Mitochondrial Proteome Changes in Rett Syndrome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Brain Tissue Isolation
2.2. Isolation of Mitochondria
2.3. Protein Determination
2.4. Citrate-Synthase Activity Assay
2.5. 1-D Electrophoresis and Western Blotting
2.6. 2-D Electrophoresis
2.7. Staining, Scanning, and Image Processing
2.8. In-Gel Digestion and Subsequent MS Analysis
2.9. Statistical Analysis
3. Results
3.1. Citrate Synthase Activities Differ among Brain Regions
3.2. The OXPHOS System Appears Largely Unaffected in a First Targeted Screening
3.3. Regulators of Mitochondrial Fusion/Fission Dynamics Are Partly Decreased in RTT Mice
3.4. The Mitochondrial Proteome Clearly Differs among Mecp2-/y and WT Mice
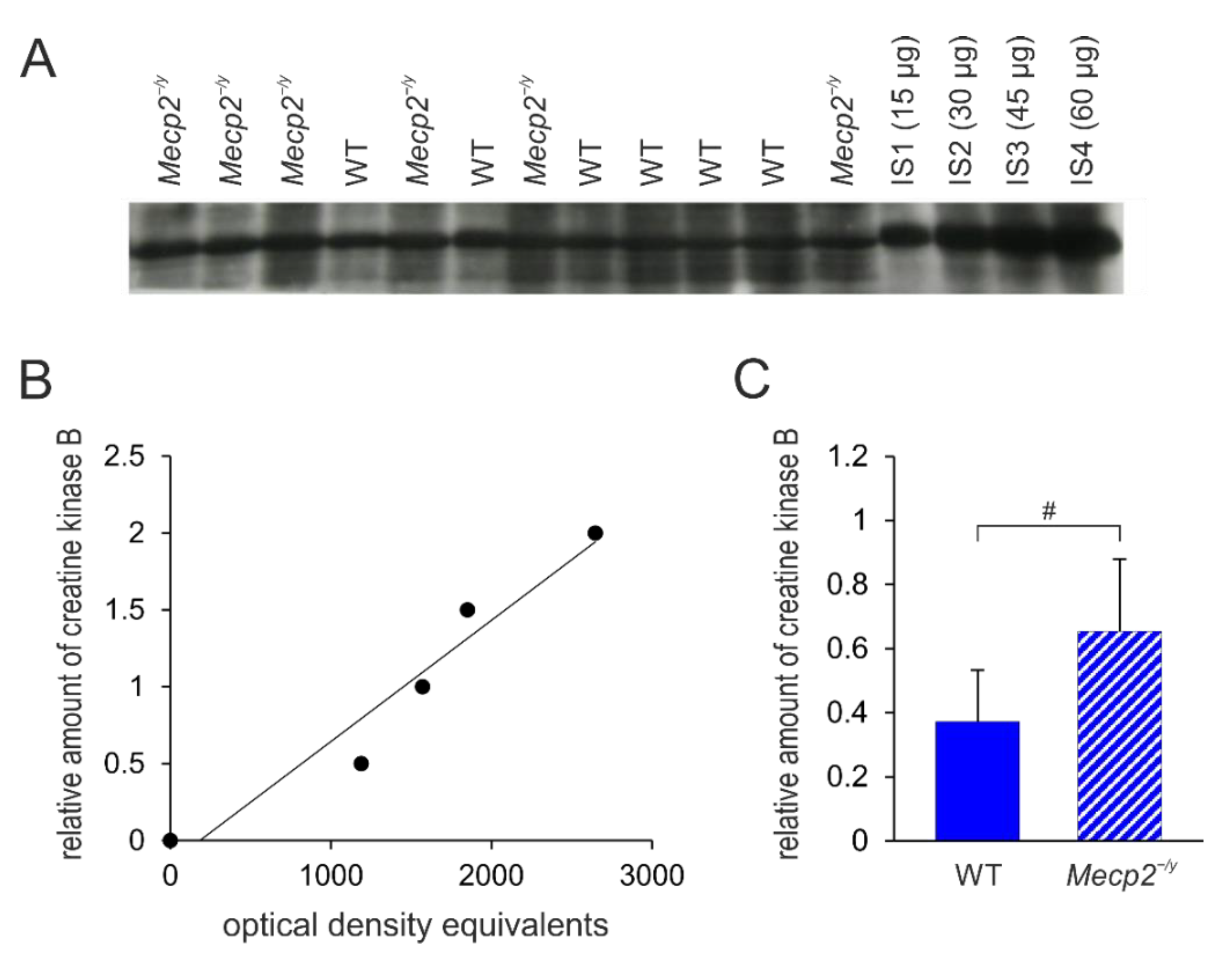
3.5. Confirmatory Western Blot Analyses of Selected Differentially Expressed Proteins
4. Discussion
4.1. Brain-Regional Differences in Mitochondrial Mass
4.2. Mitochondrial Fusion/Fission Dynamics Are Impaired in RTT
4.3. Complex I Components of the OXPHOS System
4.4. Complex III Components of the OXPHOS System
4.5. ATP Synthase Complex of the OXPHOS System
4.6. Modulatory and Metabolic Key Players
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hagberg, B.; Aicardi, J.; Dias, K.; Ramos, O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Ann. Neurol. 1983, 14, 471–479. [Google Scholar] [CrossRef]
- Rett, A. Über ein eigenartiges hirnatrophisches Syndrom bei Hyperammonämie im Kindesalter. Wien. Med. Wochenschr. 1966, 116, 723–726. [Google Scholar] [PubMed]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.; Qin, J.; Zoghbi, H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Young, J.I.; Hong, E.P.; Castle, J.C.; Crespo-Barreto, J.; Bowman, A.B.; Rose, M.F.; Kang, D.; Richman, R.; Johnson, J.M.; Berget, S.; et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc. Natl. Acad. Sci. USA 2005, 102, 17551–17558. [Google Scholar] [CrossRef]
- Lee, W.; Kim, J.; Yun, J.M.; Ohn, T.; Gong, Q. MeCP2 regulates gene expression through recognition of H3K27me3. Nat. Commun. 2020, 11, 3140. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Dunn, J.K.; Antalffy, B.; Trivedi, R. Selective dendritic alterations in the cortex of Rett syndrome. J. Neuropathol. Exp. Neurol. 1995, 54, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Skinner, S.A.; Annese, F.; Lane, J.; Heydemann, P.; Jones, M.; Kaufmann, W.E.; Glaze, D.G.; Percy, A.K. Metabolic signatures differentiate Rett syndrome from unaffected siblings. Front. Integr. Neurosci. 2020, 14, 7. [Google Scholar] [CrossRef]
- Kyle, S.M.; Vashi, N.; Justice, M.J. Rett syndrome: A neurological disorder with metabolic components. Open. Biol. 2018, 8, 170216. [Google Scholar] [CrossRef]
- Eeg-Olofsson, O.; al-Zuhair, A.G.; Teebi, A.S.; Daoud, A.S.; Zaki, M.; Besisso, M.S.; Al-Essa, M.M. Rett syndrome: A mitochondrial disease? J. Child. Neurol. 1990, 5, 210–214. [Google Scholar] [CrossRef]
- Villard, L. MECP2 mutations in males. J. Med. Genet. 2007, 44, 417–423. [Google Scholar] [CrossRef]
