Subaerial Decomposition of Small-Sized Remains in The Netherlands: Important Findings Regarding the PMI of a Four-Year Taphonomic Study
Abstract
:Simple Summary
Abstract
1. Introduction
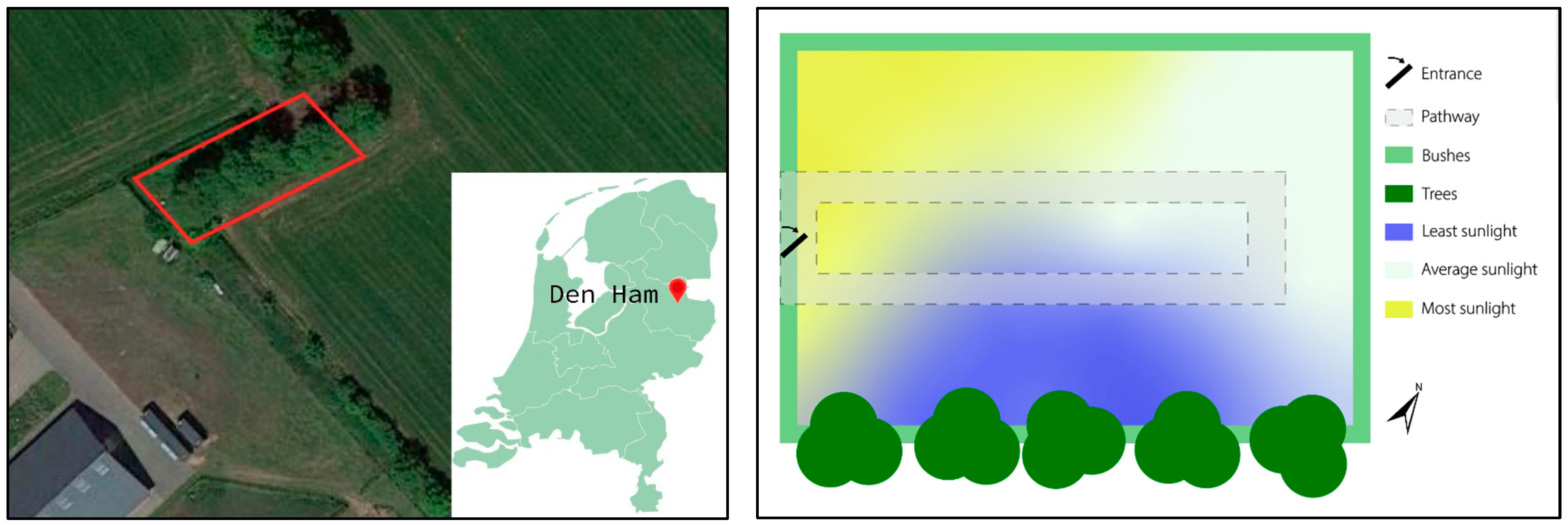
2. Materials and Methods
3. Results and Discussion
3.1. Decomposition Results and Interpretation
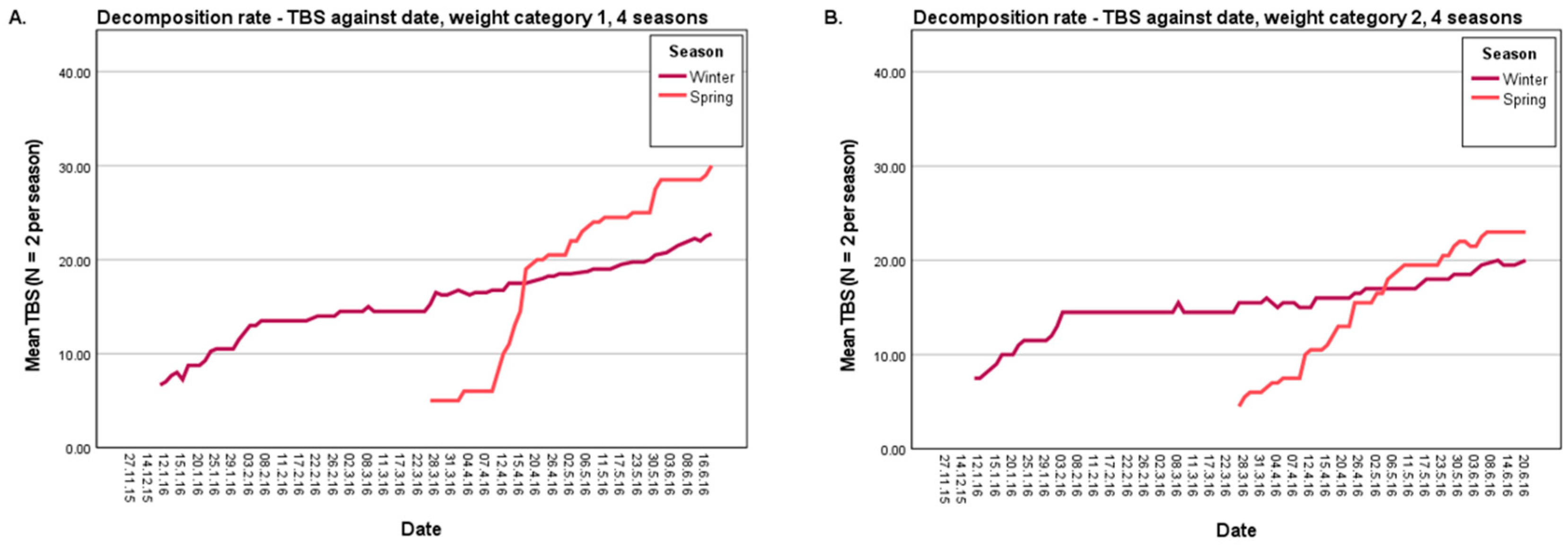
3.1.1. Decomposition Rate
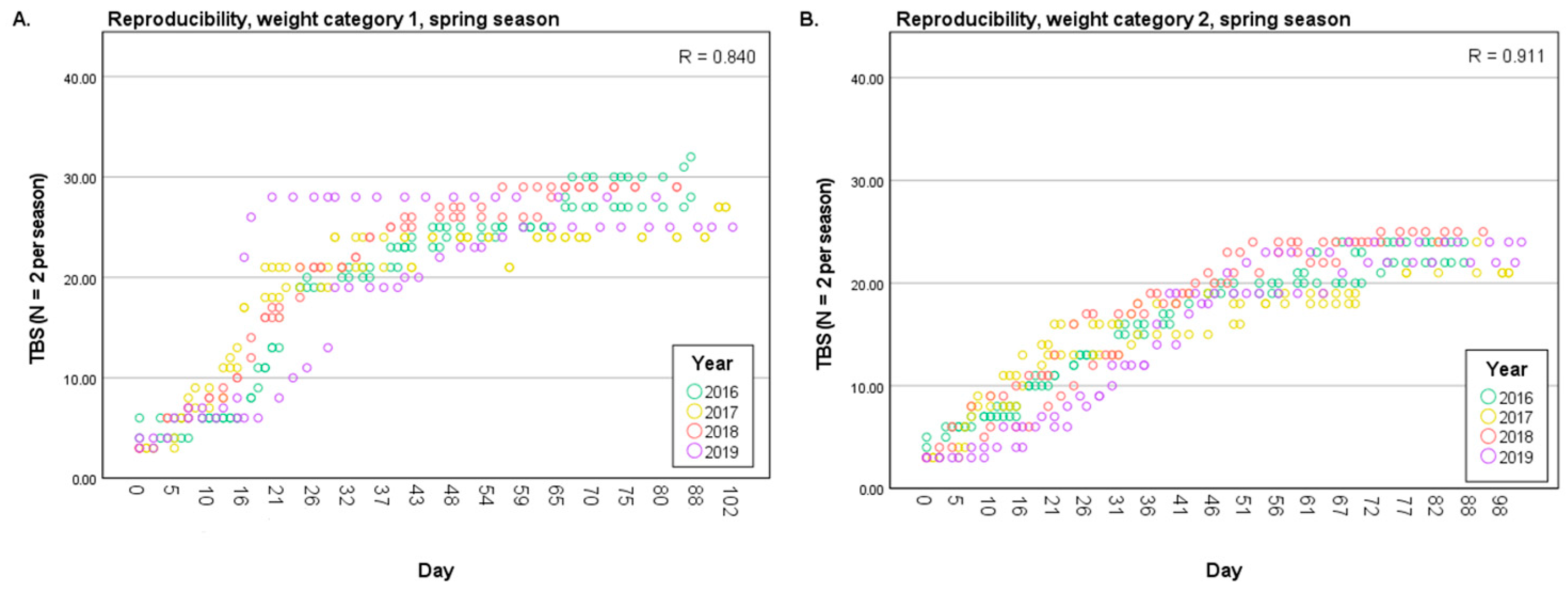
3.1.2. Reproducibility
3.1.3. Body Weight
3.1.4. Post-Mortem Movement
3.1.5. Rainfall
3.1.6. Delayed Bloating
3.1.7. Invertebrate Activity
3.2. Discussion
3.2.1. General Discussion
3.2.2. Limitations of the Study
3.2.3. Further Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nederlands Instituut Voor de Documentatie van Anoniem Afstanddoen (NIDAA). Available online: https://www.nidaa.nl/babylijkjes-in-nederland (accessed on 2 February 2021).
