Central Autonomic Network Regions and Hypertension: Unveiling Sympathetic Activation and Genetic Therapeutic Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Animals
2.3. Metabolic Evaluation
2.4. Surgical procedures
2.4.1. Implantation of Radio-Telemetry Probes
2.4.2. Viral Vector Construction and Validation
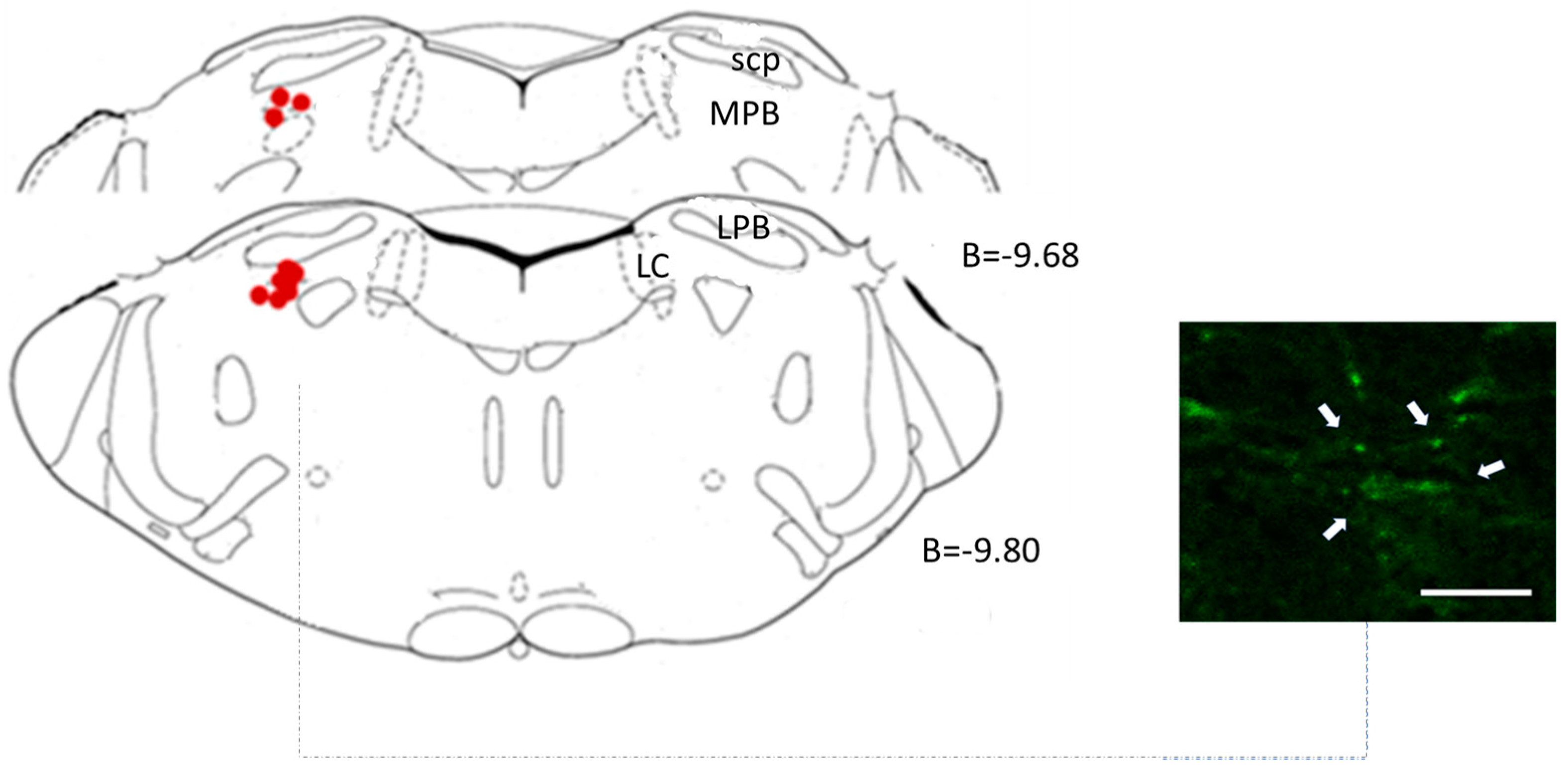
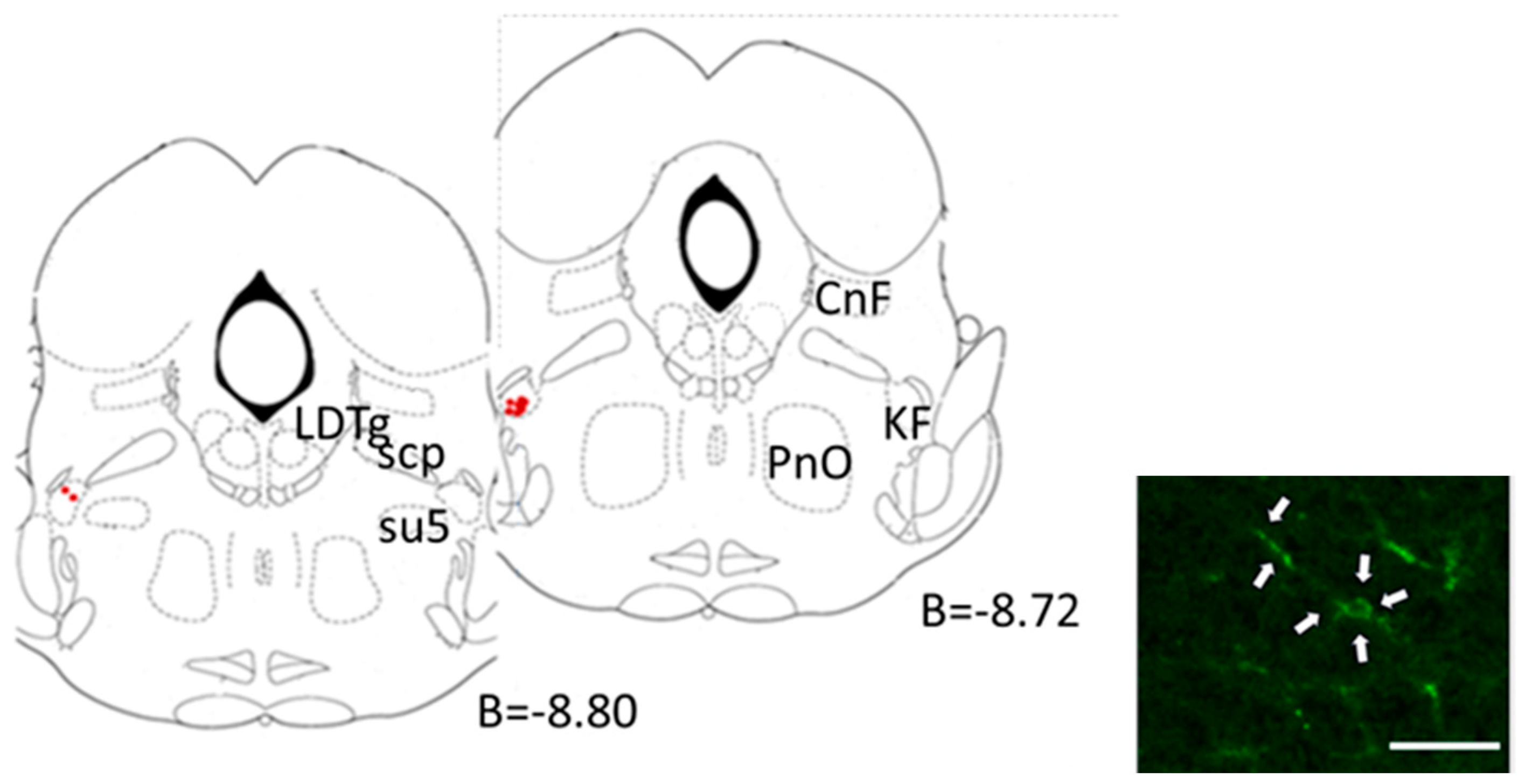
2.4.3. Central Microinjection Sites
2.4.4. Cardiorespiratory Evaluation
2.5. Morphological Studies
2.6. Data Acquisition and Analysis
2.6.1. Baroreceptor, Chemoreceptor Reflex, and Overall Autonomic Tone Evaluation
- Baroreceptor Reflex Evaluation: The baroreceptor reflex gain (BRG) was quantified using the following formula: BRG = (HRbasal-HRBPmax)/(BPmax-BPbasal) (bpm mmHg −1). This calculation provides a measure of the baroreceptor reflex sensitivity, which is a key mechanism for short-term blood pressure regulation [23].
- Chemoreceptor Reflex Evaluation: The chemoreceptor reflex (ChR) was evaluated by measuring the change in the respiratory rate (∆ChR) in response to lobeline stimulation. The respiratory rate (RespR) was derived from the tracheal pressure before and after the lobeline administration. The following formula was used: ∆ChR = RespRlobeline-RespRbasal [24].
- Autonomic Tone Evaluation: To assess the overall autonomic tone, a spectral analysis of the systolic blood pressure (SBP) and RR interval data was performed. The low-frequency (LF) band (0.15–0.6 Hz) of the SBP was used as an indicator of the sympathetic activity, while the high-frequency (HF) band (0.6–2.0 Hz) of the RR interval was used as an indicator of the parasympathetic activity. The data were analysed in the frequency domain using a Fast Fourier Transform with the in-house software Fisiosinal [25].
- Respiratory Sinus Arrhythmia: Respiratory sinus arrhythmia, a measure of parasympathetic activity, was quantified as the ratio of the longer RR interval of the ECG during expiration to the shorter RR interval during inspiration, as described previously [26].
