The Impact of Head-Up Tilt Sleeping on Orthostatic Tolerance: A Scoping Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
- (1)
- Studies of people with or without autonomic dysfunction;
- (2)
- Studies of people aged ≥6 years;
- (3)
- Articles assessing the effect of full-body head-up tilt sleeping of any angle;
- (4)
- Articles with outcome measures related to cardiovascular control (e.g., orthostatic tolerance, BP, weight, oedema and nycturia).
- (1)
- Studies simultaneously evaluating HUTS with other pharmacological treatments for OH, including salt loading;
- (2)
- The following article types: case reports, narrative reviews, expert opinions, editorials, design studies and systematic reviews.
2.2. Study Selection on Data Extraction
2.3. Applied Methods
2.4. Risk of Bias
2.5. Data Analysis
3. Results
3.1. Selection of Sources
3.2. Study Protocols and Populations
3.3. Methodological Quality
3.4. HUTS Implementation
3.5. Orthostatic Hypotension Definition
3.6. Tolerance
3.7. Compliance
3.8. Main Findings
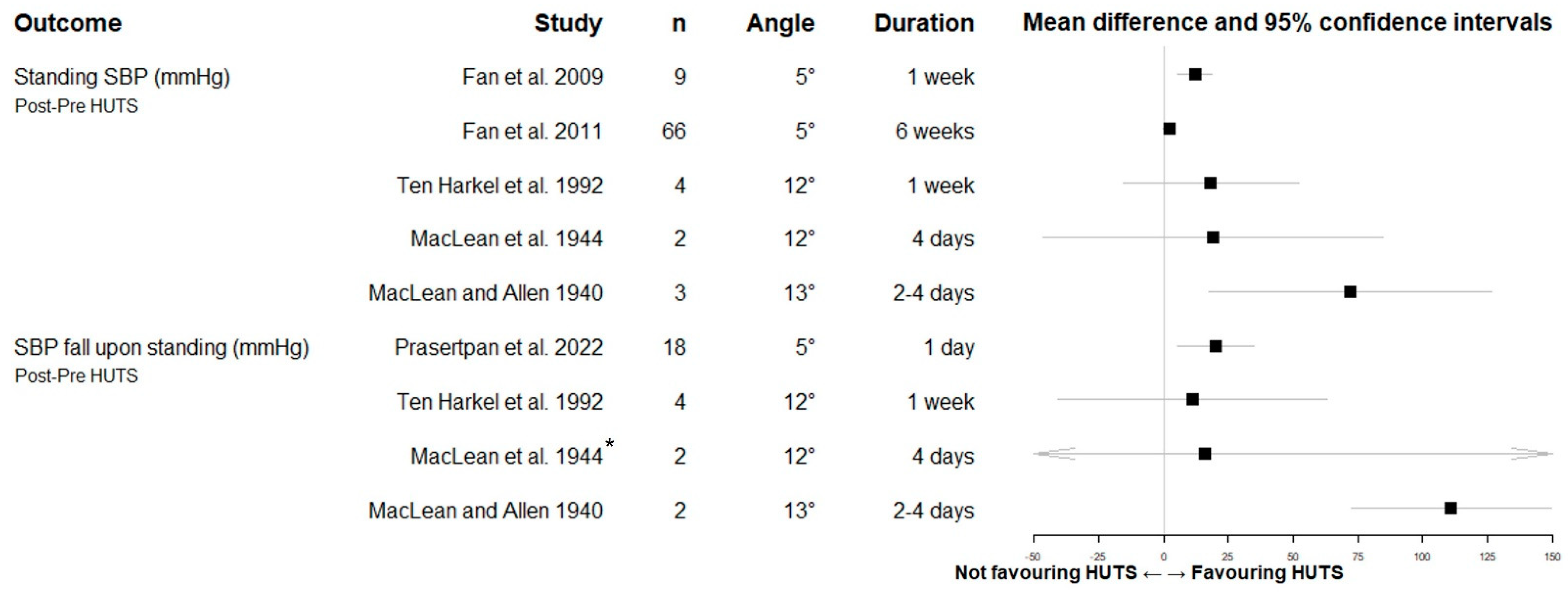
3.8.1. Orthostatic Blood Pressure
3.8.2. Orthostatic Symptoms and Syncope
3.8.3. Other Blood Pressure Data
3.8.4. Other Variables
4. Discussion
4.1. Summary of Evidence
4.2. Strengths and Weaknesses of the Review
4.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Saedon, N.I.; Tan, M.P.; Frith, J. The prevalence of orthostatic hypotension: A systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.-A.; Kaufmann, H. Epidemiology, diagnosis, and management of neurogenic orthostatic hypotension. Mov. Disord. Clin. Pract. 2017, 4, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Shibao, C.A.; Biaggioni, I. Management of orthostatic hypotension, postprandial hypotension, and supine hypertension. Semin. Neurol. 2020, 40, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Wieling, W.; Kaufmann, H.; E Claydon, V.; van Wijnen, V.K.; Harms, M.P.M.; Juraschek, S.P.; Thijs, R.D. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol. 2022, 21, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, J.G.; Van Rossum, I.A.; Thijs, R.D. Timing of circulatory and neurological events in syncope. Front. Cardiovasc. Med. 2020, 7, 36. [Google Scholar] [CrossRef]
- Jordan, J.; Fanciulli, A.; Tank, J.; Calandra-Buonaura, G.; Cheshire, W.P.; Cortelli, P.; Eschlboeck, S.; Grassi, G.; Hilz, M.J.; Kaufmann, H.; et al. Management of supine hypertension in patients with neurogenic orthostatic hypotension: Scientific statement of the american autonomic society, european federation of autonomic societies, and the european society of hypertension. J. Hypertens. 2019, 37, 1541–1546. [Google Scholar] [CrossRef]
- Palma, J.-A.; Kaufmann, H. Management of orthostatic hypotension. Contin. (Minneap. Minn.) 2020, 26, 154–177. [Google Scholar] [CrossRef]
- Wieling, W.; van Lieshout, J.J.; Hainsworth, R. Extracellular fluid volume expansion in patients with posturally related syncope. Clin. Auton. Res. 2002, 12, 242–249. [Google Scholar] [CrossRef]
- Wieling, W.; Raj, S.R.; Thijs, R.D. Are small observational studies sufficient evidence for a recommendation of head-up sleeping in all patients with debilitating orthostatic hypotension? Maclean and allen revisited after 70 years. Clin. Auton. Res. 2009, 19, 8–12. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Schmidt, P.; Biaggioni, I.; Frazier-Mills, C.; Freeman, R.; Isaacson, S.; Karabin, B.; Kuritzky, L.; Lew, M.; Low, P.; et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J. Neurol. 2017, 264, 1567–1582. [Google Scholar] [CrossRef]