- Putkonen, H.; Collander, J.; Weizmann-Henelius, G.; Eronen, M. Legal outcomes of all suspected neonaticides in Finland 1980–2000. Int. J. Law. Psychiatry 2007, 30, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.T.; Berger, W.; Valença, A.M.; Coutinho, E.S.; Jean-Louis, G.; Fontenelle, L.F.; Mendlowicz, M.V. The world-wide incidence of neonaticide: A systematic review. Arch. Women’s Ment. Health 2017, 20, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Krajčovič, J.; Janík, M.; Novomeský, F.; Straka, Ľ.; Hejna, P. Feasibility, diagnostic validity and limits of postmortem evaluation of a newborn infant following an extremely prolonged freezing interval: A thanatological case study. Leg. Med. 2014, 16, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Gelderman, H.T.; Boer, L.; Naujocks, T.; IJzermans, A.C.M.; Duijst, W.L.J.M. The development of a post-mortem interval estimation for human remains found on land in the Netherlands. Int. J. Leg. Med. 2018, 132, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Alfsdotter, C.; Petaros, A. Outdoor human decomposition in Sweden: A retrospective quantitative study of forensic-taphonomic changes and postmortem interval in terrestrial and aquatic settings. J. Forensic Sci. 2021, 66, 1348–1363. [Google Scholar] [CrossRef]
- Finaughty, D.A.; Morris, A.G. Precocious natural mummification in a temperate climate (Western Cape, South Africa). Forensic Sci. Int. 2019, 303, 109948. [Google Scholar] [CrossRef]
- Brooks, J.W. Postmortem changes in animal carcasses and estimation of the postmortem interval. Vet. Pathol. 2016, 53, 929–940. [Google Scholar] [CrossRef]
- Goff, M.L. Early post-mortem changes and stages of decomposition in exposed cadavers. Exp. Appl. Acarol. 2009, 49, 21–36. [Google Scholar] [CrossRef]
- Galloway, A.; Birkby, W.H.; Jones, A.M.; Henry, T.E.; Parks, B.O. Decay rates of human remains in an arid environment. J. Forensic Sci. 1989, 34, 607–616. [Google Scholar] [CrossRef]
- Megyesi, M.S.; Nawrocki, S.P.; Haskell, N.H. Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. J. Forensic Sci. 2005, 50, JFS2004017. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef]
- Madea, B.; Henssge, C.; Reibe, S.; Tsokos, M.; Kernbach-Wighton, G. Postmortem changes and time since death. In Handbook of Forensic Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 75–133. [Google Scholar]
- Madea, B.; Kernbach-Wighton, G.; Henssge, C.; Doberentz, E.; Musshoff, F. Autolysis, putrefactive changes and postmortem chemistry. In Estimation of the Time Since Death, 3rd ed.; Madea, B., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 161–185. [Google Scholar]
- Gelderman, H.T.; Kruiver, C.A.; Oostra, R.J.; Zeegers, M.P.; Duijst, W.L.J.M. Estimation of the postmortem interval based on the human decomposition process. J. Forensic Leg. Med. 2019, 61, 122–127. [Google Scholar] [CrossRef]
- Mann, R.W.; Bass, W.M.; Meadows, L. Time since death and decomposition of the human body: Variables and observations in case and experimental field studies. J. Forensic Sci. 1990, 35, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Anderson, B.; Carter, D.O. Seasonal variation of carcass decomposition and gravesoil chemistry in a cold (Dfa) climate. J. Forensic Sci. 2013, 58, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 1: Pattern and rate of decomposition. Forensic Sci. Int. 2010, 194, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Konwerski, S.; Frątczak, K.; Szafałowicz, M. Effect of body mass and clothing on decomposition of pig carcasses. Int. J. Leg. Med. 2014, 128, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Galloway, A. The process of decomposition: A model from the Arizona-Sonoran desert. In Forensic Taphonomy: The Postmortem Fate of Human Remains; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 1997; pp. 139–150. [Google Scholar]