2.6.2. Circadian BP and HR Profile
2.7. Statistical Analysis
3. Results
3.1. Lateral Parabrachial Nucleus
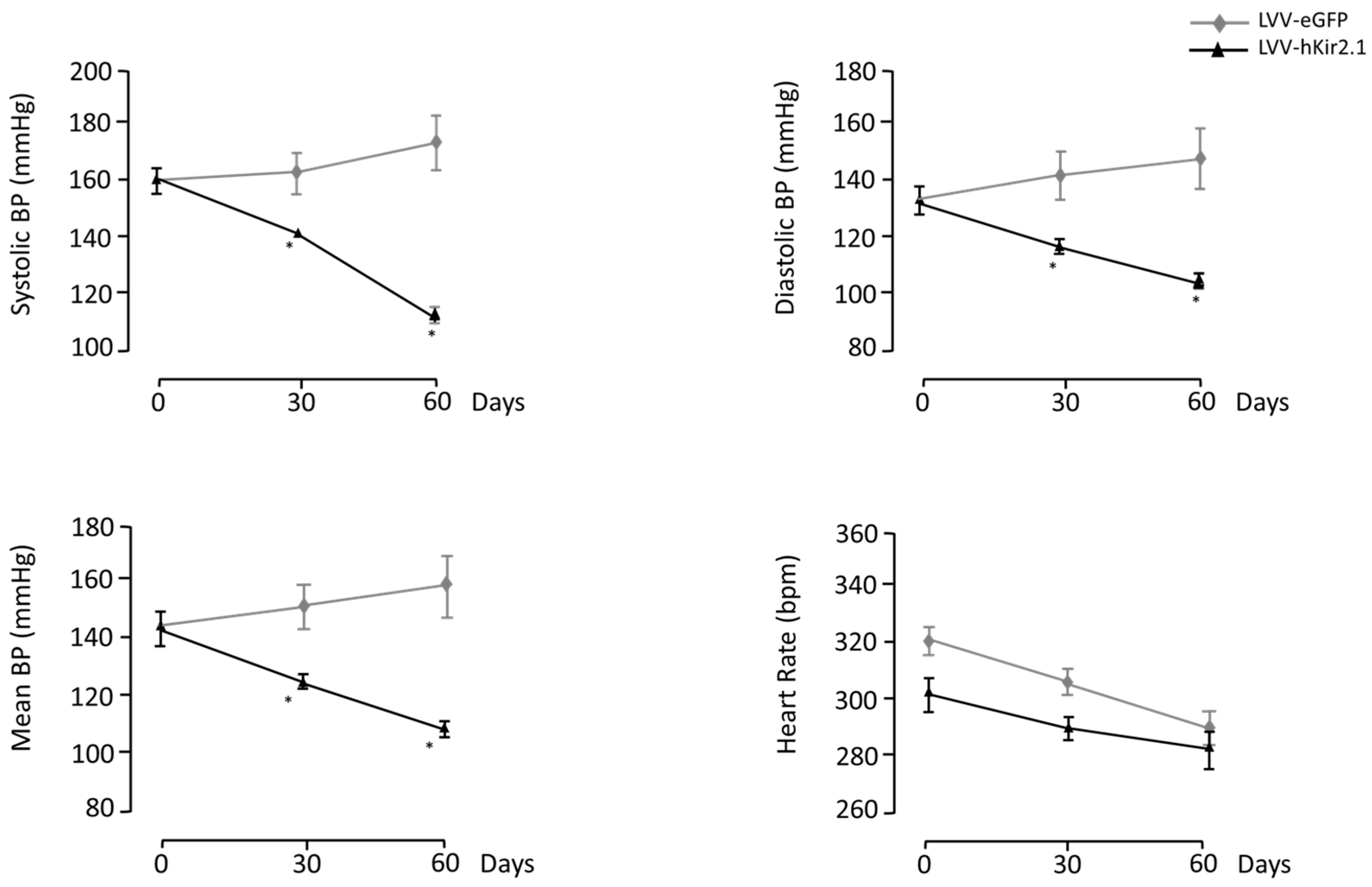
3.1.1. LPBN Lentiviral Microinjection Influence on Long-Term Blood Pressure Control
3.1.2. LPBN Lentiviral Microinjection Impact on Sympathetic Tone
3.1.3. Blood Pressure and Heart Rate Circadian Variation
3.1.4. Parasympathetic Tonus Indirect Assessment
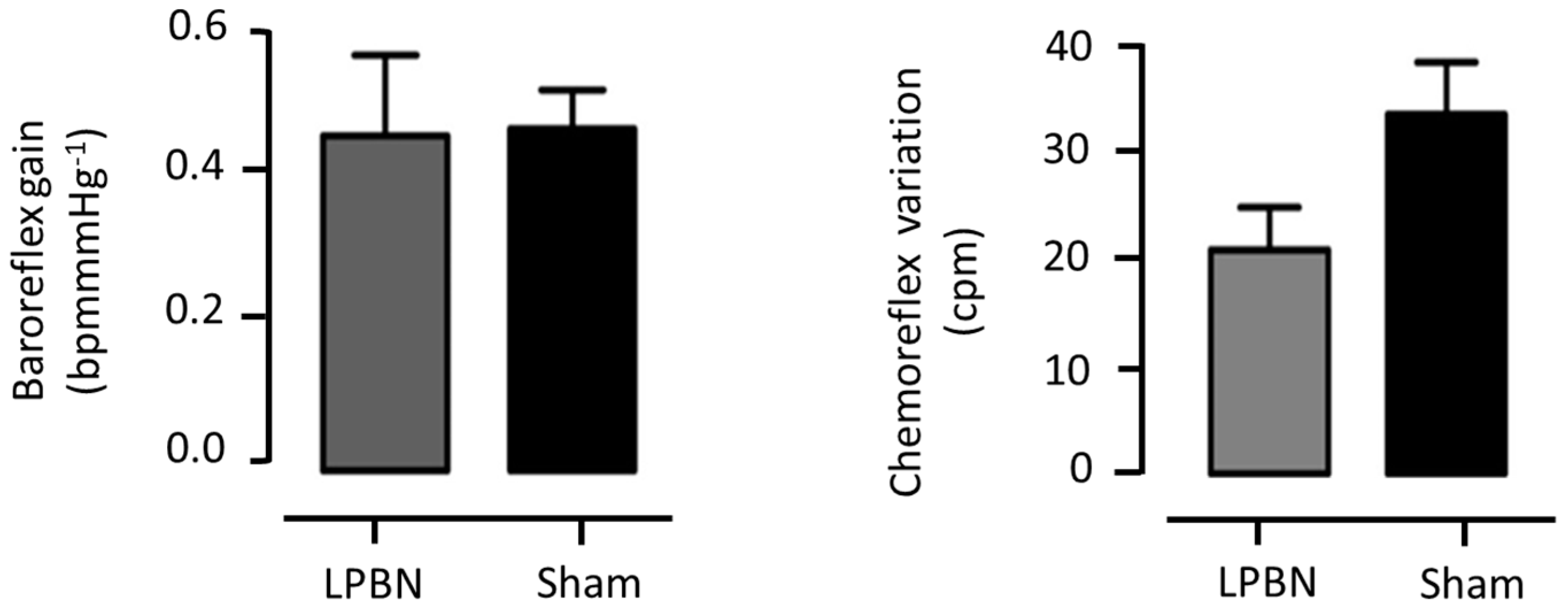
3.1.5. Cardiovascular Reflexes Evaluation
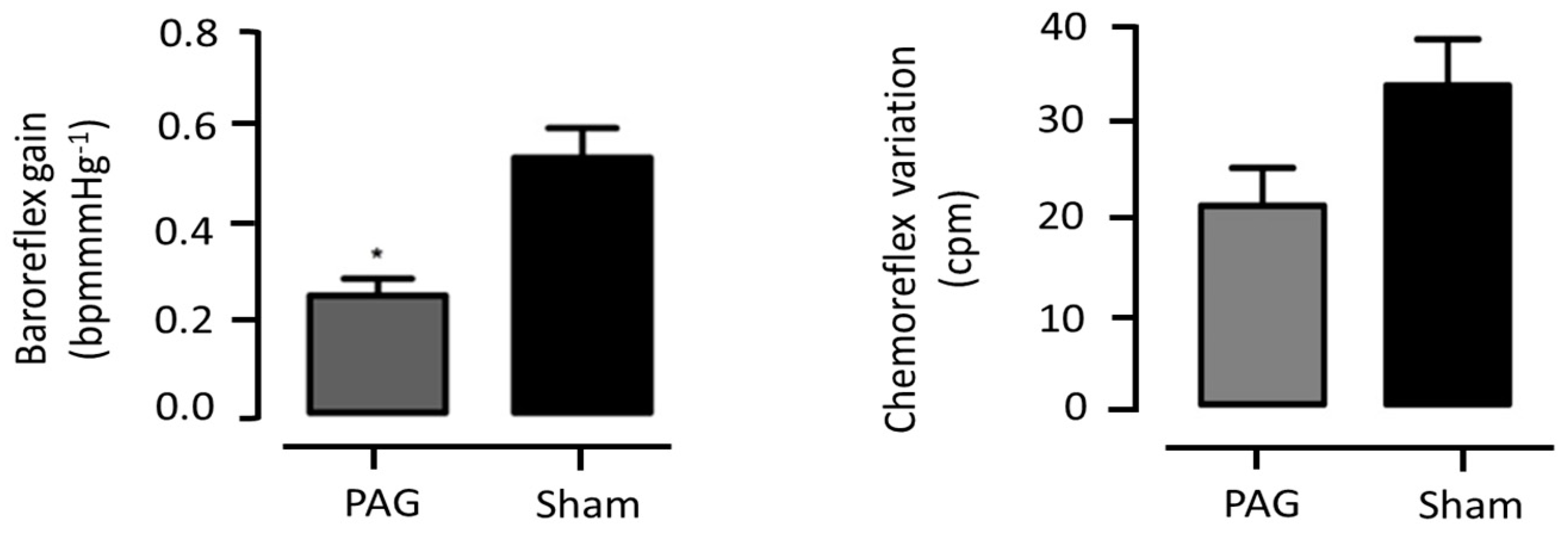
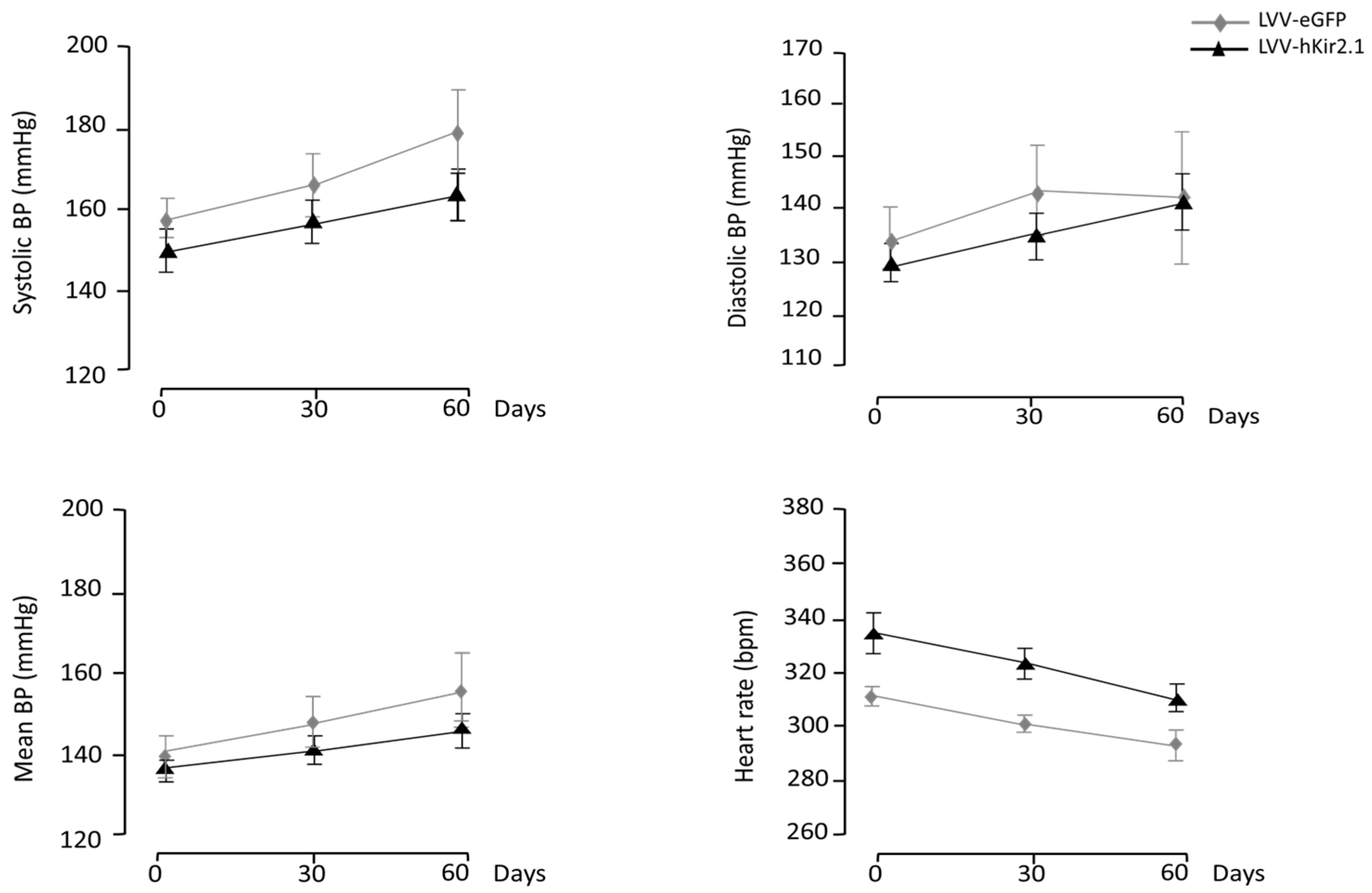
3.2. Periaqueductal Gray Matter
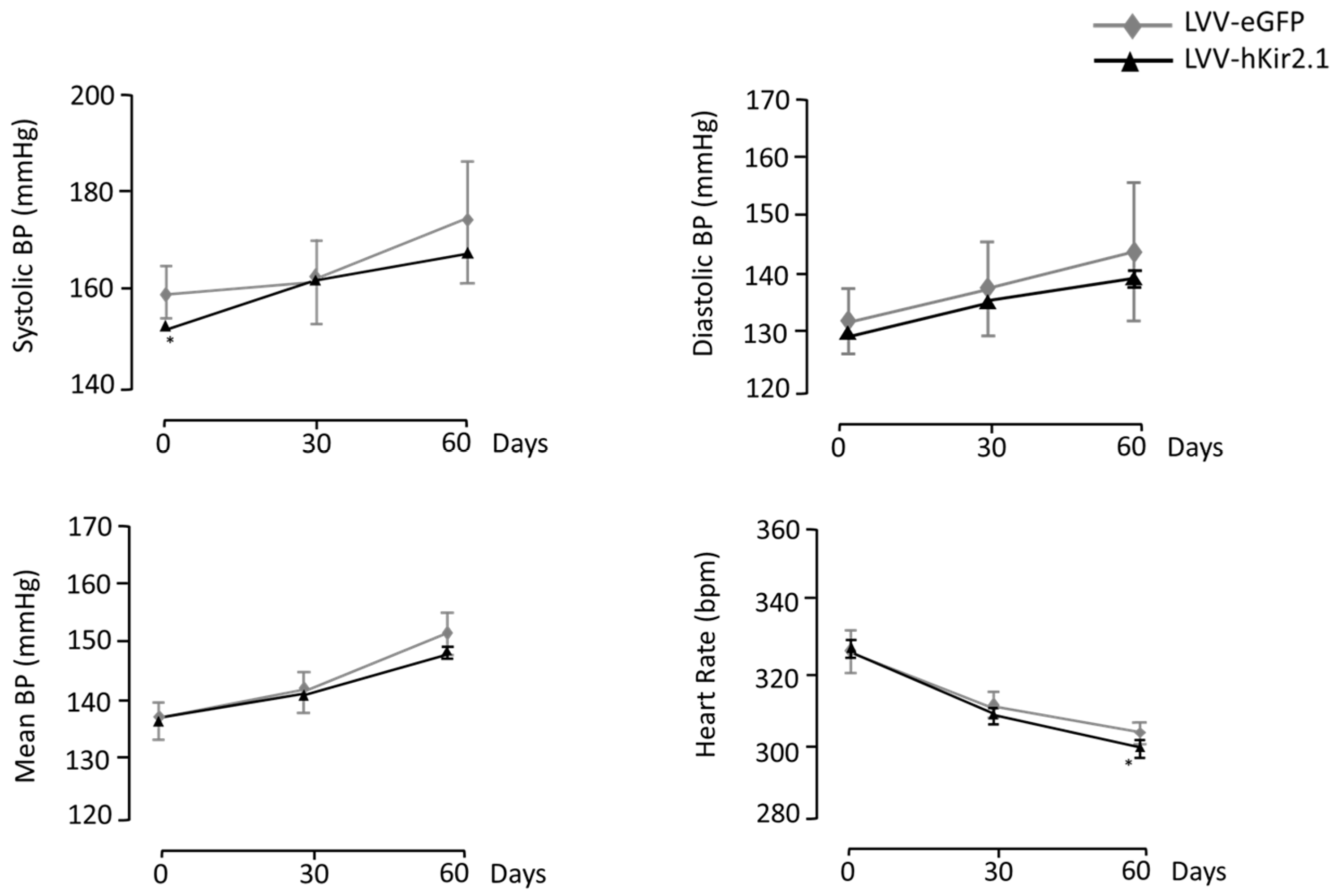
3.2.1. PAG Lentiviral Microinjection Influence on 24 h Mean Values of Blood Pressure and Heart Rate
3.2.2. PAG Lentiviral Microinjection Effect on Sympathetic Output
3.2.3. Blood Pressure and Heart Rate Circadian Variation
3.2.4. Indirect Quantification of Vagal Tonus
3.2.5. Cardiorespiratory Evaluation
3.3. Kolliker-Fuse Nucleus
3.3.1. KF Lentiviral Microinjection Influence on 24 h Mean Values of Blood Pressure and Heart Rate
3.3.2. Effect of KF LVV-hKir2.1 Microinjection on Sympathetic Output
3.3.3. Blood Pressure and Heart Rate Circadian Variation
3.3.4. Indirect Assessment of Vagal Tonus
3.3.5. Cardiorespiratory Reflex Assessment
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esler, M.; Lambert, E.; Schlaich, M. Point: Chronic activation of the sympathetic nervous system is the dominant contributor to systemic hypertension. J. Appl. Physiol. 2010, 109, 1996–1998. [Google Scholar] [CrossRef]
- Grassi, G.; Ram, V.S. Evidence for a critical role of the sympathetic nervous system in hypertension. J. Am. Soc. Hypertens. 2016, 10, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Rapp, J.P. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension 1982, 4, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Dampney, R.A. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef] [PubMed]
- Potts, P.D.; Paton, J.F. Residual depressor function within RVLM of the ‘decentralized’ rat. J. Physiol. 2006, 572, 425–437. [Google Scholar]
- Geraldes, V.; Goncalves-Rosa, N.; Liu, B.; Paton, J.F.; Rocha, I. Essential role of RVL medullary neuronal activity in the long term maintenance of hypertension in conscious SHR. Auton. Neurosci. 2014, 186, 22–31. [Google Scholar] [CrossRef]
