Extracellular Vesicles: Investigating the Pathophysiology of Diabetes-Associated Hypertension and Diabetic Nephropathy
Abstract
:Simple Summary
Abstract
1. Introduction
2. EVs in Cellular Communication and Alterations of Intracellular Signaling Pathways in Physiology and Pathophysiology
3. Salt-Sensitive Hypertension and Enrichment of Blood-Pressure-Regulating Proteins in uEVs
4. Association between Circulating EVs and Increased Blood Pressure
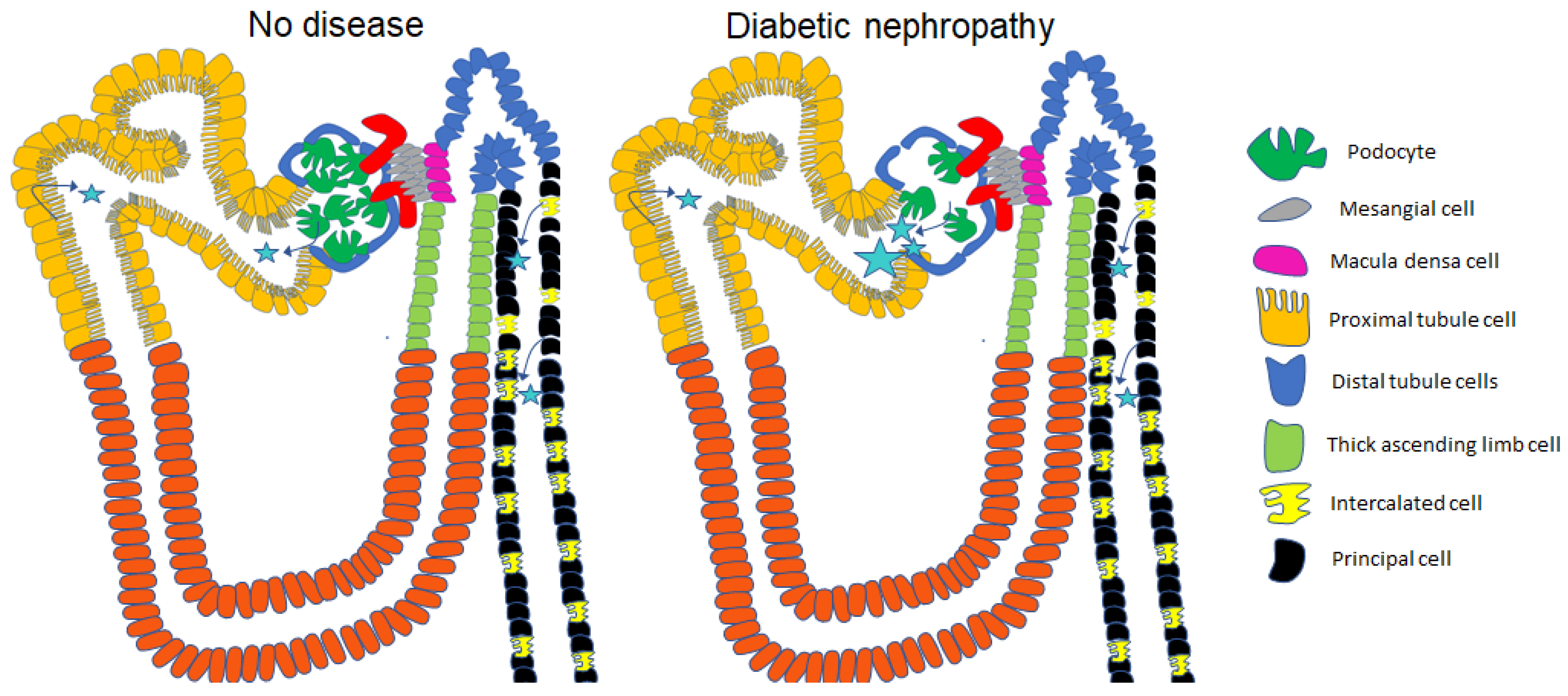
5. Urinary Excretion of Extracellular Vesicles in Diabetes and Diabetic Nephropathy
6. uEVs and Diabetes-Associated Hypertension
7. Relevance of Protease and Protease Inhibitor Enrichment in EVs to Diabetic Kidney Disease
8. Contribution of EVs in the Development of Insulin Resistance and Diabetes and Associated Complications
9. Circadian Regulation of EV Cargo Relevant to Diabetes Research
10. Renal Handling of EVs
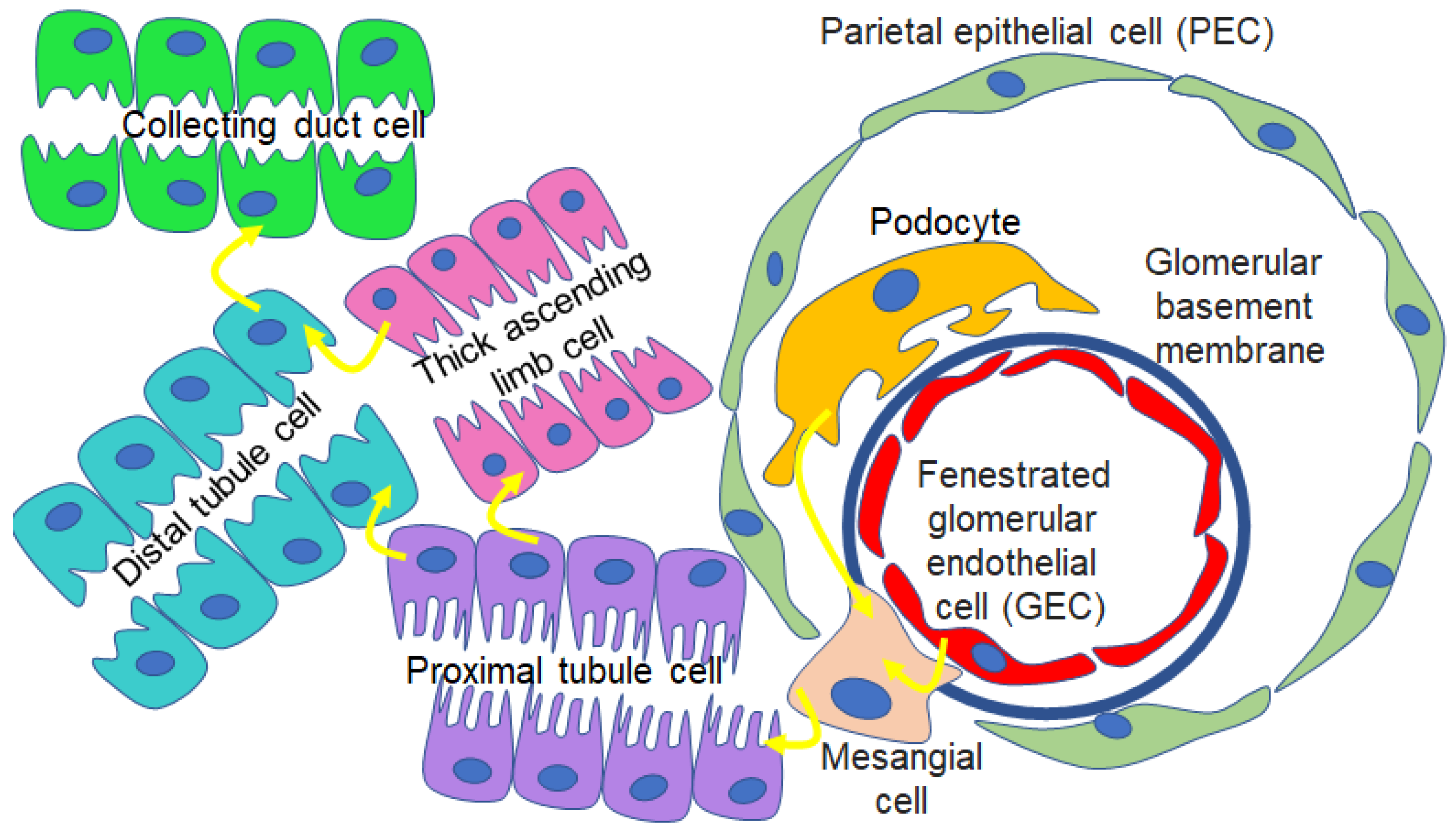
11. Proteomics of uEVs and EVs Released from Renal Cell Types
12. uEVs as Biomarkers for Various Human Diseases
13. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and characterization of urinary extracellular vesicles: Implications for biomarker discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Madison, R.D.; McGee, C.; Rawson, R.; Robinson, G.A. Extracellular vesicles from a muscle cell line (C2C12) enhance cell survival and neurite outgrowth of a motor neuron cell line (NSC-34). J. Extracell. Vesicles 2014, 3, 22865. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.K.; Yu, L.; Yue, Q.; Friedman, D.; Duke, B.J.; Alli, A.A. Exosomal GAPDH from Proximal Tubule Cells Regulate ENaC Activity. PLoS ONE 2016, 11, e0165763. [Google Scholar] [CrossRef] [PubMed]
- Pais, V.; Pais, E.S. Intercellular communication by extracellular vesicles with emphasis on the roles of cordocytes in the human brain. An ultrastructural study. Ultrastruct. Pathol. 2015, 39, 177–186. [Google Scholar] [CrossRef]
- Zieske, J.D.; Hutcheon, A.E.K.; Guo, X. Extracellular Vesicles and Cell-Cell Communication in the Cornea. Anat. Rec. (Hoboken) 2020, 303, 1727–1734. [Google Scholar] [CrossRef]
- Chen, G.; Xu, C.; Gillette, T.G.; Huang, T.; Huang, P.; Li, Q.; Li, X.; Li, Q.; Ning, Y.; Tang, R.; et al. Cardiomyocyte-derived small extracellular vesicles can signal eNOS activation in cardiac microvascular endothelial cells to protect against Ischemia/Reperfusion injury. Theranostics 2020, 10, 11754–11774. [Google Scholar] [CrossRef]
- Pekkucuksen, N.T.; Liu, L.P.; Aly, R.; Shoemaker, L.R.; Alli, A.A. Extracellular vesicles from focal segmental glomerulosclerosis pediatric patients induce STAT3 activation and mesangial cell proliferation. PLoS ONE 2022, 17, e0274598. [Google Scholar] [CrossRef]
- Wei, X.; Ye, J.; Pei, Y.; Wang, C.; Yang, H.; Tian, J.; Si, G.; Ma, Y.; Wang, K.; Liu, G. Extracellular vesicles from colorectal cancer cells promote metastasis via the NOD1 signalling pathway. J. Extracell. Vesicles 2022, 11, e12264. [Google Scholar] [CrossRef]
- Ditte, Z.; Silbern, I.; Ditte, P.; Urlaub, H.; Eichele, G. Extracellular vesicles derived from the choroid plexus trigger the differentiation of neural stem cells. J. Extracell. Vesicles 2022, 11, e12276. [Google Scholar] [CrossRef]
- Ito, A.; Kagawa, S.; Sakamoto, S.; Kuwada, K.; Kajioka, H.; Yoshimoto, M.; Kikuchi, S.; Kuroda, S.; Yoshida, R.; Tazawa, H.; et al. Extracellular vesicles shed from gastric cancer mediate protumor macrophage differentiation. BMC Cancer 2021, 21, 102. [Google Scholar] [CrossRef]