- Duijst, W.; Reijnders, U.; Reijnen, G.; Dijkhuizen, L. Ontbinding en het vaststellen van het postmortaal interval. In Handboek Forensische Geneeskunde; Gompel & Svacina: ’s-Hertogenbosch, The Netherlands, 2021; pp. 282–311. [Google Scholar]
- Bass, W.M. Outdoor decomposition rates in Tennessee. In Forensic Taphonomy: The Postmortem Fate of Human Remains; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 1997; pp. 181–186. [Google Scholar]
- Saukko, P.; Knight, B. The establishment of identity of human remains. In Knight’s Forensic Pathology; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2015; pp. 95–132. [Google Scholar]
- Micozzi, M.S. Frozen environments and soft tissue preservation. In Forensic Taphonomy: The Postmortem Fate of Human Remains; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 1997; pp. 171–180. [Google Scholar]
- Cockle, D.L.; Bell, L.S. Human decomposition and the reliability of a ‘Universal’ model for post mortem interval estimations. Forensic Sci. Int. 2015, 253, 136.e1–136.e9. [Google Scholar] [CrossRef]
- Roberts, L.G.; Dabbs, G.R.A. Taphonomic Study Exploring the Differences in Decomposition Rate and Manner between Frozen and Never Frozen Domestic Pigs (Sus scrofa). J. Forensic Sci. 2015, 60, 588–594. [Google Scholar] [CrossRef]
- Giles, S.B.; Harrison, K.; Errickson, D.; Márquez-Grant, N. The effect of seasonality on the application of accumulated degree-days to estimate the early post-mortem interval. Forensic Sci. Int. 2020, 315, 110419. [Google Scholar] [CrossRef]
- Lyu, Z.; Wan, L.H.; Yang, Y.Q.; Tang, R.; Xu, L.Z. A checklist of beetles (Insecta, Coleoptera) on pig carcasses in the sub-urban area of southwestern China: A preliminary study and its forensic relevance. J. Forensic Leg. Med. 2016, 41, 42–48. [Google Scholar] [CrossRef]
- Tembe, D.; Mukaratirwa, S. Insect succession and decomposition pattern on pig carrion during warm and cold seasons in KwaZulu-Natal Province of South Africa. J. Med. Entomol. 2021, 58, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Cockle, D.L.; Bell, L.S. The environmental variables that impact human decomposition in terrestrially exposed contexts within Canada. Sci. Justice 2017, 57, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Saukko, P.; Knight, B. The pathophysiology of death. In Knight’s Forensic Pathology; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2015; pp. 55–94. [Google Scholar]
- Reibe, S. Forensic entomology. In Estimation of the Time Since Death; Madea, B., Ed.; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2015; pp. 249–258. [Google Scholar]
- Roberts, L.G.; Spencer, J.R.; Dabbs, G.R. The effect of body mass on outdoor adult human decomposition. J. Forensic Sci. 2017, 62, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Maurer, H.H. Toxicokinetics and toxicogenetics. In Handbook of Forensic Medicine; Madea, B., Ed.; J. Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 889–899. [Google Scholar]
- Zhou, C.; Byard, R.W. Factors and processes causing accelerated decomposition in human cadavers—An overview. J. Forensic Leg. Med. 2011, 18, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.; Adlam, R.E.; Moffatt, C. Debugging decomposition data—Comparative taphonomic studies and the influence of insects and carcass size on decomposition rate. J. Forensic Sci. 2010, 55, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Gelderman, T.; Stigter, E.; Krap, T.; Amendt, J.; Duijst, W. The time of death in Dutch court; using the Daubert criteria to evaluate methods to estimate the PMI used in court. Leg. Med. 2021, 53, 101970. [Google Scholar] [CrossRef] [PubMed]