- Geraldes, V.; Gonçalves-Rosa, N.; Liu, B.; Paton, J.F.; Rocha, I. Chronic depression of hypothalamic paraventricular neuronal activity produces sustained hypotension in hypertensive rats. Exp. Physiol. 2014, 99, 89–100. [Google Scholar] [CrossRef]
- Kc, P.; Dick, T.E. Modulation of cardiorespiratory function mediated by the parabrachial nucleus: Role of nitric oxide. Respir. Physiol. Neurobiol 2010, 174, 55–64. [Google Scholar] [CrossRef]
- Silvani, A.; Calandra-Buonaura, G.; Dampney, R.A.; Cortelli, P. Brain-heart interactions: Physiology and clinical implications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150181. [Google Scholar] [CrossRef]
- Benarroch, E.E. Periaqueductal gray: An interface for behavioral control. Neurology 2012, 78, 210–217. [Google Scholar] [CrossRef]
- Sato, M.; Shirasaka, T.; Uchida, S.; Shibamoto, T. Neural Interaction between Respiratory and Autonomic Centers in the Medulla. Neurosci. Insights 2020, 15, 2633105520974660. [Google Scholar]
- Subramanian, H.H. Distinct Periaqueductal Gray Projections to Medullary Cardioinhibitory and Excitatory Autonomic Pools in the Rat. Brain Res. 2018, 1698, 28–39. [Google Scholar]
- Ciriello, J.; Caverson, M.M.; McMurray, J.C. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 2013, 236, 104–119. [Google Scholar] [CrossRef]
- Scopinho, A.A.; Antunes, V.R.; Mauad, H.; Reis, D.G.; Moraes, D.J.A. The role of the parabrachial complex in the cardiorespiratory response evoked from hypothalamic defense area stimulation. Brain Res. 2016, 1646, 457–467. [Google Scholar]
- Farnham, M.M.J.; Pilowsky, P.M. The role of the lateral parabrachial nucleus in cardiovascular regulation. Auton. Neurosci. Basic Clin. 2018, 211, 28–35. [Google Scholar]
- Hayward, L.F.; Felder, R.B. Lateral parabrachial nucleus modulates baroreflex regulation of sympathetic nerve activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 274, R1274–R1282. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, J.R.; Lara, J.P.; Fuentealba, J.A.; Bonansco, C.; Díaz-Véliz, G. Cardiovascular changes induced by microinjections of GABAergic agonists into the central nucleus of the amygdala and lateral parabrachial nucleus in the rat. Physiol. Rep. 2020, 8, e14560. [Google Scholar]
