioMed Res. Int. 2021, 2021, 6697973. [Google Scholar] [CrossRef] [PubMed]
- Shanehbandi, D.; Zarredar, H.; Asadi, M.; Zafari, V.; Esmaeili, S.; Seyedrezazadeh, E.; Soleimani, Z.; Jadid, H.S.; Eyvazi, S.; Feyziniya, S.; et al. Anticancer impacts of Terminalia catappa extract on SW480 colorectal neoplasm cell line. J. Gastrointest. Cancer 2021, 52, 99–105. [Google Scholar] [CrossRef]
- Chang, C.K.; Chu, S.C.; Huang, J.Y.; Chen, P.N.; Hsieh, Y.S. Terminalia catappa leaf extracts inhibited metastasis of A2058 and A375 melanoma cells via downregulating p-Src and β-catenin pathway in vitro. Front. Farmacol. 2022, 13, 963589. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Souza, F.; de Souza, C.D.S.F.; Taniwaki, N.N.; Silva, J.J.M.; de Oliveira, R.M.; Abreu-Silva, A.L.; da Silva Calabrese, K. Morinda citrifolia Linn. Fruit (Noni) juice induces an increase in NO production and death of Leishmania amazonensis amastigotes in peritoneal macrophages from BALB/c. Nitric Oxide 2016, 58, 51–58. [Google Scholar] [CrossRef]
- Almeida-Souza, F.; Silva, V.D.D.; Silva, G.X.; Taniwaki, N.N.; Hardoim, D.D.J.; Buarque, C.D.; Abreu-Silva, A.L.; Calabrese, K.D.S. 1,4-Disubstituted-1,2,3-Triazole compounds induce ultrastructural alterations in Leishmania amazonensis promastigote: An in vitro antileishmanial and in silico pharmacokinetic study. Int. J. Mol. Ciência 2020, 21, 6839. [Google Scholar] [CrossRef]
- Ouattara, L.P.; Sanon, S.; Mahiou-Leddet, V.; Gansané, A.; Baghdikian, B.; Traoré, A.; Azas, N.; Ollivier, E.; Sirima, S.B. In vitro antiplasmodial activity of some medicinal plants of Burkina Faso. Parasitol. Res. 2014, 113, 405–416. [Google Scholar] [CrossRef]
- Mahmoud, A.B.; Mäser, P.; Kaiser, M.; Hamburger, M.; Khalid, S. Mining sudanese medicinal plants for antiprotozoal agents. Front. Pharmacol. 2020, 11, 865. [Google Scholar] [CrossRef]
- Muganga, R.; Bero, J.; Quetin-Leclercq, J.; Angenot, L.; Tits, M.; Mouithys-Mickalad, A.; Franck, T.; Frédérich, M. In vitro Antileishmanial, Antitrypanosomal, and Anti-inflammatory-like Activity of Terminalia mollis Root Bark. Planta Med. 2021, 87, 724–731. [Google Scholar] [CrossRef]
- Djedjibegovic, J.; Marjanovic, A.; Panieri, E.; Saso, L. llagic acid-derived urolithins as modulators of oxidative stress. Oxidative Med. Cell. Longev. 2020, 2020, 5194508. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Jamieson, S.; Pandey, A.K.; Bishayee, A. Neuroprotective potential of ellagic acid: A critical review. Adv. Nutr. 2021, 12, 1211–1238. [Google Scholar] [CrossRef]
- Yoganathan, S.; Alagaratnam, A.; Acharekar, N.; Kong, J. Ellagic acid and schisandrins: Natural biaryl polyphenols with therapeutic potential to overcome multidrug resistance in cancer. Cells 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Zhang, G.; Zhang, J.; Ren, L. Synergism of ellagic acid in combination with radiotherapy and chemotherapy for cancer treatment. Phytomedicine 2021, 99, 153998. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS characterization of antibacterial, antioxidant, and antitrypanosomal activity of Syzygium aromaticum essential oil and eugenol. Evid. Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.N.; Peluffo, G.; Piacenza, L.; Radi, R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: Consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J. Biol. Chem. 2011, 286, 6627–6640. [Google Scholar] [CrossRef]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef]
