Estimation of Late Postmortem Interval: Where Do We Stand? A Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
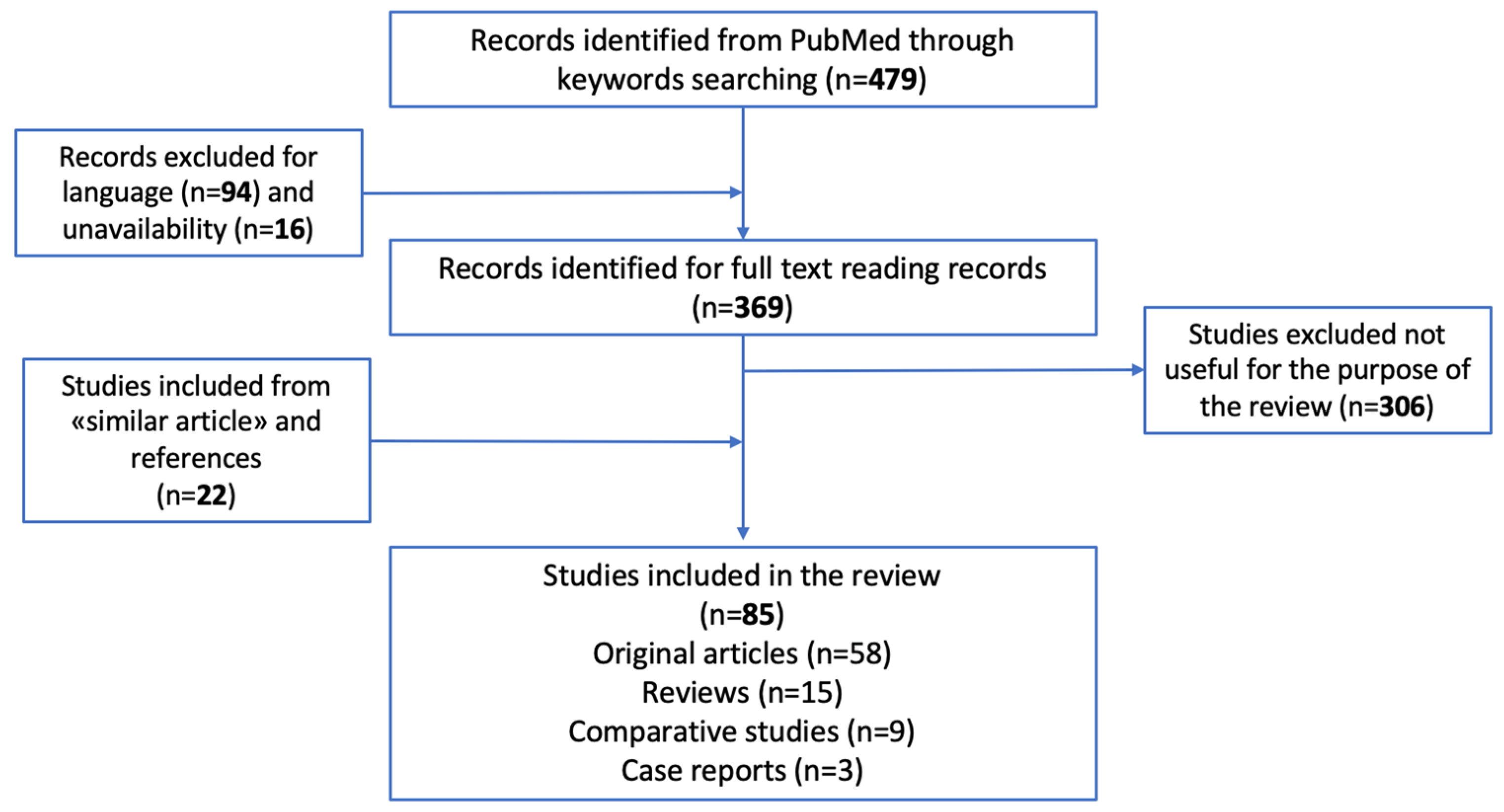
2. Methodology
2.1. Search Criteria and Critical Appraisal
((Postmortem Interval[MeSH Terms] OR Postmortem Interval[Title/Abstract]) OR (PMI[Title/Abstract] AND (skeletal remains[Title/Abstract] OR decomposed bodies[Title/Abstract]))) AND (“Methods”[MeSH Terms] OR “Methods”[Title/Abstract])
2.2. Risk of Bias
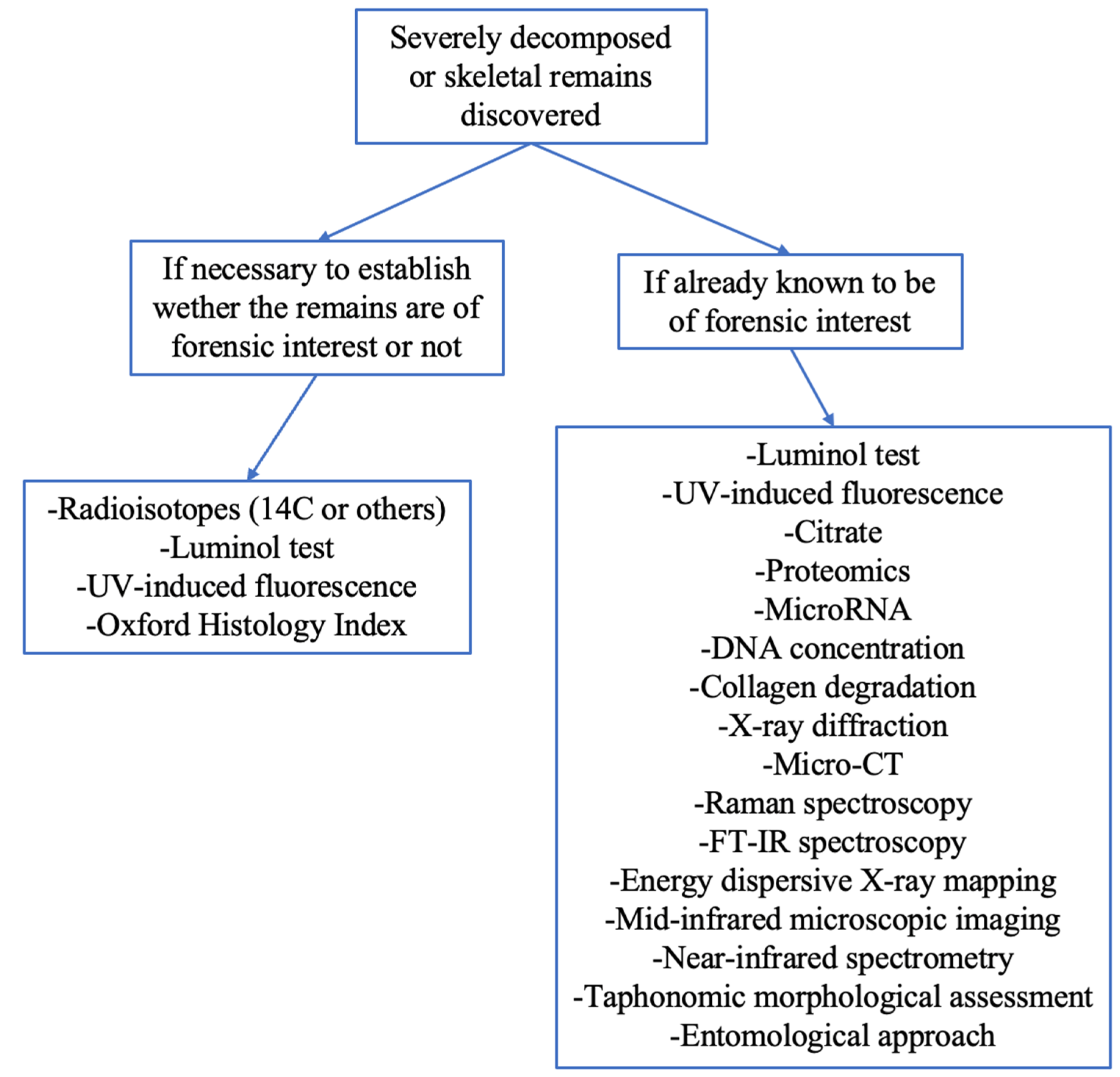
3. Results and Discussion
3.1. Radiocarbon (C14) Dating
3.2. Not Only C14: Other Radioisotopes
3.3. Fluorescence Techniques: Luminol and Ultraviolet-Induced Light
3.4. Bone Extracellular Matrix Component Analyses: Citrate
3.5. Proteomics
3.6. Small Particles for PMI: Evaluation of Bone Components including MicroRNA, DNA Concentration, and Collagen Degradation
3.7. Radiology and Spectroscopy
3.8. Taphonomic Analysis and Morphological Assessment
3.9. Entomological Approach
3.10. Comparative Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madea, B. Methods for determining time of death. Forensic Sci. Med. Pathol. 2016, 12, 451–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, H.C.; Xu, J.R.; Huang, P.; Wang, Z.Y. Current Research and Prospects on Postmortem Interval Estimation. Fa Yi Xue Za Zhi 2018, 34, 459–467. [Google Scholar] [PubMed]
- Brooks, J.W. Postmortem Changes in Animal Carcasses and Estimation of the Postmortem Interval. Veter- Pathol. 2016, 53, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, L.; Pradelli, J.; Tuccia, F.; Giordani, G.; Cattaneo, C.; Vanin, S. Comparison of Accumulated Degree-Days and Entomological Approaches in Post Mortem Interval Estimation. Insects 2021, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Maile, A.E.; Inoue, C.G.; Barksdale, L.E.; Carter, D.O. Toward a universal equation to estimate postmortem interval. Forensic Sci. Int. 2017, 272, 150–153. [Google Scholar] [CrossRef]
- Caccianiga, M.; Caccia, G.; Mazzarelli, D.; Salsarola, D.; Poppa, P.; Gaudio, D.; Cappella, A.; Franceschetti, L.; Tambuzzi, S.; Maggioni, L.; et al. Common and much less common scenarios in which botany is crucial for forensic pathologist and anthropologists: A series of eight case studies. Int. J. Leg. Med. 2021, 135, 1067–1077. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Vignali, G.; Franceschetti, L.; Lanza Attisano, G.C.; Cattaneo, C. Assessing wound vitality in decomposed bodies: A review of the literature. Int. J. Leg. Med. 2023, 137, 459–470. [Google Scholar] [CrossRef]
- Franceschetti, L.; Di Candia, D.; Giordano, G.; Carabelli, I.; Vignali, G.; Cattaneo, C. Drugs in bone: Detectability of substances of toxicological interest in different states of preservation. J. Forensic Sci. 2021, 66, 677–686. [Google Scholar] [CrossRef]
- Corrêa, H.; Cortellini, V.; Franceschetti, L.; Verzeletti, A. Large fragment demineralization: An alternative pretreatment for forensic DNA typing of bones. Int. J. Leg. Med. 2021, 135, 1417–1424. [Google Scholar] [CrossRef]
- Franceschetti, L.; Palamenghi, A.; Mazzarelli, D.; Cappella, A.; Gibelli, D.M.; De Angelis, D.; Verzeletti, A.; Cattaneo, C. Taphonomic study on drowned victims in a non-sequestered aquatic environment in the Mediterranean Sea. Int. J. Leg. Med. 2022, 136, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Ubelaker, D.H. Radiocarbon analysis of human remains: A review of forensic applications. J. Forensic Sci. 2014, 59, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Ubelaker, D.H.; Buchholz, B. Complexities in the Use of Bomb-Curve Radiocarbon to Determine Time Since Death of Human Skeletal Remains. Forensic Sci. Commun. 2005, 8, 1–12. [Google Scholar]
- Ubelaker, D.H.; Parra, R.C. Radiocarbon analysis of dental enamel and bone to evaluate date of birth and death: Perspective from the southern hemisphere. Forensic Sci. Int. 2011, 208, 103–107. [Google Scholar] [CrossRef]
- Ubelaker, D.H.; Buchholz, B.A.; Stewart, J.E. Analysis of artificial radiocarbon in different skeletal and dental tissue types to evaluate date of death. J. Forensic Sci. 2006, 51, 484–488. [Google Scholar] [CrossRef]