- Suckling, J.K.; Spradley, M.K.; Godde, K. A longitudinal study on human outdoor decomposition in Central Texas. J. Forensic Sci. 2016, 61, 19–25. [Google Scholar] [CrossRef]
- Ubelaker, D.H. Taphonomic applications in forensic anthropology. In Forensic Taphonomy: The Postmortem Fate of Human Remains; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 1997; pp. 77–90. [Google Scholar]
- Marhoff, S.J.; Fahey, P.; Forbes, S.L.; Green, H. Estimating post-mortem interval using accumulated degree-days and a degree of decomposition index in Australia: A validation study. Aust. J. Forensic Sci. 2016, 48, 24–36. [Google Scholar] [CrossRef]
- Marhoff-Beard, S.J.; Forbes, S.L.; Green, H. The validation of ‘universal’ PMI methods for the estimation of time since death in temperate Australian climates. Forensic Sci. Int. 2018, 291, 158–166. [Google Scholar] [CrossRef]
- Michaud, J.P.; Moreau, G. A statistical approach based on accumulated degree-days to predict decomposition-related processes in forensic studies. J. Forensic Sci. 2011, 56, 229–232. [Google Scholar] [CrossRef]
- Ross, A.H.; Hale, A.R. Decomposition of juvenile-sized remains: A macro-and microscopic perspective. Forensic Sci. Res. 2018, 3, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Dawson, B.M.; Barton, P.S.; Wallman, J.F. Contrasting insect activity and decomposition of pigs and humans in an Australian environment: A preliminary study. Forensic Sci. Int. 2020, 316, 110515. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Hall, M.J.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int. J. Leg. Med. 2020, 134, 793–810. [Google Scholar] [CrossRef] [PubMed]
- KNMI—Daggegevens van Het Weer in Nederland. Available online: https://www.knmi.nl/nederland-nu/klimatologie/daggegevens (accessed on 4 February 2021).
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Archdeacon, T.J. Evaluating the regression equation. In Correlation and Regression Analysis: A Historian’s Guide; University of Wisconsin Press: Madison, WI, USA, 1994; pp. 160–177. [Google Scholar]
- Sharma, R.; Garg, R.K.; Gaur, J.R. Various methods for the estimation of the post mortem interval from Calliphoridae: A review. Egypt. J. Forensic Sci. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Dautartas, A.; Kenyhercz, M.W.; Vidoli, G.M.; Meadows Jantz, L.; Mundorff, A.; Steadman, D.W. Differential decomposition among pig, rabbit, and human remains. J. Forensic Sci. 2018, 63, 1673–1683. [Google Scholar] [CrossRef]
- Keough, N.; Myburgh, J.; Steyn, M. Scoring of decomposition: A proposed amendment to the method when using a pig model for human studies. J. Forensic Sci. 2017, 62, 986–993. [Google Scholar] [CrossRef]
- Zhang, C.; Plastow, G. Genomic Diversity in Pig (Sus scrofa) and its Comparison with Human and other Livestock. Curr. Genom. 2011, 12, 138–146. [Google Scholar] [CrossRef]
- Cordeiro, C.; Ordóñez-Mayán, L.; Lendoiro, E.; Febrero-Bande, M.; Vieira, D.N.; Muñoz-Barús, J.I. A reliable method for estimating the postmortem interval from the biochemistry of the vitreous humor, temperature and body weight. Forensic Sci. Int. 2019, 295, 157–168. [Google Scholar] [CrossRef]
- Miles, K.L.; Finaughty, D.A.; Gibbon, V.E. A review of experimental design in forensic taphonomy: Moving towards forensic realism. Forensic Sci. Res. 2020, 5, 249–259. [Google Scholar] [CrossRef]