- Ma, Q.; Beal, J.R.; Bhurke, A.; Kannan, A.; Yu, J.; Taylor, R.N.; Bagchi, I.C.; Bagchi, M.K. Extracellular vesicles secreted by human uterine stromal cells regulate decidualization, angiogenesis, and trophoblast differentiation. Proc. Natl. Acad. Sci. USA 2022, 119, e2200252119. [Google Scholar] [CrossRef] [PubMed]
- Alter, C.L.; Detampel, P.; Schefer, R.B.; Lotter, C.; Hauswirth, P.; Puligilla, R.D.; Weibel, V.J.; Schenk, S.H.; Heusermann, W.; Schurz, M.; et al. High efficiency preparation of monodisperse plasma membrane derived extracellular vesicles for therapeutic applications. Commun. Biol. 2023, 6, 478. [Google Scholar] [CrossRef]
- Antich-Rossello, M.; Munar-Bestard, M.; Forteza-Genestra, M.A.; Calvo, J.; Gaya, A.; Monjo, M.; Ramis, J.M. Evaluation of Platelet-Derived Extracellular Vesicles in Gingival Fibroblasts and Keratinocytes for Periodontal Applications. Int. J. Mol. Sci. 2022, 23, 7668. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Li, Y.J.; Hu, X.B.; Huang, S.; Xiang, D.X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Deliv. 2021, 28, 162–170. [Google Scholar] [CrossRef]
- Lopez, J.P.; Nouri, M.Z.; Ebrahim, A.; Chacko, K.M.; Schramm, W.C.; Gholam, M.F.; Ozrazgat-Baslanti, T.; Denslow, N.D.; Alli, A.A. Lipid Profiles of Urinary Extracellular Vesicles Released during the Inactive and Active Phases of Aged Male Mice with Spontaneous Hypertension. Int. J. Mol. Sci. 2022, 23, 15397. [Google Scholar] [CrossRef]
- Darwish, S.; Liu, L.P.; Robinson, T.O.; Tarugu, S.; Owings, A.H.; Glover, S.C.; Alli, A.A. COVID-19 Plasma Extracellular Vesicles Increase the Density of Lipid Rafts in Human Small Airway Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 1654. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Jedrychowski, M.P.; Yanamandra, K.; Ikezu, S.; Gygi, S.P.; Ikezu, T. Proteomic Profiling of Extracellular Vesicles Derived from Cerebrospinal Fluid of Alzheimer’s Disease Patients: A Pilot Study. Cells 2020, 9, 1959. [Google Scholar] [CrossRef]
- Sequeiros, T.; Rigau, M.; Chiva, C.; Montes, M.; Garcia-Grau, I.; Garcia, M.; Diaz, S.; Celma, A.; Bijnsdorp, I.; Campos, A.; et al. Targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer. Oncotarget 2017, 8, 4960–4976. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Yang, X.; Jiang, Y.; Li, A.; Cong, J.; Li, Y.; Xie, Q.; Xu, C.; Liu, D. Identification of faecal extracellular vesicles as novel biomarkers for the non-invasive diagnosis and prognosis of colorectal cancer. J. Extracell. Vesicles 2023, 12, e12300. [Google Scholar] [CrossRef]
- Kim, S.; Kang, J.H.; Nguyen Cao, T.G.; Kang, S.J.; Jeong, K.; Kang, H.C.; Kwon, Y.J.; Rhee, W.J.; Ko, Y.T.; Shim, M.S. Extracellular vesicles with high dual drug loading for safe and efficient combination chemo-phototherapy. Biomater. Sci. 2022, 10, 2817–2830. [Google Scholar] [CrossRef]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A Biomimetic Drug Delivery System by Integrating Grapefruit Extracellular Vesicles and Doxorubicin-Loaded Heparin-Based Nanoparticles for Glioma Therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef]
- Mishra, D.D.; Sahoo, B.; Maurya, P.K.; Sharma, R.; Varughese, S.; Prasad, N.; Tiwari, S. Therapeutic potential of urine exosomes derived from rats with diabetic kidney disease. Front. Endocrinol. 2023, 14, 1157194. [Google Scholar] [CrossRef]