- Wild, E.M.; Arlamovsky, K.A.; Golser, R.; Kutschera, W.; Priller, A.; Puchegger, S.; Rom, W.; Steier, P.; Vycudilik, W. 14C dating with the bomb peak: An application to forensic medicine. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2000, 172, 944–950. [Google Scholar] [CrossRef]
- Cardoso, H.F.V.; Puentes, K.; Soares, A.M.; Santos, A.; Magalhães, T. The value of radiocarbon analysis in determining the forensic interest of human skeletal remains found in unusual circumstances. J. Forensic Leg. Med. 2012, 19, 97–100. [Google Scholar] [CrossRef]
- Schrag, B.; Uldin, T.; Mangin, P.; Bochud, F.; Froidevaux, P. Dating human skeletal remains using 90Sr and 210Pb: Case studies. Forensic Sci. Int. 2014, 234, 190.e1–190.e6. [Google Scholar] [CrossRef]
- Kandlbinder, R.; Geißler, V.; Schupfner, R.; Wolfbeis, O.; Zinka, B. Analysing of 228Th, 232Th, 228Ra in human bone tissues for the purpose of determining the post mortal interval. J. Radioanal. Nucl. Chem. 2009, 280, 113–119. [Google Scholar] [CrossRef]
- Schrag, B.; Uldin, T.; Mangin, P.; Froidevaux, P. Dating human skeletal remains using a radiometric method: Biogenic versus diagenetic 90Sr and 210Pb in vertebrae. Forensic Sci. Int. 2012, 220, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Introna, F., Jr.; Di Vella, G.; Campobasso, C.P. Determination of postmortem interval from old skeletal remains by image analysis of luminol test results. J. Forensic Sci. 1999, 44, 535–538. [Google Scholar] [CrossRef]
- Creamer, J.I.; Buck, A.M. The assaying of haemoglobin using luminol chemiluminescence and its application to the dating of human skeletal remains. Luminescence 2009, 24, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Caudullo, G.; Caruso, V.; Cappella, A.; Sguazza, E.; Mazzarelli, D.; Amadasi, A.; Cattaneo, C. Luminol testing in detecting modern human skeletal remains: A test on different types of bone tissue and a caveat for PMI interpretation. Int. J. Leg. Med. 2017, 131, 287–292. [Google Scholar] [CrossRef]
- Ramsthaler, F.; Kreutz, K.; Zipp, K.; Verhoff, M.A. Dating skeletal remains with luminol-chemiluminescence. Validity, intra- and interobserver error. Forensic Sci. Int. 2009, 187, 47–50. [Google Scholar] [CrossRef]
- Ramsthaler, F.; Ebach, S.C.; Birngruber, C.G.; Verhoff, M.A. Postmortem interval of skeletal remains through the detection of intraosseal hemin traces. A comparison of UV-fluorescence, luminol, Hexagon-OBTI®, and Combur® tests. Forensic Sci. Int. 2011, 209, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sterzik, V.; Jung, T.; Jellinghaus, K.; Bohnert, M. Estimating the postmortem interval of human skeletal remains by analyzing their optical behavior. Int. J. Leg. Med. 2016, 130, 1557–1566. [Google Scholar] [CrossRef]
- Hoke, N.; Grigat, A.; Grupe, G.; Harbeck, M. Reconsideration of bone postmortem interval estimation by UV-induced autofluorescence. Forensic Sci. Int. 2013, 228, 176.e1–176.e6. [Google Scholar] [CrossRef]
- Ermida, C.; Navega, D.; Cunha, E. Luminol chemiluminescence: Contribution to postmortem interval determination of skeletonized remains in Portuguese forensic context. Int. J. Leg. Med. 2017, 131, 1149–1153. [Google Scholar] [CrossRef]
- Sarabia, J.; Pérez-Martínez, C.; Hernández Del Rincón, J.P.; Luna, A. Study of chemiluminescence measured by luminometry and its application in the estimation of postmortem interval of bone remains. Leg. Med. 2018, 33, 32–35. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Agur, K.; Jantz, L.M. A new method for determination of postmortem interval: Citrate content of bone. J. Forensic Sci. 2010, 55, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Christensen, A.M. A test of the citrate method of PMI estimation from skeletal remains. Forensic Sci. Int. 2017, 270, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Kanz, F.; Reiter, C.; Risser, D.U. Citrate Content of Bone for Time Since Death Estimation: Results from Burials with Different Physical Characteristics and Known PMI. J. Forensic Sci. 2014, 59, 613–620. [Google Scholar] [CrossRef]