- Silva-Carvalho, L.; Dawid-Milner, M.S.; Goldsmith, G.E.; Spyer, K.M. The effects of electrical stimulation and lesions in the parabrachial area on arterial blood pressure and heart rate in anaesthetized cats. J. Auton. Nerv. Syst. 1991, 33, 123–132. [Google Scholar]
- Teschemacher, A.G.; Wang, S.; Lonergan, T.; Duale, H.; Waki, H.; Paton, J.F.R.; Kasparov, S. Targeting specific neuronal populations using adeno-and lentiviral vectors: Applications for imaging and studies of cell function. Exp. Physiol. 2005, 90, 61–69. [Google Scholar] [CrossRef]
- Duale, H.; Waki, H.; Howorth, P.; Kasparov, S.; Teschemacher, A.G.; Paton, J.F. Restraining influence of A2 neurons in chronic control of arterial pressure in spontaneously hypertensive rats. Cardiovasc. Res. 2007, 76, 184–193. [Google Scholar] [CrossRef]
- Liu, B.; Paton, J.F.; Kasparov, S. Viral vectors based on bidirectional cell-specific mammalian promoters and transcriptional amplification strategy for use in vitro and in vivo. BMC Biotechnol. 2008, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates/George Paxinos; Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Gordon, F.J.; Matsuguchi, H.; Mark, A.L. Abnormal baroreflex control of heart rate in prehypertensive and hypertensive Dahl genetically salt-sensitive rats. Hypertension 1981, 3 Pt 2, I135. [Google Scholar] [CrossRef] [PubMed]
- Silva Carvalho, L.; Moniz De Bettencourt, J.; Esguelha, N.; Marques, M.; Silva Carvalho, J. The Double Reflexogenic Action of Lobeline, Acetylcholine and Cyanides on the Carotid Body, Influence of Phentolamine and Sulpiride. In Chemoreceptors in Respiratory Control; Springer: Dordrecht, The Netherlands, 1987; pp. 334–341. [Google Scholar]
- Tavares, C.; Carneiro, R.; Laranjo, S.; Rocha, I. Computational tools for assessing cardiovascular variability. In Proceedings of the 1st Portuguese Meeting in Bioengineering, Lisbon, Portugal, 1–4 March 2011; pp. 1–6. [Google Scholar]
- Castro, R.; Ramalho, S.; Nóbrega, A. Selection of RR interval in the electrocardiogram to determine the respiratory sinus arrhythmia. Rev. Bras. Med. Esporte 2000, 6, 5–8. [Google Scholar] [CrossRef][Green Version]