- Cornford, M.E.; Philippart, M.; Jacobs, B.; Scheibel, A.B.; Vinters, H.V. Neuropathology of Rett syndrome: Case report with neuronal and mitochondrial abnormalities in the brain. J. Child Neurol. 1994, 9, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Dotti, M.T.; Manneschi, L.; Malandrini, A.; De Stefano, N.; Caznerale, F.; Federico, A. Mitochondrial dysfunction in Rett syndrome. An ultrastructural and biochemical study. Brain Dev. 1993, 15, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Großer, E.; Hirt, U.; Janc, O.A.; Menzfeld, C.; Fischer, M.; Kempkes, B.; Vogelgesang, S.; Manzke, T.U.; Opitz, L.; Salinas-Riester, G.; et al. Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome. Neurobiol. Dis. 2012, 48, 102–114. [Google Scholar] [CrossRef]
- Kriaucionis, S.; Paterson, A.; Curtis, J.; Guy, J.; Macleod, N.; Bird, A. Gene expression analysis exposes mitochondrial abnormalities in a mouse model of Rett syndrome. Mol. Cell. Biol. 2006, 26, 5033–5042. [Google Scholar] [CrossRef]
- Gold, W.A.; Williamson, S.L.; Kaur, S.; Hargreaves, I.P.; Land, J.M.; Pelka, G.J.; Tam, P.P.; Christodoulou, J. Mitochondrial dysfunction in the skeletal muscle of a mouse model of Rett syndrome (RTT): Implications for the disease phenotype. Mitochondrion 2014, 15, 10–17. [Google Scholar] [CrossRef]
- Saywell, V.; Viola, A.; Confort-Gouny, S.; Le Fur, Y.; Villard, L.; Cozzone, P.J. Brain magnetic resonance study of Mecp2 deletion effects on anatomy and metabolism. Biochem. Biophys. Res. Commun. 2006, 340, 776–783. [Google Scholar] [CrossRef]
- Golubiani, G.; Lagani, V.; Solomonia, R.; Müller, M. Metabolomic fingerprint of Mecp2-deficient mouse cortex: Evidence for a pronounced multi-facetted metabolic component in Rett syndrome. Cells 2021, 10, 2494. [Google Scholar] [CrossRef]
- Liu, S.; Pei, P.; Li, L.; Wu, H.; Zheng, X.; Wang, S.; Xiao, Y.; Pan, H.; Bao, X.; Qi, Y.; et al. Mitochondrial DNA copy number in Rett syndrome caused by methyl-CpG-binding protein-2 variants. J. Pediatr. 2022, 241, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.H.; Slobedman, B.; Karikrishnan, N.H.; Williamson, S.L.; Minchenko, D.; El-Osta, A.; Stern, J.L.; Christodoulou, J. Downstream targets of methyl CpG binding protein 2 and their abnormal expression in the frontal cortex of the human Rett syndrome brain. BMC Neurosci. 2010, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Can, K.; Menzfeld, C.; Rinne, L.; Rehling, P.; Kügler, S.; Golubiani, G.; Dudek, J.; Müller, M. Neuronal redox-imbalance in Rett syndrome affects mitochondria as well as cytosol, and is accompanied by intensified mitochondrial O2 consumption and ROS release. Front. Physiol. 2019, 10, 479. [Google Scholar] [CrossRef]
- Müller, M. Disturbed redox homeostasis and oxidative stress: Potential players in the developmental regression in Rett syndrome. Neurosci. Biobehav. Rev. 2019, 98, 154–163. [Google Scholar] [CrossRef]