- Paiva, C.N.; Medei, E.; Bozza, M.T. ROS and Trypanosoma cruzi: Fuel to infection, poison to the heart. PLoS. Pathog. 2018, 14, e1006928. [Google Scholar] [CrossRef]
- Montenote, M.C.; Wajsman, V.Z.; Konno, Y.T.; Ferreira, P.C.; Silva, R.M.G.; Therezo, A.L.S.; Silva, L.P.; Martins, L.P.A. Antioxidant effect of Morus nigra on Chagas disease progression. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e73. [Google Scholar] [CrossRef]
- Quintero, W.L.; Moreno, E.M.; Pinto, S.M.L.; Sanabria, S.M.; Stashenko, E.; García, L.T. Immunomodulatory, trypanocide, and antioxidant properties of essential oil fractions of Lippia alba (Verbenaceae). BMC Complement. Med. Ther. 2021, 21, 187. [Google Scholar] [CrossRef]
- Sánchez-Villamil, J.P.; Bautista-Niño, P.K.; Serrano, N.C.; Rincon, M.Y.; Garg, N.J. Potential role of antioxidants as adjunctive therapy in Chagas disease. Oxidative Med. Cell. Longev. 2020, 2020, 9081813. [Google Scholar] [CrossRef]
- Maldonado, E.; Rojas, D.A.; Urbina, F.; Solari, A. The use of antioxidants as potential co-adjuvants to treat chronic chagas disease. Antioxidants 2021, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Hwang, Y.S.C. Antibacterial and antioxidant effect of ethanol extracts of Terminalia chebula on Streptococcus mutans. Clin. Exp. Dent. Res. 2021, 7, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.N.; Ahmad, S.; Tousif, M.I.; Ahmad, I.; Rao, H.; Ahmad, B.; Basit, A. Profiling of phytochemicals from aerial parts of Terminalia neotaliala using LC-ESI-MS2 and determination of antioxidant and enzyme inhibition activities. PLoS ONE 2022, 17, e0266094. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Wakeel, A.; Shinwari, Z.J.; Sohail, K.A.; Khalil, A.; Ali, M. Antibacterial and antifungal activity of Isatis tinctoria L. (Brassicaceae) using the micro-plate method. Pak. J. Bot. 2017, 49, 1949–1957. [Google Scholar]
- Chekroun-Bechlaghem, N.; Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Nanni, V.; Di Marco, G.; Canuti, L.; Canini, A.; El Haci, I.A.; Atik Bekkara, F. Phytochemical analysis and antioxidant activity of Tamarix africana, Arthrocnemum macrostachyum and Suaeda fruticosa, three halophyte species from Algeria. Plant Biosyst. 2019, 153, 843–852. [Google Scholar] [CrossRef]
- Dehkharghanian, M.; Adenier, H.; Vijayalakshmi, M.A. Study of flavonoids in aqueous spinach extract using positive electrospray ionisation tandem quadrupole mass spectrometry. Food Chem. 2010, 121, 863–870. [Google Scholar] [CrossRef]
- Rufin Marie, T.K.; Mbetyoumoun Mfouapon, H.; Madiesse Kemgne, E.A.; Jiatsa Mbouna, C.D.; Tsouh Fokou, P.V.; Sahal, D.; Fekam Boyom, F. Anti-plasmodium falciparum activity of extracts from 10 Cameroonian medicinal plants. Medicines 2018, 5, 115. [Google Scholar] [CrossRef]
- Ohashi, M.; Amoa-Bosompem, M.; Kwofie, K.D.; Agyapong, J.; Adegle, R.; Sakyiamah, M.M.; Ayertey, F.; Owusu, K.B.; Tuffour, I.; Atchoglo, P.; et al. In vitro antiprotozoan activity and mechanisms of action of selected Ghanaian medicinal plants against Trypanosoma, Leishmania, and Plasmodium parasites. Phytother. Res. 2018, 32, 1617–1630. [Google Scholar] [CrossRef]