- Dampney, R.A. Central Neural Control of the Cardiovascular System: Current Perspectives. Adv. Physiol. Educ. 2016, 40, 283–296. [Google Scholar] [CrossRef]
- Mortensen, L.H.; Ohman, L.E.; Haywood, J.R. Effects of lateral parabrachial nucleus lesions in chronic renal hypertensive rats. Hypertension 1994, 23 Pt 1, 774–780. [Google Scholar] [CrossRef][Green Version]
- Lovick, T.A. The periaqueductal gray-rostral medulla connection in the defence reaction: Efferent pathways and descending control mechanisms. Behav. Brain Res. 1993, 58, 19–25. [Google Scholar] [CrossRef]
- Carrive, P. The periaqueductal gray and defensive behavior: Functional representation and neuronal organization. Behav. Brain Res. 1993, 58, 27–47. [Google Scholar] [CrossRef]
- Schenberg, L.C.; Lucas Brandão, C.A.; Vasquez, E.C. Role of periaqueductal gray matter in hypertension in spontaneously hypertensive rats. Hypert 1995, 26, 1125–1128. [Google Scholar] [CrossRef]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar] [CrossRef]
- Jun, S.; Ou, X.; Shi, L.; Yu, H.; Deng, T.; Chen, J.; Nie, X.; Hao, Y.; Shi, Y.; Liu, W.; et al. Circuit-Specific Control of Blood Pressure by PNMT-Expressing Nucleus Tractus Solitarii Neurons. Neurosci. Bull. 2023, 39, 1193–1209. [Google Scholar] [CrossRef]
- Kubo, T.; Fukumori, R.; Kobayashi, M.; Yamaguchi, H. Evidence suggesting that lateral parabrachial nucleus is responsible for enhanced medullary cholinergic activity in hypertension. Hypertens. Res. 1998, 21, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Poon, C.S. Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir. Physiol. Neurobiol. 2009, 165, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chamberlin, N.L.; Saper, C.B. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 1994, 14, 6500–6510. [Google Scholar] [CrossRef] [PubMed]
- Molkov, Y.I.; Bacak, B.J.; Dick, T.E.; Rybak, I.A. Control of breathing by interacting pontine and pulmonary feedback loops. Front. Neural Circ. 2017, 7, 16. [Google Scholar]
- Morris, K.F.; Nuding, S.C.; Segers, L.S.; Baekey, D.M.; Shannon, R.; Lindsey, B.G.; Dick, T.E. Respiratory and Mayer wave-related discharge patterns of raphe and pontine neurons change with vagotomy. J. Appl. Physiol. 2010, 109, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, R.S.; Takakura, A.C.; Moreira, T.S. Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R57–R67. [Google Scholar] [CrossRef]