- Brown, M.A.; Bunch, A.W.; Froome, C.; Gerling, R.; Hennessy, S.; Ellison, J. Citrate Content of Bone as a Measure of Postmortem Interval: An External Validation Study. J. Forensic Sci. 2018, 63, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Zissler, A.; Stoiber, W.; Steinbacher, P.; Geissenberger, J.; Monticelli, F.C.; Pittner, S. Postmortem Protein Degradation as a Tool to Estimate the PMI: A Systematic Review. Diagnostics 2020, 10, 1014. [Google Scholar] [CrossRef]
- Procopio, N.; Williams, A.; Chamberlain, A.T.; Buckley, M. Forensic proteomics for the evaluation of the post-mortem decay in bones. J. Proteom. 2018, 177, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Procopio, N.; Chamberlain, A.T.; Buckley, M. Intra- and Interskeletal Proteome Variations in Fresh and Buried Bones. J. Proteome Res. 2017, 16, 2016–2029. [Google Scholar] [CrossRef]
- Costa, I.; Carvalho, F.; Magalhães, T.; Guedes de Pinho, P.; Silvestre, R.; Dinis-Oliveira, R.J. Promising blood-derived biomarkers for estimation of the postmortem interval. Toxicol. Res. 2015, 4, 1443–1452. [Google Scholar] [CrossRef]
- Prieto-Bonete, G.; Pérez-Cárceles, M.D.; Maurandi-López, A.; Pérez-Martínez, C.; Luna, A. Association between protein profile and postmortem interval in human bone remains. J. Proteom. 2019, 192, 54–63. [Google Scholar] [CrossRef]
- Akçan, R.; Taştekin, B.; Yildirim, M.Ş.; Aydogan, H.C.; Sağlam, N. Omics era in forensic medicine: Towards a new age. Turk. J. Med Sci. 2020, 50, 1480–1490. [Google Scholar] [CrossRef]
- Choi, K.M.; Zissler, A.; Kim, E.; Ehrenfellner, B.; Cho, E.; Lee, S.I.; Steinbacher, P.; Yun, K.N.; Shin, J.H.; Kim, J.Y.; et al. Postmortem proteomics to discover biomarkers for forensic PMI estimation. Int. J. Leg. Med. 2019, 133, 899–908. [Google Scholar] [CrossRef]
- Pittner, S.; Ehrenfellner, B.; Monticelli, F.C.; Zissler, A.; Sänger, A.M.; Stoiber, W.; Steinbacher, P. Postmortem muscle protein degradation in humans as a tool for PMI delimitation. Int. J. Leg. Med. 2016, 130, 1547–1555. [Google Scholar] [CrossRef]
- Geissenberger, J.; Ehrenfellner, B.; Monticelli, F.C.; Pittner, S.; Steinbacher, P. Dismembered porcine limbs as a proxy for postmortem muscle protein degradation. Int. J. Leg. Med. 2021, 135, 1627–1636. [Google Scholar] [CrossRef]
- Pittner, S.; Monticelli, F.C.; Pfisterer, A.; Zissler, A.; Sänger, A.M.; Stoiber, W.; Steinbacher, P. Postmortem degradation of skeletal muscle proteins: A novel approach to determine the time since death. Int. J. Leg. Med. 2016, 130, 421–431. [Google Scholar] [CrossRef]
- Ehrenfellner, B.; Zissler, A.; Steinbacher, P.; Monticelli, F.C.; Pittner, S. Are animal models predictive for human postmortem muscle protein degradation? Int. J. Leg. Med. 2017, 131, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Bonicelli, A.; Mickleburgh, H.L.; Chighine, A.; Locci, E.; Wescott, D.J.; Procopio, N. The ‘ForensOMICS’ approach for postmortem interval estimation from human bone by integrating metabolomics, lipidomics, and proteomics. eLife. 2022, 11, e83658. [Google Scholar] [CrossRef]
- Na, J.-Y. Estimation of the post-mortem interval using microRNA in the bones. J. Forensic Leg. Med. 2020, 75, 102049. [Google Scholar] [CrossRef]
- Montanari, E.; Giorgetti, R.; Busardò, F.P.; Giorgetti, A.; Tagliabracci, A.; Alessandrini, F. Suitability of miRNA assessment in postmortem interval estimation. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1774–1787. [Google Scholar] [PubMed]
- Nagai, M.; Sakurada, K.; Imaizumi, K.; Ogawa, Y.; Uo, M.; Funakoshi, T.; Uemura, K. Evaluation of Parameters for Estimating the Postmortem Interval of Skeletal Remains Using Bovine Femurs: A Pilot Study. Diagnostics 2020, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Du, T.S.; Mengxi, M.M.; Ye, X.; Tu, C.Y.; Jin, K.D.; Chen, S.W.; Liu, N.G.; Xie, J.H.; Shen, Y.W. Research Progress of Metabolomics in Forensic Pathology. Fa Yi Xue Za Zhi 2020, 36, 347–353. [Google Scholar]