- Lahrmann, H.; Cortelli, P.; Hilz, M.; Mathias, C.J.; Struhal, W.; Tassinari, M. Efns guidelines on the diagnosis and management of orthostatic hypotension. Eur. J. Neurol. 2006, 13, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.W.; Coakley, D.; Walsh, J.B.; Cunningham, C.J. Postal questionnaire survey: The use of sleeping with the head of the bed tilted upright for treatment of orthostatic hypotension in clinical practice. Age Ageing 2006, 35, 529–532. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. Prisma extension for scoping reviews (prisma-scr): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Fanciulli, A.; Jordan, J.; Biaggioni, I.; Calandra–Buonaura, G.; Cheshire, W.P.; Cortelli, P.; Eschlboeck, S.; Grassi, G.; Hilz, M.J.; Kaufmann, H.; et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the american autonomic society (aas) and the european federation of autonomic societies (efas): Endorsed by the european academy of neurology (ean) and the european society of hypertension (esh). Clin. Auton. Res. 2018, 28, 355–362. [Google Scholar] [CrossRef]
- Gronseth, G.S.; Cox, J.; Gloss, D.; Merillat, S.; Dittman, J.; Armstrong, M.; Getchius, T. Clinical Practice Guideline Process Manual; American Academy of Neurology: Minneapolis, MN, USA, 2017. [Google Scholar]
- Mohr, R.; Smolinsky, A.; Goor, D. Treatment of nocturnal angina with 10 degrees reverse trendelenburg bed position. Lancet 1982, 1, 1325–1327. [Google Scholar] [CrossRef]
- Ten Harkel, A.D.; Lieshout, J.J.; Wieling, W. Treatment of orthostatic hypotension with sleeping in the head-up tilt position, alone and in combination with fludrocortisone. J. Intern. Med. 1992, 232, 139–145. [Google Scholar] [CrossRef]
- Cooper, V.L.; Hainsworth, R. Head-up sleeping improves orthostatic tolerance in patients with syncope. Clin. Auton. Res. 2008, 18, 318–324. [Google Scholar] [CrossRef]
- Fan, C.W.; O’sullivan, E.; Healy, M.; Gasparro, D.; Crowley, V.; Cunningham, C.J. Physiological effects of sleeping with the head of the bed elevated 18 in. In young healthy volunteers. Ir. J. Med. Sci. 2008, 177, 371–377. [Google Scholar] [CrossRef]
- Fan, C.-W.; Gasparro, D.; Crowley, V.; Cunningham, C.J. Acute haemodynamic response to sleeping head-up at 6 inches in older inpatients. Clin. Auton. Res. 2009, 19, 51–57. [Google Scholar] [CrossRef]
- Fan, C.W.; Walsh, C.; Cunningham, C.J. The effect of sleeping with the head of the bed elevated six inches on elderly patients with orthostatic hypotension: An open randomised controlled trial. Age Ageing 2011, 40, 187–192. [Google Scholar] [CrossRef]
- Prasertpan, T.; Chokesuwattanaskul, R.; Sringean, J.; Siwanogsatham, S.; Bhidayasiri, R. What is the appropriate sleep position for parkinson’s disease patients with orthostatic hypotension in the morning? In Movement Disorders; Wiley: Hoboken, NJ, USA; Madrid, Spain, 2022. [Google Scholar]
- Pham, L.V.; Goodman, D.; Aguilar, T.; Polotsky, V.Y.; Checkley, W.; Schwartz, A.R. A cross-over trial of postural therapy for sleep disordered breathing in native highlanders. Am. J. Respir. Crit. Care Med. 2019, 199, A2593. [Google Scholar]