- Sutherland, A.; Myburgh, J.; Steyn, M.; Becker, P.J. The effect of body size on the rate of decomposition in a temperate region of South Africa. Forensic Sci. Int. 2013, 231, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Chatrabgoun, O.; Akbarzadeh, K.; Oshaghi, M.; Falahati, M.H.; Rafizadeh, S.; Yusuf, M.A.; Rassi, Y. Evaluation of insect succession patterns and carcass weight loss for the estimation of postmortem interval. J. Med. Entomol. 2018, 55, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Neilsen, P.; Berry, R.; Seckiner, D.; Mallett, X. Quantifying human post-mortem movement resultant from decomposition processes. Forensic Sci. Int. Synerg. 2020, 2, 248–261. [Google Scholar] [CrossRef]
- Díaz-Aranda, L.M.; Martín-Vega, D.; Gómez-Gómez, A.; Cifrián, B.; Baz, A. Annual variation in decomposition and insect succession at a periurban area of central Iberian Peninsula. J. Forensic Leg. Med. 2018, 56, 21–31. [Google Scholar] [CrossRef]
- Šuláková, H.; Barták, M. Forensically important Calliphoridae (Diptera) associated with animal and human decomposition in the Czech Republic: Preliminary results. Acta Musei Sil. Sci. Nat. 2013, 62, 255–266. [Google Scholar] [CrossRef]
- Hackshaw, A. Small studies: Strengths and limitations. Eur. Respir. J. 2008, 32, 1141–1143. [Google Scholar] [CrossRef]
- Archer, M.S. Rainfall and temperature effects on the decomposition rate of exposed neonatal remains. Sci. Justice J. Forensic Sci. Soc. 2004, 44, 35–41. [Google Scholar] [CrossRef]
- DuPriest, E.A.; Kupfer, P.; Lin, B.; Sekiguchi, K.; Morgan, T.K.; Saunders, K.E.; Chatkupt, T.T.; Denisenko, O.N.; Purnell, J.Q.; Bagby, S.P. Altered adipocyte structure and function in nutritionally programmed microswine offspring. J. Dev. Orig. Health Dis. 2012, 3, 198–209. [Google Scholar] [CrossRef]
- Kuzawa, C.W. Adipose tissue in human infancy and childhood: An evolutionary perspective. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 1998, 107, 177–209. [Google Scholar] [CrossRef]




| Categories and Stages of Decomposition for the Head and Neck |
| A. Fresh - (1 pt) Fresh, no discolortion |
| B. Early decomposition - (2 pts) Pink-white appearance with skin slippage and some hair loss. - (3 pts) Gray to green discoloration: some flesh still relatively fresh. - (4 pts) Discoloration and/or brownish shades particularly at edges, drying of nose, ears and lips. - (5 pts) Purging of decompositional fluids out of eyes, ears, nose, mouth, some bloating of neck and face may be present. - (6 pts) Brown to black discoloration of flesh. |
| C. Advanced - (7 pts) Caving in of the flesh and tissues of eyes and throat. - (8 pts) Moist decomposition with bone exposure less than one half that of the area being scored. - (9 pts) Mummification with bone exposure less than one half that of the area being scored. |
| D. Skeletonisation - (10 pts) Bone exposure of more than half of the area being scored with greasy substances and decomposed tissue. - (11 pts) Bone exposure of more than half the area being scored with desiccated or mummified tissue. - (12 pts) Bones largely dry, but retaining some grease. - (13 pts) Dry bone. |
| Categories and Stages of Decomposition for the Torso |
| A. Fresh - (1 pt) Fresh, no discolortion |
| B. Early decomposition - (2 pts) Pink-white appearance with skin slippage and marbling present. - (3 pts) Gray to green discoloration: some flesh relatively fresh. - (4 pts) Bloating with green discoloration and purging of decompositional fluids. - (5 pts) Post-bloating following release of the abdominal gases, with discoloration changing from green to black. |
| C. Advanced - (2 pts) Pink-white appearance with skin slippage and marbling present. - (3 pts) Gray to green discoloration: some flesh relatively fresh. - (4 pts) Bloating with green discoloration and purging of decompositional fluids. - (5 pts) Post-bloating following release of the abdominal gases, with discoloration changing from green to black. |