- Pérez-Martínez, C.; Pérez-Cárceles, M.D.; Legaz, I.; Prieto-Bonete, G.; Luna, A. Quantification of nitrogenous bases, DNA and Collagen type I for the estimation of the postmortem interval in bone remains. Forensic Sci. Int. 2017, 281, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.; Bachmeier, B.; Conrad, C.; Nerlich, A.; Bratzke, H.; Eisenmenger, W.; Peschel, O. Molecular study of time dependent changes in DNA stability in soil buried skeletal residues. Forensic Sci. Int. 2008, 177, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Hagelberg, E.; Bell, L.S.; Allen, T.; Boyde, A.; Jones, S.J.; Clegg, J.B. Analysis of ancient bone DNA: Techniques and applications. Philos. Trans. R. Soc. B: Biol. Sci. 1991, 333, 399–407. [Google Scholar]
- Boaks, A.; Siwek, D.; Mortazavi, F. The temporal degradation of bone collagen: A histochemical approach. Forensic Sci. Int. 2014, 240, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Jellinghaus, K.; Urban, P.K.; Hachmann, C.; Bohnert, M.; Hotz, G.; Rosendahl, W.; Wittwer-Backofen, U. Collagen degradation as a possibility to determine the post-mortem interval (PMI) of human bones in a forensic context—A survey. Leg. Med. 2019, 36, 96–102. [Google Scholar] [CrossRef]
- Prieto-Castelló, M.J.; Hernández del Rincón, J.P.; Pérez-Sirvent, C.; Alvarez-Jiménez, P.; Pérez-Cárceles, M.D.; Osuna, E.; Luna, A. Application of biochemical and X-ray diffraction analyses to establish the postmortem interval. Forensic Sci. Int. 2007, 172, 112–118. [Google Scholar] [CrossRef]
- Liu, Q.; Cai, X.; Liu, Y.; Zhou, L.; Yi, S.; Liu, L. Spectrophotometric determination of trimethylamine-nitrogen in cadaver tissues for the estimation of late postmortem interval: A pilot study. J. Huazhong Univ. Sci. Technol. 2008, 28, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Doty, K.C.; Muro, C.K.; Bueno, J.; Halámková, L.; Lednev, I.K. What can Raman spectroscopy do for criminalistics? J. Raman Spectrosc. 2016, 47, 39–50. [Google Scholar] [CrossRef]
- McLaughlin, G.; Lednev, I.K. Potential application of Raman spectroscopy for determining burial duration of skeletal remains. Anal. Bioanal. Chem. 2011, 401, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Bertoluzza, A.; Brasili, P.; Castrì, L.; Facchini, F.; Fagnano, C.; Tinti, A. Preliminary Results in Dating Human Skeletal Remains by Raman Spectroscopy. J. Raman Spectrosc. 1997, 28, 185–188. [Google Scholar] [CrossRef]
- Creagh, D.; Cameron, A. Estimating the Post-Mortem Interval of skeletonized remains: The use of Infrared spectroscopy and Raman spectro-microscopy. Radiat. Phys. Chem. 2017, 137, 225–229. [Google Scholar] [CrossRef]
- Ortiz-Herrero, L.; Uribe, B.; Hidalgo Armas, L.; Alonso, M.L.; Sarmiento, A.; Irurita, J.; Alonso, R.M.; Maguregui, M.I.; Etxeberria, F.; Bartolomé, L. Estimation of the post-mortem interval of human skeletal remains using Raman spectroscopy and chemometrics. Forensic Sci. Int. 2021, 329, 111087. [Google Scholar] [CrossRef]
- Falgayrac, G.; Vitale, R.; Delannoy, Y.; Behal, H.; Penel, G.; Duponchel, L.; Colard, T. Critical aspects of Raman spectroscopy as a tool for postmortem interval estimation. Talanta 2022, 249, 123589. [Google Scholar] [CrossRef]
- Nagy, G.; Lorand, T.; Patonai, Z.; Montsko, G.; Bajnoczky, I.; Marcsik, A.; Mark, L. Analysis of pathological and non-pathological human skeletal remains by FT-IR spectroscopy. Forensic Sci. Int. 2008, 175, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Patonai, Z.; Maasz, G.; Avar, P.; Schmidt, J.; Lorand, T.; Bajnoczky, I.; Mark, L. Novel dating method to distinguish between forensic and archeological human skeletal remains by bone mineralization indexes. Int. J. Leg. Med. 2013, 127, 529–533. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Lin, H.; Zha, S.; Fang, R.; Wei, X.; Fan, S.; Wang, Z. Estimation of the late postmortem interval using FTIR spectroscopy and chemometrics in human skeletal remains. Forensic Sci. Int. 2017, 281, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Howes, J.M.; Stuart, B.H.; Thomas, P.S.; Raja, S.; O’Brien, C. An investigation of model forensic bone in soil environments studied using infrared spectroscopy. J. Forensic Sci. 2012, 57, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Leskovar, T.; Zupanič Pajnič, I.; Geršak, Ž.M.; Jerman, I.; Črešnar, M. ATR-FTIR spectroscopy combined with data manipulation as a pre-screening method to assess DNA preservation in skeletal remains. Forensic Sci. Int.: Genet. 2020, 44, 102196. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, Y.Y.; Deng, K.F.; Luo, Y.W.; Sun, Q.R.; Li, Z.R.; Huang, P.; Zhang, J.; Cai, H.X. Relationship between Postmortem Interval and FTIR Spectroscopy Changes of the Rat Skin. Fa Yi Xue Za Zhi 2020, 36, 187–191. [Google Scholar]
- Alkhuder, K. Attenuated total reflection-Fourier transform infrared spectroscopy: A universal analytical technique with promising applications in forensic analyses. Int. J. Leg. Med. 2022, 136, 1717–1736. [Google Scholar] [CrossRef]
- Woess, C.; Unterberger, S.H.; Roider, C.; Ritsch-Marte, M.; Pemberger, N.; Cemper-Kiesslich, J.; Hatzer-Grubwieser, P.; Parson, W.; Pallua, J.D. Assessing various Infrared (IR) microscopic imaging techniques for post-mortem interval evaluation of human skeletal remains. PLoS ONE 2017, 12, e0174552. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, X.; Huang, J.; Lin, H.; Deng, K.; Li, Z.; Shao, Y.; Zou, D.; Chen, Y.; Huang, P.; et al. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectral prediction of postmortem interval from vitreous humor samples. Anal. Bioanal. Chem. 2018, 410, 7611–7620. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.; Wang, Q.; Wei, X.; Feng, W.; Chen, Y.; Huang, P.; Wang, Z. Application of Fourier transform infrared spectroscopy with chemometrics on postmortem interval estimation based on pericardial fluids. Sci. Rep. 2017, 7, 18013. [Google Scholar] [CrossRef]
- Longato, S.; Wöss, C.; Hatzer-Grubwieser, P.; Bauer, C.; Parson, W.; Unterberger, S.H.; Kuhn, V.; Pemberger, N.; Pallua, A.K.; Recheis, W.; et al. Post-mortem Interval Estimation of Human Skeletal Remains by Micro-Computed Tomography, Mid-Infrared Microscopic Imaging and Energy Dispersive X-ray Mapping. Anal. Methods 2015, 7, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, N.; Çetin, S.; Bilecenoğlu, B.; Altan, A.; Akbulut, S.; Ocak, M.; Orhan, K. The micro-CT evaluation of enamel-cement thickness, abrasion, and mineral density in teeth in the postmortem interval (PMI): New parameters for the determination of PMI. Int. J. Leg. Med. 2020, 134, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Le Garff, E.; Mesli, V.; Marchand, E.; Behal, H.; Demondion, X.; Becart, A.; Hedouin, V. Is bone analysis with μCT useful for short postmortem interval estimation? Int. J. Leg. Med. 2018, 132, 269–277. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Zelger, P.; Wöss, C.; Huck, C.W.; Arora, R.; Bechtel, E.; Stahl, A.; Brunner, A.; Zelger, B.; Schirmer, M.; et al. Post-Mortem Interval of Human Skeletal Remains Estimated with Handheld NIR Spectrometry. Biology 2022, 11, 1020. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Zelger, P.; Woess, C.; Pallua, A.K.; Arora, R.; Degenhart, G.; Brunner, A.; Zelger, B.; Schirmer, M.; Rabl, W.; et al. Application of Micro-Computed Tomography for the Estimation of the Post-Mortem Interval of Human Skeletal Remains. Biology 2022, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Megyesi, M.S.; Nawrocki, S.P.; Haskell, N.H. Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. J. Forensic Sci. 2005, 50, 618–626. [Google Scholar] [CrossRef]
- Galloway, A.; Birkby, W.H.; Jones, A.M.; Henry, T.E.; Parks, B.O. Decay rates of human remains in an arid environment. J. Forensic Sci. 1989, 34, 607–616. [Google Scholar] [CrossRef]
- Adlam, R.E.; Simmons, T. The effect of repeated physical disturbance on soft tissue decomposition--are taphonomic studies an accurate reflection of decomposition? J. Forensic Sci. 2007, 52, 1007–1014. [Google Scholar] [CrossRef]