- Filosa, S.; Pecorelli, A.; D’Esposito, M.; Valacchi, G.; Hayek, J. Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome. Free Radic. Biol. Med. 2015, 88, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Shulyakova, N.; Andreazza, A.C.; Mills, L.R.; Eubanks, J.H. Mitochondrial dysfunction in the pathogenesis of Rett syndrome: Implications for mitochondria-targeted therapies. Front. Cell. Neurosci. 2017, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, B.; Valenti, D.; de Bari, L.; De Rasmo, D.; Musto, M.; Fabbri, A.; Ricceri, L.; Fiorentini, C.; Laviola, G.; Vacca, R.A. Mitochondrial free radical overproduction due to respiratory chain impairment in the brain of a mouse model of Rett syndrome: Protective effect of CNF1. Free Radic. Biol. Med. 2015, 83, 167–177. [Google Scholar] [CrossRef]
- Cervellati, C.; Sticozzi, C.; Romani, A.; Belmonte, G.; De Rasmo, D.; Signorile, A.; Cervellati, F.; Milanese, C.; Mastroberardino, P.G.; Pecorelli, A.; et al. Impaired enzymatic defensive activity, mitochondrial dysfunction and proteasome activation are involved in RTT cell oxidative damage. Biochim. Biophys. Acta 2015, 1852, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Formichi, P.; Battisti, C.; Dotti, M.T.; Hayek, G.; Zappella, M.; Federico, A. Vitamin E serum levels in Rett syndrome. J. Neurol. Sci. 1998, 156, 227–230. [Google Scholar] [CrossRef]
- Sierra, C.; Vilaseca, M.A.; Brandi, N.; Artuch, R.; Mira, A.; Nieto, M.; Pineda, M. Oxidative stress in Rett syndrome. Brain Dev. 2001, 23 (Suppl. 1), S236–S239. [Google Scholar] [CrossRef]
- Festerling, K.; Can, K.; Kügler, S.; Müller, M. Overshooting subcellular redox-responses in Rett-mouse hippocampus during neurotransmitter stimulation. Cells 2020, 9, 2539. [Google Scholar] [CrossRef]
- De Felice, C.; Ciccoli, L.; Leoncini, S.; Signorini, C.; Rossi, M.; Vannuccini, L.; Guazzi, G.; Latini, G.; Comporti, M.; Valacchi, G.; et al. Systemic oxidative stress in classic Rett syndrome. Free Radic. Biol. Med. 2009, 47, 440–448. [Google Scholar] [CrossRef]
- Jagtap, S.; Thanos, J.M.; Fu, T.; Wang, J.; Lalonde, J.; Dial, T.O.; Feiglin, A.; Chen, J.; Kohane, I.; Lee, J.T.; et al. Aberrant mitochondrial function in patient-derived neural cells from CDKL5 deficiency disorder and Rett syndrome. Hum. Mol. Genet. 2019, 28, 3625–3636. [Google Scholar] [CrossRef] [PubMed]
- Belichenko, P.V.; Wright, E.E.; Belichenko, N.P.; Masliah, E.; Li, H.H.; Mobley, W.C.; Francke, U. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: Evidence for disruption of neuronal networks. J. Comp. Neurol. 2009, 514, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.; Hendrich, B.; Holmes, M.; Martin, J.E.; Bird, A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 2001, 27, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Meparishvili, M.; Nozadze, M.; Margvelani, G.; McCabe, B.J.; Solomonia, R.O. A proteomic study of memory after imprinting in the domestic chick. Front. Behav. Neurosci. 2015, 9, 319. [Google Scholar] [CrossRef]