- Varga, A.G.; Whitaker-Fornek, J.R.; Maletz, S.N.; Levitt, E.S. Activation of orexin-2 receptors in the Kӧlliker-Fuse nucleus of anesthetized mice leads to transient slowing of respiratory rate. Front. Physiol. 2022, 13, 977569. [Google Scholar] [CrossRef]
- Damasceno, R.S.; Takakura, A.C.; Moreira, T.S. Respiratory and sympathetic chemoreflex regulation by Kölliker-Fuse neurons in rats. Pflüg. Arch. Eur. J. Physiol. 2015, 467, 231–239. [Google Scholar] [CrossRef]
- González-García, M.; Carrillo-Franco, L.; Peinado-Aragonés, C.A.; Díaz-Casares, A.; Gago, B.; López-González, M.V.; Dawid-Milner, M.S. Impact of the glutamatergic neurotransmission within the A5 region on the cardiorespiratory response evoked from the midbrain dlPAG. Pflüg. Arch. Eur. J. Physiol. 2023, 475, 505–516. [Google Scholar] [CrossRef]
- Koshiya, N.; Guyenet, P.G. Role of the pons in the carotid sympathetic chemoreflex. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1994, 267, R508–R518. [Google Scholar] [CrossRef]
- Rosin, D.L.; Chang, D.A.; Guyenet, P.G. Afferent and efferent connections of the rat retrotrapezoid nucleus. J. Comp. Neurol. 2006, 499, 64–89. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.N.; Lucena, E.V.; Silva, T.M.; Damasceno, R.S.; Takakura, A.C.; Moreira, T.S. Inhibition of the pontine Kölliker-Fuse nucleus reduces genioglossal activity elicited by stimulation of the retrotrapezoid chemoreceptor neurons. Neuroscience 2016, 328, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Guyenet, P.G.; Stornetta, R.L. Rostral ventrolateral medulla, retropontine region and autonomic regulations. Auton. Neurosci. 2022, 237, 102922. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Nabika, T. Genetic Modifications to Alter Blood Pressure Level. Biomedicines 2022, 10, 1855. [Google Scholar] [CrossRef]
- Bonis, J.M.; Neumueller, S.E.; Krause, K.L.; Kiner, T.; Smith, A.; Marshall, B.D.; Qian, B.; Pan, L.G.; Forster, H.V. A role for the Kölliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J. Appl. Physiol. 2010, 109, 159–170. [Google Scholar] [CrossRef]