- Cross, P.; Simmons, T. The influence of penetrative trauma on the rate of decomposition. J. Forensic Sci. 2010, 55, 295–301. [Google Scholar] [CrossRef]
- Pittner, S.; Bugelli, V.; Weitgasser, K.; Zissler, A.; Sanit, S.; Lutz, L.; Monticelli, F.; Campobasso, C.P.; Steinbacher, P.; Amendt, J. A field study to evaluate PMI estimation methods for advanced decomposition stages. Int. J. Leg. Med. 2020, 134, 1361–1373. [Google Scholar] [CrossRef]
- Pittner, S.; Bugelli, V.; Benbow, M.E.; Ehrenfellner, B.; Zissler, A.; Campobasso, C.P.; Oostra, R.J.; Aalders, M.C.G.; Zehner, R.; Lutz, L.; et al. The applicability of forensic time since death estimation methods for buried bodies in advanced decomposition stages. PLoS ONE 2020, 15, e0243395. [Google Scholar] [CrossRef]
- Fancher, J.P.; Aitkenhead-Peterson, J.A.; Farris, T.; Mix, K.; Schwab, A.P.; Wescott, D.J.; Hamilton, M.D. An evaluation of soil chemistry in human cadaver decomposition islands: Potential for estimating postmortem interval (PMI). Forensic Sci. Int. 2017, 279, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Suckling, J.K.; Spradley, M.K.; Godde, K. A longitudinal study on human outdoor decomposition in Central Texas. J. Forensic Sci. 2016, 61, 19–25. [Google Scholar] [CrossRef] [PubMed]
- De Donno, A.; Campobasso, C.P.; Santoro, V.; Leonardi, S.; Tafuri, S.; Introna, F. Bodies in sequestered and non-sequestered aquatic environments: A comparative taphonomic study using decompositional scoring system. Sci. Justice 2014, 54, 439–446. [Google Scholar] [CrossRef]
- Cockle, D.L.; Bell, L.S. Human decomposition and the reliability of a ‘Universal’ model for post mortem interval estimations. Forensic Sci. Int. 2015, 253, 136.e1–136.e9. [Google Scholar] [CrossRef] [PubMed]
- Nawrocka, M.; Frątczak, K.; Matuszewski, S. Inter-Rater Reliability of Total Body Score-A Scale for Quantification of Corpse Decomposition. J. Forensic Sci. 2016, 61, 798–802. [Google Scholar] [CrossRef]
- Bugelli, V.; Gherardi, M.; Focardi, M.; Pinchi, V.; Vanin, S.; Campobasso, C.P. Decomposition pattern and insect colonization in two cases of suicide by hanging. Forensic Sci. Res. 2018, 3, 94–102. [Google Scholar] [CrossRef]
- Gelderman, H.T.; Kruiver, C.A.; Oostra, R.J.; Zeegers, M.P.; Duijst, W.L.J.M. Estimation of the postmortem interval based on the human decomposition process. J. Forensic Leg. Med. 2019, 61, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Dautartas, A.; Kenyhercz, M.W.; Vidoli, G.M.; Meadows Jantz, L.; Mundorff, A.; Steadman, D.W. Differential Decomposition Among Pig, Rabbit, and Human Remains. J. Forensic Sci. 2018, 63, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.; Simmons, T.; Lynch-Aird, J. An Improved Equation for TBS and ADD: Establishing a Reliable Postmortem Interval Framework for Casework and Experimental Studies. J. Forensic Sci. 2016, 61 (Suppl. S1), S201–S207. [Google Scholar] [CrossRef] [PubMed]
- Heaton, V.; Lagden, A.; Moffatt, C.; Simmons, T. Predicting the postmortem submersion interval for human remains recovered from U.K. waterways. J. Forensic Sci. 2010, 55, 302–307. [Google Scholar] [CrossRef]
- Matuszewski, S.; Hall, M.J.R.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int. J. Leg. Med. 2020, 134, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Stokes, S.; Márquez-Grant, N.; Greenwood, C. Establishing a minimum PMI for bone sun bleaching in a UK environment with a controlled desert-simulated comparison. Int J Legal Med. 2020, 134, 2297–2306. [Google Scholar] [CrossRef]
- Myburgh, J.; L’Abbé, E.N.; Steyn, M.; Becker, P.J. Estimating the postmortem interval (PMI) using accumulated degree-days (ADD) in a temperate region of South Africa. Forensic Sci. Int. 2013, 229, 165.e1–165.e6. [Google Scholar] [CrossRef] [PubMed]
- Marhoff-Beard, S.J.; Forbes, S.L.; Green, H. The validation of ‘universal’ PMI methods for the estimation of time since death in temperate Australian climates. Forensic Sci. Int. 2018, 291, 158–166. [Google Scholar] [CrossRef]