- Camara, A.; Haddad, M.; Traore, M.S.; Chapeland-Leclerc, F.; Ruprich-Robert, G.; Fourasté, I.; Balde, M.A.; Royo, J.; Parny, M.; Batigne, P.; et al. Variation in chemical composition and antimalarial activities of two samples of Terminalia albida collected from separate sites in Guinea. BMC Complement. Med. Ther. 2021, 21, 64. [Google Scholar] [CrossRef]
- Griebler, A.; Banhuk, F.W.; Staffen, I.V.; Bortoluzzi, A.A.M.; Ayala, T.S.; Gandra, R.F.; Schuquel, I.T.A.; da Silva, A.E.A.; Marinho Jorge, M.T.C.; Menolli, R.A. Anti-Trypanosoma cruzi activity, cytotoxicity and, chemical characterization of extracts from seeds of Lonchocarpus cultratus. J. Infect. Dev. Ctries. 2021, 15, 270–279. [Google Scholar] [CrossRef]
- Chaves, A.C.T.A.; Rocha, V.P.C.; Bastos, T.M.; Soares, M.B.P.; Rodrigues, A.C.B.D.C.; Bezerra, D.P.; Silva Júnior, L.J.C.; Paula, V.F.; Silva, E.R.; Queiroz, R.F. Antioxidant, antibacterial, leishmanicidal and trypanocidal activities of extract and fractions of Manilkara rufula stem bark. Int. J. Adv. Eng. Res. Sci. 2019, 6, 672–687. [Google Scholar] [CrossRef]
- García-Huertas, P.; Olmo, F.; Sánchez-Moreno, M.; Dominguez, J.; Chahboun, R.; Triana-Chávez, O. Activity in vitro and in vivo against Trypanosoma cruzi of a furofuran lignan isolated from Piper jericoense. Exp. Parasitol. 2018, 189, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.M.C.; Pedra-Rezende, Y.; Pereira, L.D.; de Melo, T.G.; Barbosa, H.S.; Lannes-Vieira, J.; de Castro, S.L.; Daliry, A.; Salomão, K. Benznidazole and amiodarone combined treatment attenuates cytoskeletal damage in Trypanosoma cruzi-infected cardiac cells. Front. Cell. Infect. Microbiol. 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]


| Compound | Mode | Fragments | ||
|---|---|---|---|---|
| 1 | Hexahydroxydiphenyldigalloylglucose acid | − | MS2 | 785 [M-H]−/765; 633 [M-H-152]−; 483 [M-H-152-132-18]−; 419; 301 |
| MS3 | 785→483/331 [M-H-152-132-18-152]−; 313 [M-H-152-132-18-152-18]−; 193; 169 | |||
| + | MS2 | 805 [M + H2O]+/787 [M + H-18]+; 617 [M + H-18-152-18]+; 467; 303 | ||
| MS3 | 805→787/769; 617; 467; 449; 303 [M + H-152-152-162-18]+; 277 | |||
| 2 | Trigaloyl-hexoside | − | MS2 | 635 [M-H]−/483 [M-H-152]−; 465 [M-H-152-18]−; 313 [M-H-152-18-152]−; |
| MS3 | 635→465/447; 313 [M-H-152-18-152]−; 295 [M-H-152-132-18-18]−; 169 | |||
| + | MS2 | 659 [M + Na]+/489 [M + Na-152-18]+; 319 | ||
| MS3 | 659→489/471; 337; 319 [M + Na-152-18-152-18]+ | |||
| 3 | Galoyl-HHDP-hexoside | − | MS2 | 633 [M-H]−/463 [M-H-152-18]−; 301 [M-H-152-18-162]−; 275 |
| MS3 | 633→301/301 [M-H-152-18-162]− | |||
| 4 | Luteolin 8-C-hexoside | − | MS2 | 447 [M-H]−/357 [M-H-90]−; 327 [M-H-120]− |
| MS3 | 447→327/327 [M-H-120]−; 299 [M-H-120-28]− | |||
| 5 | Luteolin 6-C-hexoside | − | MS2 | 447 [M-H]−/429 [M-H-18]−; 357 [M-H-90]−; 327 [M-H-120]− |
| MS3 | 447→327/327 [M-H-120]−; 299 | |||
| 6 | Apigenin 8-C-hexoside | − | MS2 | 431 [M-H]−/341 [M-H-90]−; 311 [M-H-120]−; 283 [M-H-120-18]− |
| MS3 | 431→311/311 [M-H-120-18]−; 283 | |||
| 7 | Luteolin-6-C-(2″-galloyl)-hexoside | − | MS2 | 599 [M-H]−/555 [M-H-44]−; 447 [M-H-152]−; 429 [M-H-152-18]−; 327 [M-H-152-120]− |
| MS3 | 599→447/429; 357; 327 [M-H-152-120]− | |||
| + | MS2 | 601 [M + H]+/583 [M + H-18]+; 481 [M + H-120]+; 449; 431 [M + H-170]+; 383; 329; 311 | ||
| MS3 | 601→431/413;395; 383; 353; 311 [M + H-18-152-120]+ | |||
| 8 | Apigenin 6-C-hexoside | − | MS2 | 431 [M-H]−/413 [M-H-18]−; 341 [M-H-90]−; 311 [M-H-120]− |
| MS3 | 431→311/311; 283 [M-H-120-28]− | |||