- Kontopoulou, E.; Strachan, S.; Reinhardt, K.; Kunz, F.; Walter, C.; Walkenfort, B.; Jastrow, H.; Hasenberg, M.; Giebel, B.; von Neuhoff, N.; et al. Evaluation of dsDNA from extracellular vesicles (EVs) in pediatric AML diagnostics. Ann. Hematol. 2020, 99, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, H.; Chen, T.; Jin, J.; Ruan, L.; Hu, L.; Chen, L. Extracellular vesicles metabolic changes reveals plasma signature in stage-dependent diabetic kidney disease. Ren. Fail. 2022, 44, 1840–1849. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jo, H.; Park, S.; Kim, H.; Kim, S.I.; Han, Y.; Lee, J.; Seol, A.; Kim, J.; Lee, M.; et al. Integrated analysis of ascites and plasma extracellular vesicles identifies a miRNA-based diagnostic signature in ovarian cancer. Cancer Lett. 2022, 542, 215735. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, B.; Zeng, F.; He, B.; Gao, Y.; Liu, X.; Song, Y. Magnetic Colloid Antibodies Accelerate Small Extracellular Vesicles Isolation for Point-of-Care Diagnostics. Nano Lett. 2021, 21, 2001–2009. [Google Scholar] [CrossRef]
- Dooley, K.; McConnell, R.E.; Xu, K.; Lewis, N.D.; Haupt, S.; Youniss, M.R.; Martin, S.; Sia, C.L.; McCoy, C.; Moniz, R.J.; et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. 2021, 29, 1729–1743. [Google Scholar] [CrossRef]
- Esteves, M.; Abreu, R.; Fernandes, H.; Serra-Almeida, C.; Martins, P.A.T.; Barao, M.; Cristovao, A.C.; Saraiva, C.; Ferreira, R.; Ferreira, L.; et al. MicroRNA-124-3p-enriched small extracellular vesicles as a therapeutic approach for Parkinson’s disease. Mol. Ther. 2022, 30, 3176–3192. [Google Scholar] [CrossRef]
- Lu, M.; Huang, B.; Hanash, S.M.; Onuchic, J.N.; Ben-Jacob, E. Modeling putative therapeutic implications of exosome exchange between tumor and immune cells. Proc. Natl. Acad. Sci. USA 2014, 111, E4165–E4174. [Google Scholar] [CrossRef] [PubMed]
- Trac, D.; Hoffman, J.R.; Bheri, S.; Maxwell, J.T.; Platt, M.O.; Davis, M.E. Predicting Functional Responses of Progenitor Cell Exosome Potential with Computational Modeling. Stem Cells Transl. Med. 2019, 8, 1212–1221. [Google Scholar] [CrossRef]
- Lennon, K.M.; Saftics, A.; Abuelreich, S.; Sahu, P.; Lehmann, H.I.; Maddox, A.L.; Bagabas, R.; Januzzi, J.L.; Van Keuren-Jensen, K.; Shah, R.; et al. Cardiac troponin T in extracellular vesicles as a novel biomarker in human cardiovascular disease. Clin. Transl. Med. 2022, 12, e979. [Google Scholar] [CrossRef] [PubMed]
- Paluschinski, M.; Loosen, S.; Kordes, C.; Keitel, V.; Kuebart, A.; Brandenburger, T.; Scholer, D.; Wammers, M.; Neumann, U.P.; Luedde, T.; et al. Extracellular Vesicles as Markers of Liver Function: Optimized Workflow for Biomarker Identification in Liver Disease. Int. J. Mol. Sci. 2023, 24, 9631. [Google Scholar] [CrossRef]
- Takizawa, K.; Ueda, K.; Sekiguchi, M.; Nakano, E.; Nishimura, T.; Kajiho, Y.; Kanda, S.; Miura, K.; Hattori, M.; Hashimoto, J.; et al. Urinary extracellular vesicles signature for diagnosis of kidney disease. iScience 2022, 25, 105416. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Gao, Y.B.; Cui, F.Q.; Zhang, N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol. Open 2016, 5, 484–491. [Google Scholar] [CrossRef]
- Borges, F.T.; Melo, S.A.; Ozdemir, B.C.; Kato, N.; Revuelta, I.; Miller, C.A.; Gattone, V.H., 2nd; LeBleu, V.S.; Kalluri, R. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J. Am. Soc. Nephrol. 2013, 24, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Kim, E.; Bae, Y.U.; Yang, W.M.; Lee, H.; Kim, H.; Noh, H.; Han, D.C.; Ryu, S.; Kwon, S.H. microRNA in Extracellular Vesicles Released by Damaged Podocytes Promote Apoptosis of Renal Tubular Epithelial Cells. Cells 2020, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Pizzolo, F.; Chiecchi, L.; Morandini, F.; Channavajjhala, S.K.; Guarini, P.; Salvagno, G.; Olivieri, O. Circadian exosomal expression of renal thiazide-sensitive NaCl cotransporter (NCC) and prostasin in healthy individuals. Proteomics Clin. Appl. 2015, 9, 623–629. [Google Scholar] [CrossRef]