- Campobasso, C.P.; De Micco, F.; Bugelli, V.; Cavezza, A.; Rodriguez, W.C., III.; Della Pietra, B. Undetected traumatic diastasis of cranial sutures: A case of child abuse. Forensic Sci. Int. 2019, 298, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Stork, N.E. How many Species of Insects and Other Terrestrial Arthropods are there on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef]
- Deel, H.; Emmons, A.L.; Kiely, J.; Damann, F.E.; Carter, D.O.; Lynne, A.; Knight, R.; Xu, Z.Z.; Bucheli, S.; Metcalf, J.L. A Pilot Study of Microbial Succession in Human Rib Skeletal Remains during Terrestrial Decomposition. mSphere 2021, 6, e00455-21. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Shang, Y.; Chen, W.; Meng, F.; Cai, J.; Zhu, G.; Chen, L.; Wang, Y.; Deng, J.; Guo, Y. A brief review of forensically important flesh flies (Diptera: Sarcophagidae). Forensic Sci. Res. 2018, 3, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Gemmellaro, M.D.; Hamilton, G.C.; Ware, J.L. Review of Molecular Identification Techniques for Forensically Important Diptera. J. Med. Enthomol. 2019, 56, 887–902. [Google Scholar] [CrossRef]
- Kashyap, V.K.; Pillay, V.V. Efficacy of entomological method in estimation of postmortem interval: A comparative analysis. Forensic Sci. Int. 1989, 40, 245–250. [Google Scholar] [CrossRef]
- Acosta, X.; Corronca, J.A.; González-Reyes, A.X.; Centeno, N.D. Postmortem Interval Estimation and Validation Through a Comparative Study of South American Flies Reared in the Field Versus Laboratory Conditions. J. Med. Enthomol. 2022, 59, 147–161. [Google Scholar] [CrossRef]
- Bugelli, V.; Tarozzi, I.; Galante, N.; Bartolini, S.; Franceschetti, L. Review on forensic importance of myiasis: Focus on medicolegal issues on post-mortem interval estimation and neglect evaluation. Leg. Med. 2023, 63, 102263. [Google Scholar] [CrossRef]
- Moreau, G. The Pitfalls in the Path of Probabilistic Inference in Forensic Entomology: A Review. Insects 2021, 12, 240. [Google Scholar] [CrossRef]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J. European Association for Forensic Entomology. Best practice in forensic entomology--standards and guidelines. Int. J. Leg. Med. 2007, 121, 90–104. [Google Scholar] [CrossRef]
- Goff, M.; Gherardi, M.; Campobasso, C. Forensic Implications of Myiasis. In Current Concepts in Forensic Entomology; Amendt, J., Campobasso, C.P., Goff, M.L., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 313–325. [Google Scholar]
- Anderson, G.S.; VanLaerhoven, S.L. Initial Studies on Insect Succession on Carrion in southwestern British Columbia. J. Forensic Sci. 1996, 41, 617–625. [Google Scholar] [CrossRef]
- Arnaldos, I.; Romera, E.; Garcìa, M.D.; Luna, A. An initial study on the succession of sarcosaprophagous Diptera (Insecta) on carrion in the southeastern Iberian Peninsula. Int. J. Leg. Med. 2001, 114, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.S. Annual variation in arrival and departure times of carrion insects at carcasses: Implications for succession studies in forensic entomology. Aust. J. Zool. 2004, 51, 569–576. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, D. Insect faunal succession on decaying rabbit carcasses in Punjab, India. J. Forensic Sci. 2003, 48, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Grassberger, M.; Frank, C. Initial study of arthropod succession on pig carrion in a central European urban habitat. J. Med. Entomol. 2004, 41, 511–523. [Google Scholar] [CrossRef]
- Watson, E.J.; Carlton, C.E. Insect succession and decomposition of wildlife carcasses during fall and winter in Louisiana. J. Med. Entomol. 2005, 42, 193–203. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Elliot, D.A. A preliminary investigation of insect colonization and succession on remains in New Zealand. Forensic Sci. Int. 2008, 176, 217–223. [Google Scholar] [CrossRef]
- Goff, L.M. Estimation of postmortem interval using arthropod development and successional patterns. Forensic Sci. Rev. 1996, 5, 81–94. [Google Scholar]