| Light Phase | Dark Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | sBP | dBP | mBP | HR | sBP | dBP | mBP | HR |
| Basal conditions | ||||||||
| PAG | 149 ± 2 | 126 ± 3 | 134 ± 3 | 309 ± 6 | 156 ± 3 | 133 ± 5 | 141 ± 4 | 343 ± 8 |
| SHAM | 157 ± 5 | 130 ± 5 | 139 ± 5 | 289 ± 7 | 160 ± 5 | 133 ± 6 | 142 ± 6 | 316 ± 8 |
| 60 days after microinjection | ||||||||
| PAG | 164 ± 2 *** | 140 ± 4 * | 148 ± 3 ** | 284 ± 4 *** | 172 ± 3 ** | 148 ± 7 | 156 ± 5 * | 322 ± 5 ** |
| SHAM | 170 ± 12 | 141 ± 12 | 151 ± 11 | 268 ± 4 | 175 ± 11 | 147 ± 11 | 156 ± 11 | 311 ± 3 |
| Light Phase | Dark Phase | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | sBP | dBP | mBP | HR | sBP | dBP | mBP | HR |
| Basal conditions | ||||||||
| KF | 148 ± 2 | 127 ± 3 | 134 ± 3 | 322 ± 9 | 152 ± 5 | 131 ± 4 | 138 ± 4 | 353 ± 8 |
| SHAM | 157 ± 5 | 131 ± 6 | 140 ± 5 | 295 ± 5 | 160 ± 5 | 134 ± 6 | 143 ± 6 | 321 ± 6 |
| 60 days after microinjection | ||||||||
| KF | 161 ± 6 * | 138 ± 5 * | 146 ± 5 * | 294 ± 14 * | 166 ± 7 * | 142 ± 6 * | 150 ± 6 * | 327 ± 7 * |
| SHAM | 177 ± 10 | 148 ± 12 | 158 ± 11 | 266 ± 5 | 181 ± 11 | 153 ± 13 | 163 ± 12 | 305 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geraldes, V.; Laranjo, S.; Nunes, C.; Rocha, I. Central Autonomic Network Regions and Hypertension: Unveiling Sympathetic Activation and Genetic Therapeutic Perspectives. Biology 2023, 12, 1153. https://doi.org/10.3390/biology12081153
Geraldes V, Laranjo S, Nunes C, Rocha I. Central Autonomic Network Regions and Hypertension: Unveiling Sympathetic Activation and Genetic Therapeutic Perspectives. Biology. 2023; 12(8):1153. https://doi.org/10.3390/biology12081153
Chicago/Turabian StyleGeraldes, Vera, Sérgio Laranjo, Catarina Nunes, and Isabel Rocha. 2023. "Central Autonomic Network Regions and Hypertension: Unveiling Sympathetic Activation and Genetic Therapeutic Perspectives" Biology 12, no. 8: 1153. https://doi.org/10.3390/biology12081153
APA StyleGeraldes, V., Laranjo, S., Nunes, C., & Rocha, I. (2023). Central Autonomic Network Regions and Hypertension: Unveiling Sympathetic Activation and Genetic Therapeutic Perspectives. Biology, 12(8), 1153. https://doi.org/10.3390/biology12081153