- MacLean, A.R.; Allen, E.V. Orthostatic hypotension and orthostatic tacchycardia: Treatment with the ‘head-up’ bed. JAMA 1940, 115, 2162–2167. [Google Scholar] [CrossRef]
- MacLean, A.R.; Allen, E.V.; Magath, T.B. Orthostatic tachycardia and orthostatic hypotension: Defects in the return of venous blood to the heart. Am. Heart J. 1944, 27, 145–163. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology 1996, 46, 1470. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef]
- van Lieshout, J.J.; Harkel, A.D.J.T.; Wieling, W. Fludrocortisone and sleeping in the head-up position limit the postural decrease in cardiac output in autonomic failure. Clin. Auton. Res. 2000, 10, 35–42. [Google Scholar] [CrossRef]
- Ten Harmsen, B.L.; van Rumund, A.; Aerts, M.B.; Bergkamp, M.I.; Esselink, R.A.; Richard, E.; Meijer, F.J.; Bloem, B.R.; van Wamelen, D.J. Clinical correlates of cerebral white matter abnormalities in patients with parkinson’s disease. Park. Relat. Disord. 2018, 49, 28–33. [Google Scholar] [CrossRef]


| First Author and Year | Study Type | Population | Cases n | Age (y; Mean (SD)) | Female n (%) | HUTS Angle (°) | HUTS Duration | Collected Data | Method Orthostatic BP Measurement | Details of OH Assessment c | Risk of Bias Class d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fan et al., 2009 [22] | Prospective cohort | Elderly with symptomatic OH of all causes | 9 | 76 (5) | 5 (55) | 5 | 1 w | Orth. symptoms, orth. BP, ABPM, weight, lab | Active standing, beat-to-beat BP (Finapres). Supine 5 m; stand 120 s. | NR | IV |
| Fan et al., 2011 [23] | Randomised controlled trial | Elderly with symptomatic OH of all causes | 100 - HUTS 66 - contr. 34 | (Median, IQR) 76 (71, 80) 76 (72, 83) | 37 (56) 19 (56) | 5 | 6 w | Orth. symptoms, orth. BP, ABPM, weight, urine volume and Na, oedema | Active standing, beat-to-beat BP (Finapres). Supine 5 m; stand 120 s. | Both HUTS and non-HUTS group increased water intake to 2 L a day. | II |
| Prasertpan et al., 2022 a [24] | Prospective cohort | nOH in PD | 18 | 69 (5.6) | 11 (61) | 6 | 1 d | Orth. BP, ABPM | NR | Morning immediately after awaking. | IV |
| Ten Harkel et al., 1992 [19] | Prospective cohort | nOH | 4 b | 23; 44; 59; 65 | 3 (50) | 12 | 1 w FU 8–70 m | Orth. symptoms, orth. BP, weight, urine K/Na/Creatinine | Active standing, beat-to-beat BP (Finapres). Supine 20 m; stand max 10 m or until symptoms. | At 08.00 h after an overnight fast. High salt intake of 150–200 mmol Na+/d and water intake of ≥2 L started 1w before HUTS. | IV |
| MacLean et al., 1944 [26] | Case series | Non-nOH | 2 | 35; 57 | 0 (0) | 12 | 4 d FU 3–6 m | Orth. symptoms, orth. BP, oedema, plasma volume, lab | Active standing. Supine before arising; stand various 1–25 m. | Before arising in the morning after overnight fast. Intake of water was controlled (not specified). | IV |
| MacLean and Allen 1940 [27] | Case series | nOH and non-nOH | 4 | 59; 30; 34; 47 | 2 (50) | 13 | 2–4 d FU (n = 3) 2–6 m | Orth. symptoms, syncope, orth. BP, oedema, plasma volume, lab, sweating | Active standing. Supine duration NR; stand 1–60 m or duration NR. | NR | IV |