| D. Skeletonisation - (2 pts) Pink-white appearance with skin slippage and marbling present. - (3 pts) Gray to green discoloration: some flesh relatively fresh. - (4 pts) Bloating with green discoloration and purging of decompositional fluids. - (5 pts) Post-bloating following release of the abdominal gases, with discoloration changing from green to black. |
| Categories and Stages of Decomposition for the Limbs |
| A. Fresh - (1 pt) Fresh, no discolortion |
| B. Early decomposition - (2 pts) Pink-white appearance with skin slippage of hands and/or feet. - (3 pts) Gray to green discoloration; marbling; some flesh still relatively fresh. - (4 pts) Discoloration and/or brownish shades particularly at edges, drying of fingers, toes, and other projecting extremities. - (5 pts) Brown to black discoloration, skin having a leathery appearance. |
| C. Advanced - (6 pts) Moist decomposition with bone exposure less than one half that of the area being scored. - (7 pts) Mummification with bone exposure of less than one half that of the area being scored. |
| D. Skeletonisation - (8 pts) Bone exposure over one half the area being scored, some decomposed tissue and body fluids remaining. - (9 pts) Bones largely dry, but retaining some grease. - (10 pts) Dry bone. |
| Weight Category | Season | Correlation | Residual Sum of Squares | Slope |
|---|---|---|---|---|
| 1 (800–2700 g) | Summer | 0.656 | 7745.968 | 0.225 |
| Autumn * | 0.796 | 9443.602 | 0.271 | |
| Winter * | 0.877 | 5750.624 | 0.115 | |
| Spring * | 0.840 | 6476.971 | 0.278 | |
| 2 (4230–10,500 g) | Summer * | 0.740 | 5816.077 | 0.264 |
| Autumn | 0.513 | 12,270.024 | 0.042 | |
| Winter | 0.862 | 4391.745 | 0.097 | |
| Spring | 0.911 | 2504.902 | 0.228 |
| Weight Category | Season | Average Maximum Reached TBS Excluding Plateau Phase | Correlation | Residual Sum of Squares | Slope |
|---|---|---|---|---|---|
| 1 (800–2700 g) | Summer | 29 | 0.669 | 5743.8 | 0.261 |
| Autumn * | 30 | 0.816 | 7088.1 | 0.332 | |
| Winter * | 28 | 0.877 | 5750.6 | 0.115 | |
| Spring * | 28 | 0.858 | 4956.8 | 0.294 | |
| 2 (4230–10,500 g) | Summer * | 28 | 0.747 | 4068.6 | 0.316 |
| Autumn | 25 | 0.516 | 11,992.7 | 0.042 | |
| Winter | 26 | 0.862 | 4391.8 | 0.097 | |
| Spring | 23 | 0.908 | 2263.9 | 0.240 |
| Weight Category | Season | Average (Days) | SEM | Minimum (Days) | Maximum (Days) |
|---|---|---|---|---|---|
| 1 | Summer | 1 | 0.263 | 1 | 2 |
| Autumn | 1 | 0.398 | 1 | 3 | |
| Winter | 7 | 0.385 | 1 | 25 | |
| Spring | 3 | 1.150 | 1 | 10 | |
| 2 | Summer | 4 | 0.780 | 3 | 7 |
| Autumn | 21 | 10.873 | 2 | 87 | |
| Winter | 11 | 3.020 | 2 | 20 | |
| Spring | 8 | 1.711 | 4 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sluis, I.; Duijst, W.; Krap, T. Subaerial Decomposition of Small-Sized Remains in The Netherlands: Important Findings Regarding the PMI of a Four-Year Taphonomic Study. Biology 2023, 12, 1164. https://doi.org/10.3390/biology12091164
Sluis I, Duijst W, Krap T. Subaerial Decomposition of Small-Sized Remains in The Netherlands: Important Findings Regarding the PMI of a Four-Year Taphonomic Study. Biology. 2023; 12(9):1164. https://doi.org/10.3390/biology12091164
Chicago/Turabian StyleSluis, Iris, Wilma Duijst, and Tristan Krap. 2023. "Subaerial Decomposition of Small-Sized Remains in The Netherlands: Important Findings Regarding the PMI of a Four-Year Taphonomic Study" Biology 12, no. 9: 1164. https://doi.org/10.3390/biology12091164
APA StyleSluis, I., Duijst, W., & Krap, T. (2023). Subaerial Decomposition of Small-Sized Remains in The Netherlands: Important Findings Regarding the PMI of a Four-Year Taphonomic Study. Biology, 12(9), 1164. https://doi.org/10.3390/biology12091164