- Hall, M.J.R.; Whitaker, A.; Richards, C. Forensic entomology. In Forensic Ecology Handbook: From Crime Scene to Court; Márquez-Grant, N., Roberts, J., Eds.; John Wiley and Sons Limited: Chichester, UK, 2012; pp. 111–140. [Google Scholar]
- Stevens, J.; Wall, R. The evolution of ectoparasitism in the genus Lucilia (Diptera:Calliphoridae). Int. J. Parasitol. 1997, 27, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Richards, C.S.; Campobasso, C.P. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef]
- Introna, F.; Campobasso, C.P. Forensic dipterology. In Contributions to A Manual of Palaearctic Diptera. 1. General and Applied Dipterology; Papp, L., Darvas, B., Eds.; Science Herald: Budapest, Hungary, 2000; pp. 793–846. [Google Scholar]
- Matuszewski, S.; Konwerski, S.; Frątczak, K.; Szafałowicz, M. Effect of body mass and clothing on decomposition of pig carcasses. Int. J. Leg. Med. 2014, 128, 1039–1048. [Google Scholar] [CrossRef]
- Michaud, J.P.; Moreau, G. Predicting the visitation of carcasses by carrion-related insects under different rates of degree-day accumulation. Forensic Sci. Int. 2009, 185, 78–83. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: Composition and residency patterns of carrion fauna. Forensic Sci. Int. 2010, 195, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S. Estimating the pre-appearance interval from temperature in Necrodes littoralis L. (Coleoptera: Silphidae). Forensic Sci. Int. 2011, 212, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Szafałowicz, M. Temperature-dependent appearance of forensically useful beetles on carcasses. Forensic Sci. Int. 2013, 229, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, S.; Mądra, A. Factors affecting quality of temperature models for the pre-appearance interval of forensically useful insects. Forensic Sci. Int. 2015, 247, 28–35. [Google Scholar] [CrossRef]
- Vanin, S.; Bonizzoli, M.; Migliaccio, M.L.; Buoninsegni, L.T.; Bugelli, V.; Pinchi, V.; Focardi, M. A case of insect colonization before the death. J. Forensic Sci. 2017, 62, 1665–1667. [Google Scholar] [CrossRef] [PubMed]
- Introna, F.; Cattaneo, C.; Mazzarelli, D.; De Micco, F.; Campobasso, C.P. Unusual Application of Insect-Related Evidence in Two European Unsolved Murders. Insects 2021, 12, 444. [Google Scholar] [CrossRef]
- Amadasi, A.; Cappella, A.; Cattaneo, C.; Cofrancesco, P.; Cucca, L.; Merli, D.; Milanese, C.; Pinto, A.; Profumo, A.; Scarpulla, V.; et al. Determination of the post mortem interval in skeletal remains by the comparative use of different physico-chemical methods: Are they reliable as an alternative to (14)C? Homo 2017, 68, 213–221. [Google Scholar] [CrossRef]
- Cappella, A.; Gibelli, D.; Muccino, E.; Scarpulla, V.; Cerutti, E.; Caruso, V.; Sguazza, E.; Mazzarelli, D.; Cattaneo, C. The comparative performance of PMI estimation in skeletal remains by three methods (C-14, luminol test and OHI): Analysis of 20 cases. Int. J. Leg. Med. 2018, 132, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Madea, B.; Ortmann, J.; Doberentz, E. Estimation of the time since death-Even methods with a low precision may be helpful in forensic casework. Forensic Sci. Int. 2019, 302, 109879. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschetti, L.; Amadasi, A.; Bugelli, V.; Bolsi, G.; Tsokos, M. Estimation of Late Postmortem Interval: Where Do We Stand? A Literature Review. Biology 2023, 12, 783. https://doi.org/10.3390/biology12060783
Franceschetti L, Amadasi A, Bugelli V, Bolsi G, Tsokos M. Estimation of Late Postmortem Interval: Where Do We Stand? A Literature Review. Biology. 2023; 12(6):783. https://doi.org/10.3390/biology12060783
Chicago/Turabian StyleFranceschetti, Lorenzo, Alberto Amadasi, Valentina Bugelli, Giulia Bolsi, and Michael Tsokos. 2023. "Estimation of Late Postmortem Interval: Where Do We Stand? A Literature Review" Biology 12, no. 6: 783. https://doi.org/10.3390/biology12060783
APA StyleFranceschetti, L., Amadasi, A., Bugelli, V., Bolsi, G., & Tsokos, M. (2023). Estimation of Late Postmortem Interval: Where Do We Stand? A Literature Review. Biology, 12(6), 783. https://doi.org/10.3390/biology12060783