| Cooper and Hainsworth 2008 [20] | Prospective cohort | VVS and poor orthostatic tolerance | 12 | 42 (5) | 6 (50) | 10 | 3–4 m | Orth. symptoms, syncope, orth. BP, plasma volume | Orthostatic stress test: supine 20 m; tilt 60° for 20 m; lower body negative pressure until pre-syncope. | NR | IV |
| Fan et al., 2008 [21] | Prospective cohort | Healthy college students | 29 | 22 (1.9) | 16 (55) | 13 | 1 w | Orth. symptoms, orth. BP, ABPM, oedema, weight, urine volume and Na, lab | Active standing, beat-to-beat BP (Finapres). Supine 5–10 m; stand 2 m. | Morning 9:00–11:00. Water intake of ≥2 L started 1 w before HUTS. | IV |
| Pham et al., 2019 a [25] | Cross-over | Healthy Peruvian highlanders | 29 | 62.3 (8.9) | 11 (38) | 15 | 1 d | Sleep, respiratory variables, heart rate | NA | NA | II or III e |
| Mohr et al., 1982 [18] | Prospective cohort | Refractory nocturnal angina | 10 | 56.4 (4,8) | 2 (20) | 10 | 2 d | Aortic pressure, central venous pressure, pulmonary artery pressure | NA | NA | IV |
| Fan et al., 2009 [22] | Fan et al., 2011 [23] | Prasertpan et al., 2022 [24] | Ten Harkel et al., 1992 [19] | McLean et al., 1944 [26] | McLean and Allen 1940 [27] | Cooper and Hainsworth 2008 [20] | Fan et al., 2008 [21] | Pham et al., 2019 [25] | Mohr et al., 1982 [18] | Total Score (n) | Total Score (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OH populations | ||||||||||||
| Report of OH aetiology | ● | ● | ● | ● | ● | ● | NA | NA | NA | NA | 5 | 83 |
| Presence of SH mentioned | ● | ● | ● | ● | ● | ● | NA | NA | NA | NA | 1 | 17 |
| Orthostatic BP protocol | ||||||||||||
| Supine rest ≥ 5 m | ● | ● | ● | ● | ● | ● | ● | ● | NA | NA | 6 | 75 |
| Standing ≥ 3 m | ● | ● | ● | ● | ● | ● | ● | ● | NA | NA | 2 | 25 |
| Constant time of day | ● | ● | ● | ● | ● | ● | ● | ● | NA | NA | 4 | 50 |
| Accounting for hydration state | ● | ● | ● | ● | ● | ● | ● | ● | NA | NA | 4 | 50 |
| Accounting for fasting state | ● | ● | ● | ● | ● | ● | ● | ● | NA | NA | 2 | 25 |
| Before/after drug administration | ● | ● | ● | ● | ● | ● | ● | ● | NA | NA | 0 | 0 |
| HUTS reporting | ||||||||||||
| HUTS duration | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 10 | 100 |
| HUTS angle | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 10 | 100 |
| HUTS tolerance | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 6 | 60 |
| HUTS compliance | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 1 | 10 |
| Quantitative symptom evaluation | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 2 | 20 |
| Nocturia: urine volume | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 2 | 20 |
| Overnight ∆ body weight | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 1 | 10 |
| Sleep quality | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | 1 | 10 |
| Total score (n) | 5 | 7 | 4 | 11 | 9 | 4 | 4 | 7 | 4 | 2 | ||
| Total score (%) | 31 | 43 | 25 | 68 | 56 | 25 | 28 | 50 | 50 | 25 |
| Variable | First Author and Year | Population (n) | Method | Outcome |
|---|---|---|---|---|
| Plasma volume, pre and post HUTS | Cooper and Hainsworth 2008 [20] | VVS (8) | Evans blue dye dilution method, 8 out of 12 cases | 3.18 to 3.40 L/kg * |
| MacLean et al., 1944 [27] | OH (1) | Unknown method, in 1 case | 38.6 to 43.0 cc/kg | |
| MacLean and Allen 1940 [26] | OH (1) | Congo red method, in 1 case | 45 to 51 cc/kg | |