- Ochiai-Homma, F.; Kuribayashi-Okuma, E.; Tsurutani, Y.; Ishizawa, K.; Fujii, W.; Odajima, K.; Kawagoe, M.; Tomomitsu, Y.; Murakawa, M.; Asakawa, S.; et al. Characterization of pendrin in urinary extracellular vesicles in a rat model of aldosterone excess and in human primary aldosteronism. Hypertens. Res. 2021, 44, 1557–1567. [Google Scholar] [CrossRef]
- Pathare, G.; Dhayat, N.; Mohebbi, N.; Wagner, C.A.; Cheval, L.; Neuhaus, T.J.; Fuster, D.G. Acute regulated expression of pendrin in human urinary exosomes. Pflugers Arch. 2018, 470, 427–438. [Google Scholar] [CrossRef]
- Pisitkun, T.; Johnstone, R.; Knepper, M.A. Discovery of urinary biomarkers. Mol. Cell. Proteom. 2006, 5, 1760–1771. [Google Scholar] [CrossRef]
- Hirohama, D.; Nishimoto, M.; Ayuzawa, N.; Kawarazaki, W.; Fujii, W.; Oba, S.; Shibata, S.; Marumo, T.; Fujita, T. Activation of Rac1-Mineralocorticoid Receptor Pathway Contributes to Renal Injury in Salt-Loaded db/db Mice. Hypertension 2021, 78, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Lugo, C.I.; Liu, L.P.; Bala, N.; Morales, A.G.; Gholam, M.F.; Abchee, J.C.; Elmoujahid, N.; Elshikha, A.S.; Avdiaj, R.; Searcy, L.A.; et al. Human Alpha-1 Antitrypsin Attenuates ENaC and MARCKS and Lowers Blood Pressure in Hypertensive Diabetic db/db Mice. Biomolecules 2022, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Scindia, Y.M.; Gholam, M.F.; Waleed, A.; Liu, L.P.; Chacko, K.M.; Desai, D.; Lopez, J.P.; Malik, Z.; Schramm, W.C.; Morales, A.G.; et al. Metformin Alleviates Diabetes-Associated Hypertension by Attenuating the Renal Epithelial Sodium Channel. Biomedicines 2023, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Veiras, L.C.; Shen, J.Z.Y.; Bernstein, E.A.; Regis, G.C.; Cao, D.; Okwan-Duodu, D.; Khan, Z.; Gibb, D.R.; Dominici, F.P.; Bernstein, K.E.; et al. Renal Inflammation Induces Salt Sensitivity in Male db/db Mice through Dysregulation of ENaC. J. Am. Soc. Nephrol. 2021, 32, 1131–1149. [Google Scholar] [CrossRef]
- Otani, K.; Yokoya, M.; Kodama, T.; Hori, K.; Matsumoto, K.; Okada, M.; Yamawaki, H. Plasma exosomes regulate systemic blood pressure in rats. Biochem. Biophys. Res. Commun. 2018, 503, 776–783. [Google Scholar] [CrossRef]
- Good, M.E.; Musante, L.; La Salvia, S.; Howell, N.L.; Carey, R.M.; Le, T.H.; Isakson, B.E.; Erdbrugger, U. Circulating Extracellular Vesicles in Normotension Restrain Vasodilation in Resistance Arteries. Hypertension 2020, 75, 218–228. [Google Scholar] [CrossRef]
- Sun, I.O.; Santelli, A.; Abumoawad, A.; Eirin, A.; Ferguson, C.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Puranik, A.S.; Lerman, L.O. Loss of Renal Peritubular Capillaries in Hypertensive Patients Is Detectable by Urinary Endothelial Microparticle Levels. Hypertension 2018, 72, 1180–1188. [Google Scholar] [CrossRef]
- Rai, A.; Fang, H.; Claridge, B.; Simpson, R.J.; Greening, D.W. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J. Extracell. Vesicles 2021, 10, e12164. [Google Scholar] [CrossRef]
- Jia, Y.; Guan, M.; Zheng, Z.; Zhang, Q.; Tang, C.; Xu, W.; Xiao, Z.; Wang, L.; Xue, Y. miRNAs in Urine Extracellular Vesicles as Predictors of Early-Stage Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 7932765. [Google Scholar] [CrossRef]
- Mohan, A.; Singh, R.S.; Kumari, M.; Garg, D.; Upadhyay, A.; Ecelbarger, C.M.; Tripathy, S.; Tiwari, S. Urinary Exosomal microRNA-451-5p Is a Potential Early Biomarker of Diabetic Nephropathy in Rats. PLoS ONE 2016, 11, e0154055. [Google Scholar] [CrossRef]