| 9 | Apigenin 6-C-(2″-galloyl)-hexoside | − | MS2 | 583 [M-H]−/431 [M-H-152]−; 413 [M-H-152-18]−; 311 [M-H-152-120]−; 169 |
| MS3 | 583→431/341; 311 [M-H-152-120]−; 283 | |||
| + | MS2 | 585 [M + H]+/567; 465; 415 [M + H-170]+; 379; 367; 313 | ||
| MS3 | 585→415/397; 379 [M + H-170-18-18]+; 325; 295; 283 | |||
| 10 | Quercetin 3-O-hexoside | − | MS2 | 463 [M-H]−/343 [M-H-120]−; 301 [M-H-162]− |
| MS3 | 463→301/301 [M-H-162]−; 179; 151 | |||
| + | MS2 | 465 [M + H]+/303 [M + H-162]+ | ||
| 11 | Ellagic acid | − | MS2 | 301 [M-H]−/301 |
| + | MS2 | 627 [2M + Na]+/325 [2M + Na-302]+ | ||
| 12 | Kaempferol 3-O-(6″-deoxyhexosyl) -hexoside | − | MS2 | 593 [M-H]−/447 [M-H-146]−; 429 [M-H-146-18]−; 309 [M-H-146-18-120]−; 285 [M-H-146-162]− |
| MS3 | 593→429/309 [M-H-146-18-120]− | |||
| + | MS2 | 595 [M + H]+/577; 449; 287 [M + H-146-162]+ | ||
| Terminalia catappa | DPPH IC50 (µg/mL) | FRAP (µM TE/mg Extract) |
|---|---|---|
| Hydroalcoholic extract | 221.70 ± 2.43 | 953.02 ± 0.16 |
| Hexanic fraction | 84.77 ± 1.05 | 153.52 ± 0.02 |
| Ethyl acetate fraction | 7.77 ± 1.61 | 687.61 ± 0.26 |
| Aqueous fraction | 5.26 ± 1.26 | 1009.32 ± 0.13 |
| Trolox | 12.80 ± 1.09 | - |
| T. catappa | Cytotoxicity CC50 (µg/mL) | T. cruzi IC50 (µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| Vero | Epimastigote | SIepi | Tripomastigote | SItri | Intracellular Amastigote | SIama | |
| Hydroalcoholic extract | >1000 | 70.85 ± 1.10 | >14.11 | 25.42 ± 1.37 | >39.33 | - | - |
| Hexanic fraction | 222.40 ± 1.51 | 148.40 ± 1.20 | 1.49 | 34.51 ± 1.15 | 6.44 | - | - |
| Ethyl acetate fraction | >1000 | 8.86 ± 1.13 | >112.86 | 24.91 ± 1.15 | >40.14 | 85.01 ± 1.21 | >11.76 |
| Aqueous fraction | >1000 | 104.50 ± 1.11 | >9.56 | 54.59 ± 1.13 | >18.31 | - | - |
| Ellagic acid | >1000 | 215.18 ± 1.57 | >4.64 | >500 | nd | - | - |
| Benznidazole | >1000 | 8.66 ± 1.22 | >115.47 | 9.29 ± 1.28 | 107.64 | 12.84 ± 1.08 | 77.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, S.A.d.; Lima, A.d.S.; Rocha, C.Q.d.; Previtalli-Silva, H.; Hardoim, D.d.J.; Taniwaki, N.N.; Calabrese, K.d.S.; Almeida-Souza, F.; Abreu-Silva, A.L. In Vitro Antioxidant and Antitrypanosomal Activities of Extract and Fractions of Terminalia catappa. Biology 2023, 12, 895. https://doi.org/10.3390/biology12070895
Araújo SAd, Lima AdS, Rocha CQd, Previtalli-Silva H, Hardoim DdJ, Taniwaki NN, Calabrese KdS, Almeida-Souza F, Abreu-Silva AL. In Vitro Antioxidant and Antitrypanosomal Activities of Extract and Fractions of Terminalia catappa. Biology. 2023; 12(7):895. https://doi.org/10.3390/biology12070895
Chicago/Turabian StyleAraújo, Sandra Alves de, Aldilene da Silva Lima, Cláudia Quintino da Rocha, Henrique Previtalli-Silva, Daiana de Jesus Hardoim, Noemi Nosomi Taniwaki, Kátia da Silva Calabrese, Fernando Almeida-Souza, and Ana Lucia Abreu-Silva. 2023. "In Vitro Antioxidant and Antitrypanosomal Activities of Extract and Fractions of Terminalia catappa" Biology 12, no. 7: 895. https://doi.org/10.3390/biology12070895
APA StyleAraújo, S. A. d., Lima, A. d. S., Rocha, C. Q. d., Previtalli-Silva, H., Hardoim, D. d. J., Taniwaki, N. N., Calabrese, K. d. S., Almeida-Souza, F., & Abreu-Silva, A. L. (2023). In Vitro Antioxidant and Antitrypanosomal Activities of Extract and Fractions of Terminalia catappa. Biology, 12(7), 895. https://doi.org/10.3390/biology12070895