| Body weight, pre and post HUTS | Ten Harkel et al., 1992 [19] Fan et al., 2009 [22] Fan et al., 2011 [23] MacLean et al., 1944 [27] Fan et al., 2008 [21] | OH (4) OH (9) OH (100) OH (1) Healthy (29) | Measured post-voiding at 22:00 and 8:00 Unknown method Unknown method, controls compared to HUTS group Day before and after 3 days of HUTS, in 1 case Measured post-voiding at 8:00 | Morning weight: 0.5 kg increase * Evening-morning difference: no change 70.0 to 70.7 kg No change 86.2 to 87.1 kg 66.1 to 66.5 kg * |
| Urine, Pre and post HUTS | Fan et al., 2008 [21] Fan et al., 2011 [23] Ten Harkel et al., 1992 [19] | Healthy (29) OH (100) OH (4) | Volume and sodium excretion 24 h volume and sodium excretion Creatinine, sodium, and potassium as day/night ratio | Night-time volume: 622 to 477 mL * Day-time volume: 1510 to 1562 mL Sodium excretion: 373 to 382 mmol Volume and sodium excretion: No change Creatinine and Potassium, no change. Sodium: 0.63 to 0.81 |
| Oedema | Fan et al., 2008 [21] Fan et al., 2011 [23] MacLean et al., 1944 [27] MacLean and Allen 1940 [26] | Healthy (29) OH (100) OH (1) OH (1) | Measured calf and ankle circumference pre- and post-HUTS Unknown method Observation, 1 case Observation, 1 case | Ankle: 255 to 263 mm * Calf: 371 to 373 mm HUTS: 41%, controls: 19% * “slight pitting oedema” “slight oedema of the lower extremities” |
| Laboratory blood values Pre and Post HUTS | Fan et al., 2009 [22] Fan et al., 2008 [21] MacLean et al., 1944 MacLean and Allen 1940 [27] | OH (9) Healthy (29) OH (1) OH (4) | Haematocrit, plasma renin, electrolyte, aldosterone, creatinine Supine haematocrit, plasma renin, electrolytes, aldosterone, pro-ANP Haematocrit, chloride, protein Haematocrit, haemoglobin, and erythrocyte count | Creatinine: 101 to 95.6 mmol/L * All others: no change Haemoglobin 13.6 to 13.3 g/dL * All others: no change Haematocrit: 35.5% to 34.8% Chloride: 99.3 to 103.8 mEq/L Protein: 6.40 to 6.45 Gm/cL Haematocrit: 36% to 34% Haemoglobin: 8.5 to 8.1 Gm/cL Erythrocytes: 3.3 to 4.1 × 106 per mL |
| Respiratory | Pham et al., 2019 [25] | Healthy (11) | Hypoxia burden during HUTS compared to flat sleeping | SpO2: 83.6% to 85.5% * RDI: 21.5 to 17.8/h * |
| Sleep | Pham et al., 2019 [25] | Healthy (11) | Total monitored sleep time, during HUTS compared to flat sleeping | Sleep time: 380 to 375 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Stam, A.H.; Shmuely, S.; de Vries, N.M.; Bloem, B.R.; Thijs, R.D. The Impact of Head-Up Tilt Sleeping on Orthostatic Tolerance: A Scoping Review. Biology 2023, 12, 1108. https://doi.org/10.3390/biology12081108
van der Stam AH, Shmuely S, de Vries NM, Bloem BR, Thijs RD. The Impact of Head-Up Tilt Sleeping on Orthostatic Tolerance: A Scoping Review. Biology. 2023; 12(8):1108. https://doi.org/10.3390/biology12081108
Chicago/Turabian Stylevan der Stam, Amber H., Sharon Shmuely, Nienke M. de Vries, Bastiaan R. Bloem, and Roland D. Thijs. 2023. "The Impact of Head-Up Tilt Sleeping on Orthostatic Tolerance: A Scoping Review" Biology 12, no. 8: 1108. https://doi.org/10.3390/biology12081108
APA Stylevan der Stam, A. H., Shmuely, S., de Vries, N. M., Bloem, B. R., & Thijs, R. D. (2023). The Impact of Head-Up Tilt Sleeping on Orthostatic Tolerance: A Scoping Review. Biology, 12(8), 1108. https://doi.org/10.3390/biology12081108