- Prabu, P.; Rome, S.; Sathishkumar, C.; Gastebois, C.; Meugnier, E.; Mohan, V.; Balasubramanyam, M. MicroRNAs from urinary extracellular vesicles are non-invasive early biomarkers of diabetic nephropathy in type 2 diabetes patients with the ‘Asian Indian phenotype’. Diabetes Metab. 2019, 45, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Delic, D.; Eisele, C.; Schmid, R.; Baum, P.; Wiech, F.; Gerl, M.; Zimdahl, H.; Pullen, S.S.; Urquhart, R. Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. PLoS ONE 2016, 11, e0150154. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Tricarico, M.; Corbelli, A.; Annaratone, L.; Pinach, S.; Grimaldi, S.; Bruno, G.; Cimino, D.; Taverna, D.; Deregibus, M.C.; et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS ONE 2013, 8, e73798. [Google Scholar] [CrossRef]
- Kalani, A.; Mohan, A.; Godbole, M.M.; Bhatia, E.; Gupta, A.; Sharma, R.K.; Tiwari, S. Wilm’s tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS ONE 2013, 8, e60177. [Google Scholar] [CrossRef]
- Zubiri, I.; Posada-Ayala, M.; Benito-Martin, A.; Maroto, A.S.; Martin-Lorenzo, M.; Cannata-Ortiz, P.; de la Cuesta, F.; Gonzalez-Calero, L.; Barderas, M.G.; Fernandez-Fernandez, B.; et al. Kidney tissue proteomics reveals regucalcin downregulation in response to diabetic nephropathy with reflection in urinary exosomes. Transl. Res. 2015, 166, 474–484.e4. [Google Scholar] [CrossRef]
- Musante, L.; Tataruch, D.; Gu, D.; Liu, X.; Forsblom, C.; Groop, P.H.; Holthofer, H. Proteases and protease inhibitors of urinary extracellular vesicles in diabetic nephropathy. J. Diabetes Res. 2015, 2015, 289734. [Google Scholar] [CrossRef]
- Gholam, M.F.; Liu, L.P.; Searcy, L.A.; Denslow, N.D.; Alli, A.A. Dapagliflozin Treatment Augments Bioactive Phosphatidylethanolamine Concentrations in Kidney Cortex Membrane Fractions of Hypertensive Diabetic db/db Mice and Alters the Density of Lipid Rafts in Mouse Proximal Tubule Cells. Int. J. Mol. Sci. 2023, 24, 1408. [Google Scholar] [CrossRef]
- Rice, G.E.; Scholz-Romero, K.; Sweeney, E.; Peiris, H.; Kobayashi, M.; Duncombe, G.; Mitchell, M.D.; Salomon, C. The Effect of Glucose on the Release and Bioactivity of Exosomes From First Trimester Trophoblast Cells. J. Clin. Endocrinol. Metab. 2015, 100, E1280–E1288. [Google Scholar] [CrossRef]
- Wen, J.; Ma, Z.; Livingston, M.J.; Zhang, W.; Yuan, Y.; Guo, C.; Liu, Y.; Fu, P.; Dong, Z. Decreased secretion and profibrotic activity of tubular exosomes in diabetic kidney disease. Am. J. Physiol. Renal Physiol. 2020, 319, F664–F673. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.P.; Masson, N.; Hud, E.; Ziyadeh, F.; Han, D.C.; Clements, R.S. Inhibiting albumin glycation ameliorates diabetic nephropathy in the db/db mouse. Exp. Nephrol. 2000, 8, 135–143. [Google Scholar] [CrossRef]
- Stas, S.N.; El-Atat, F.A.; Sowers, J.R. Pathogenesis of hypertension in diabetes. Rev. Endocr. Metab. Disord. 2004, 5, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Bagi, Z.; Erdei, N.; Toth, A.; Li, W.; Hintze, T.H.; Koller, A.; Kaley, G. Type 2 diabetic mice have increased arteriolar tone and blood pressure: Enhanced release of COX-2-derived constrictor prostaglandins. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Xie, Z.; Guo, Z.; Duncan, M.J.; Lutshumba, J.; Gong, M.C. Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H621–H633. [Google Scholar] [CrossRef] [PubMed]
- Bodary, P.F.; Shen, Y.; Ohman, M.; Bahrou, K.L.; Vargas, F.B.; Cudney, S.S.; Wickenheiser, K.J.; Myers, M.G., Jr.; Eitzman, D.T. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 70–76. [Google Scholar] [CrossRef]
- Bellini, S.; Saraceno, C.; Benussi, L.; Geviti, A.; Longobardi, A.; Nicsanu, R.; Cimini, S.; Ricci, M.; Canafoglia, L.; Coppola, C.; et al. Plasma Small Extracellular Vesicle Cathepsin D Dysregulation in GRN/C9orf72 and Sporadic Frontotemporal Lobar Degeneration. Int. J. Mol. Sci. 2022, 23, 10693. [Google Scholar] [CrossRef]
- Downs, C.A.; Dang, V.D.; Johnson, N.M.; Denslow, N.D.; Alli, A.A. Hydrogen Peroxide Stimulates Exosomal Cathepsin B Regulation of the Receptor for Advanced Glycation End-Products (RAGE). J. Cell. Biochem. 2018, 119, 599–606. [Google Scholar] [CrossRef]
- Khodayari, N.; Oshins, R.; Alli, A.A.; Tuna, K.M.; Holliday, L.S.; Krotova, K.; Brantly, M. Modulation of calreticulin expression reveals a novel exosome-mediated mechanism of Z variant alpha(1)-antitrypsin disposal. J. Biol. Chem. 2019, 294, 6240–6252. [Google Scholar] [CrossRef]
- Araujo, T.F.; Cordeiro, A.V.; Vasconcelos, D.A.A.; Vitzel, K.F.; Silva, V.R.R. The role of cathepsin B in autophagy during obesity: A systematic review. Life Sci. 2018, 209, 274–281. [Google Scholar] [CrossRef]
- Taleb, S.; Cancello, R.; Poitou, C.; Rouault, C.; Sellam, P.; Levy, P.; Bouillot, J.L.; Coussieu, C.; Basdevant, A.; Guerre-Millo, M.; et al. Weight loss reduces adipose tissue cathepsin S and its circulating levels in morbidly obese women. J. Clin. Endocrinol. Metab. 2006, 91, 1042–1047. [Google Scholar] [CrossRef]
- Karimkhanloo, H.; Keenan, S.N.; Sun, E.W.; Wattchow, D.A.; Keating, D.J.; Montgomery, M.K.; Watt, M.J. Circulating cathepsin S improves glycaemic control in mice. J. Endocrinol. 2021, 248, 167–179. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Winberg, J.O.; Reinholt, F.P.; Larsen, T.; Uhlin-Hansen, L.; Jenssen, T.; Berg, E.; Kolset, S.O. Proteases in Plasma and Kidney of db/db Mice as Markers of Diabetes-Induced Nephropathy. ISRN Endocrinol. 2011, 2011, 832642. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Y.; Liu, Y.; Lu, X.; Guo, S.; Wu, M.; Wang, M.; Yan, L.; Wang, Q.; Zhao, X.; et al. Exogenous kallikrein protects against diabetic nephropathy. Kidney Int. 2016, 90, 1023–1036. [Google Scholar] [CrossRef]
- Kumar, A.; Sundaram, K.; Mu, J.; Dryden, G.W.; Sriwastva, M.K.; Lei, C.; Zhang, L.; Qiu, X.; Xu, F.; Yan, J.; et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat. Commun. 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Abdelsaid, K.; Sudhahar, V.; Harris, R.A.; Das, A.; Youn, S.W.; Liu, Y.; McMenamin, M.; Hou, Y.; Fulton, D.; Hamrick, M.W.; et al. Exercise improves angiogenic function of circulating exosomes in type 2 diabetes: Role of exosomal SOD3. FASEB J. 2022, 36, e22177. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.S.; Pereira, D.G.; Zhang, J.; Huang, W.; Beg, M.A.; Knaack, D.A.; de Souza Goncalves, B.; Sahoo, D.; Silverstein, R.L.; Shapiro, J.I.; et al. Contribution of adipocyte Na/K-ATPase alpha1/CD36 signaling induced exosome secretion in response to oxidized LDL. Front. Cardiovasc. Med. 2023, 10, 1046495. [Google Scholar] [CrossRef]
- Yeung, C.C.; Dondelinger, F.; Schoof, E.M.; Georg, B.; Lu, Y.; Zheng, Z.; Zhang, J.; Hannibal, J.; Fahrenkrug, J.; Kjaer, M. Circadian regulation of protein cargo in extracellular vesicles. Sci. Adv. 2022, 8, eabc9061. [Google Scholar] [CrossRef]
- Koritzinsky, E.H.; Street, J.M.; Chari, R.R.; Glispie, D.M.; Bellomo, T.R.; Aponte, A.M.; Star, R.A.; Yuen, P.S.T. Circadian variation in the release of small extracellular vesicles can be normalized by vesicle number or TSG101. Am. J. Physiol. Renal Physiol. 2019, 317, F1098–F1110. [Google Scholar] [CrossRef]
- Su, W.; Guo, Z.; Randall, D.C.; Cassis, L.; Brown, D.R.; Gong, M.C. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1634–H1641. [Google Scholar] [CrossRef]
- Grosbellet, E.; Dumont, S.; Schuster-Klein, C.; Guardiola-Lemaitre, B.; Pevet, P.; Criscuolo, F.; Challet, E. Circadian phenotyping of obese and diabetic db/db mice. Biochimie 2016, 124, 198–206. [Google Scholar] [CrossRef]
- Hou, T.; Su, W.; Guo, Z.; Gong, M.C. A Novel Diabetic Mouse Model for Real-Time Monitoring of Clock Gene Oscillation and Blood Pressure Circadian Rhythm. J. Biol. Rhythms 2019, 34, 51–68. [Google Scholar] [CrossRef]
- Senador, D.; Kanakamedala, K.; Irigoyen, M.C.; Morris, M.; Elased, K.M. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp. Physiol. 2009, 94, 648–658. [Google Scholar] [CrossRef]
- Tung, C.W.; Hsu, Y.C.; Shih, Y.H.; Chang, P.J.; Lin, C.L. Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology 2018, 23 (Suppl. S4), 32–37. [Google Scholar] [CrossRef] [PubMed]
- Bolten, J.S.; Pratsinis, A.; Alter, C.L.; Fricker, G.; Huwyler, J. Zebrafish (Danio rerio) larva as an in vivo vertebrate model to study renal function. Am. J. Physiol. Renal Physiol. 2022, 322, F280–F294. [Google Scholar] [CrossRef]
- Ruggiero, A.; Villa, C.H.; Bander, E.; Rey, D.A.; Bergkvist, M.; Batt, C.A.; Manova-Todorova, K.; Deen, W.M.; Scheinberg, D.A.; McDevitt, M.R. Paradoxical glomerular filtration of carbon nanotubes. Proc. Natl. Acad. Sci. USA 2010, 107, 12369–12374. [Google Scholar] [CrossRef]
- Dang, V.D.; Jella, K.K.; Ragheb, R.R.T.; Denslow, N.D.; Alli, A.A. Lipidomic and proteomic analysis of exosomes from mouse cortical collecting duct cells. FASEB J. 2017, 31, 5399–5408. [Google Scholar] [CrossRef]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-scale proteomics and phosphoproteomics of urinary exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, K.; Lay, A.C.; Leparc, G.; Tran, V.D.T.; Rosler, M.; Dayalan, L.; Burdet, F.; Ibberson, M.; Coward, R.J.M.; Huber, T.B.; et al. An in vitro approach to understand contribution of kidney cells to human urinary extracellular vesicles. J. Extracell. Vesicles 2023, 12, e12304. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wilkinson, R.; Kildey, K.; Ungerer, J.P.J.; Hill, M.M.; Shah, A.K.; Mohamed, A.; Dutt, M.; Molendijk, J.; Healy, H.; et al. Molecular and functional profiling of apical versus basolateral small extracellular vesicles derived from primary human proximal tubular epithelial cells under inflammatory conditions. J. Extracell. Vesicles 2021, 10, e12064. [Google Scholar] [CrossRef]
- Prunotto, M.; Farina, A.; Lane, L.; Pernin, A.; Schifferli, J.; Hochstrasser, D.F.; Lescuyer, P.; Moll, S. Proteomic analysis of podocyte exosome-enriched fraction from normal human urine. J. Proteom. 2013, 82, 193–229. [Google Scholar] [CrossRef]
- Hayakawa, T.; Fukuhara, A.; Saiki, A.; Otsuki, M.; Shimomura, I. gammaENaC/CD9 in urinary extracellular vesicles as a potential biomarker of MR activity. J. Endocrinol. 2021, 252, 81–90. [Google Scholar] [CrossRef]
- Hu, C.C.; Katerelos, M.; Choy, S.W.; Crossthwaite, A.; Walker, S.P.; Pell, G.; Lee, M.; Cook, N.; Mount, P.F.; Paizis, K.; et al. Pre-eclampsia is associated with altered expression of the renal sodium transporters NKCC2, NCC and ENaC in urinary extracellular vesicles. PLoS ONE 2018, 13, e0204514. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.W.B.; Chen, Y.Y.; Huang, J.X.; Wang, K.Y.; Xu, H.S.; Lin, D. Significance of Mannan Binding Lectin-Associated Serine Protease 2 in Urinary Extracellular Vesicles in IgA Nephropathy. Clin. Investig. Med. 2022, 45, E47–E54. [Google Scholar] [CrossRef] [PubMed]
| mIR | Finding | Reference |
|---|---|---|
| miR-192, miR-194, miR-215 | increased in type 2 diabetic patients with microalbuminuria compared to patients with normoalbuminuria | [49] |
| miR-451-5p, miR-16 | protective effect in diabetes-induced renal fibrosis | [50] |
| miR-30a-5p | greater in uEVs isolated from macroalbuminuria patients but not patients with T2DM and normoalbuminuria or T2DM and microalbuminuria | [51] |
| miR-320c, miR-6068 | upregulated in uEVs from diabetic nephropathy patients | [52] |
| miR-145 | increased in mesangial cells and exosomes derived from mesangial cells after high-glucose treatment | [53] |
| Cell Type | Podocytes | Mesangial Cells | Glomerular Endothelial Cells | Proximal Tubule Cells | Principal Cells |
|---|---|---|---|---|---|
| Podocalyxin, Nephrin (Prunotto et al. [89]) Proteasome subunit beta type-2, Cullin-2, Complement C3, Cystatin-A, Keratin, type I cytoskeletal 14, Putative phospholipase B-like 2, Endoplasmic reticulum resident protein 44 (Barreiro et al. [87]) | Creatine kinase, Cysteine-rich motor neuron 1 protein (Barreiro et al. [87]) | EGF containing fibulin-like extracellular matrix protein 1 isoform 1, Prosaposin, Pentraxin-related protein, Latenttransforming growth factor beta-binding protein 2, Thioredoxin reductase 1, cytoplasmic, Chondroitin sulphate proteoglycan 4 (Barreiro et al. [87]) | CD63, CD9, CD81, TSG101, ALIX, VPS4B, HSP70, HSP84, 60S ribosomal protein L27, V-set domain-containing T-cell activation inhibitor 1, Protein lin-7 homolog C, HLA class I histocompatibility antigen, A-24 alpha chain, TNFAIP3-interacting protein 1, Laminin subunit alpha-3, Beta (β)-2-macroglobulin, Interferon-induced transmembrane protein 1 TNF alpha-induced protein 3, Myosin-10, Serum amyloid A-1 protein, Intercellular adhesion molecule 1, C-X-C motif chemokine 10, Alcohol dehydrogenase, Hyaluronan and proteoglycan link protein 3, Guanylate-binding protein 5, Indoleamine 2,3-dioxygenase 1, Tryptophan–tRNA ligase, cytoplasmic 1, Interferon-induced guanylate-binding protein 1, Tumour necrosis factor receptor superfamily member 5, ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1, Tumour necrosis factor alpha-induced protein (Wang et al. [88]) Annexin A5, Annexin A11, Adenylyl cyclase 7, GTPase-activating protein subunit alpha-2, Cofilin-2, Heat shock cognate 71 kDa protein, Phosphoglycerate kinase 1, 14-3-3 protein theta, 14-3-3 protein gamma, 14-3-3 protein zeta/delta, retinoic acid-induced protein 3 (Wen et al. [59]) Betahexosaminidase subunit beta, Elongation factor 1-gamma, Serine/threonineprotein phosphatase PP1-alpha catalytic subunit, Serine/threonineprotein phosphatase PP1-beta catalytic subunit, Brain acid soluble protein 1 (Barreiro et al. [87]) | α-actinin-1, moesin, 14-3-3 protein ζ/δ, annexin A1/A3/A4/A5/A6, clathrin heavy chain 1, GAPDH, α-enolase, filamin-A, HSP 90 (Dang et al. [85]) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alli, A.A. Extracellular Vesicles: Investigating the Pathophysiology of Diabetes-Associated Hypertension and Diabetic Nephropathy. Biology 2023, 12, 1138. https://doi.org/10.3390/biology12081138
Alli AA. Extracellular Vesicles: Investigating the Pathophysiology of Diabetes-Associated Hypertension and Diabetic Nephropathy. Biology. 2023; 12(8):1138. https://doi.org/10.3390/biology12081138
Chicago/Turabian StyleAlli, Abdel A. 2023. "Extracellular Vesicles: Investigating the Pathophysiology of Diabetes-Associated Hypertension and Diabetic Nephropathy" Biology 12, no. 8: 1138. https://doi.org/10.3390/biology12081138
APA StyleAlli, A. A. (2023). Extracellular Vesicles: Investigating the Pathophysiology of Diabetes-Associated Hypertension and Diabetic Nephropathy. Biology, 12(8), 1138. https://doi.org/10.3390/biology12081138