- Li, R.; Shen, Y. An old method facing a new challenge: Re-visiting housekeeping proteins as internal reference control for neuroscience research. Life Sci. 2013, 92, 747–751. [Google Scholar] [CrossRef]
- Satoh, J.; Onoue, H.; Arima, K.; Yamamura, T. The 14-3-3 protein forms a molecular complex with heat shock protein Hsp60 and cellular prion protein. J. Neuropathol. Exp. Neurol. 2005, 64, 858–868. [Google Scholar] [CrossRef]
- Bross, P.; Magnoni, R.; Bie, A.S. Molecular chaperone disorders: Defective Hsp60 in neurodegeneration. Curr. Top. Med. Chem. 2012, 12, 2491–2503. [Google Scholar] [CrossRef]
- Weese-Mayer, D.E.; Lieske, S.P.; Boothby, C.M.; Kenny, A.S.; Bennett, H.L.; Silvestri, J.M.; Ramirez, J.M. Autonomic nervous system dysregulation: Breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr. Res. 2006, 60, 443–449. [Google Scholar] [CrossRef]
- Bebensee, D.F.; Can, K.; Müller, M. Increased mitochondrial mass and cytosolic redox imbalance in hippocampal astrocytes of a mouse model of Rett syndrome: Subcellular changes revealed by ratiometric imaging of JC-1 and roGFP1 fluorescence. Oxid. Med. Cell. Longev. 2017, 2017, 3064016. [Google Scholar] [CrossRef]
- Simmons, E.C.; Scholpa, N.E.; Schnellmann, R.G. Mitochondrial biogenesis as a therapeutic target for traumatic and neurodegenerative CNS diseases. Exp. Neurol. 2020, 329, 113309. [Google Scholar] [CrossRef]
- Rahman, M.H.; Suk, K. Mitochondrial dynamics and bioenergetic alteration during inflammatory activation of astrocytes. Front. Aging Neurosci. 2020, 12, 614410. [Google Scholar] [CrossRef] [PubMed]
- Margvelani, G.; Meparishvili, M.; Tevdoradze, E.; McCabe, B.J.; Solomonia, R. Mitochondrial fusion and fission proteins and the recognition memory of imprinting in domestic chicks. Neuroreport 2018, 29, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Moretti, P.; Levenson, J.M.; Battaglia, F.; Atkinson, R.; Teague, R.; Antalffy, B.; Armstrong, D.; Arancio, O.; Sweatt, J.D.; Zoghbi, H.Y. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 2006, 26, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Crivellari, I.; Pecorelli, A.; Cordone, V.; Marchi, S.; Pinton, P.; Hayek, J.; Cervellati, C.; Valacchi, G. Impaired mitochondrial quality control in Rett Syndrome. Arch. Biochem. Biophys. 2021, 700, 108790. [Google Scholar] [CrossRef]
- Janssen, R.J.; Nijtmans, L.G.; van den Heuvel, L.P.; Smeitink, J.A. Mitochondrial complex I: Structure, function and pathology. J. Inherit. Metab. Dis. 2006, 29, 499–515. [Google Scholar] [CrossRef]
- Tatarkova, Z.; Kovalska, M.; Timkova, V.; Racay, P.; Lehotsky, J.; Kaplan, P. The effect of aging on mitochondrial complex I and the extent of oxidative stress in the rat brain cortex. Neurochem. Res. 2016, 41, 2160–2172. [Google Scholar] [CrossRef]
- Kim, S.H.; Vlkolinsky, R.; Cairns, N.; Fountoulakis, M.; Lubec, G. The reduction of NADH ubiquinone oxidoreductase 24- and 75-kDa subunits in brains of patients with Down syndrome and Alzheimer’s disease. Life Sci. 2001, 68, 2741–2750. [Google Scholar] [CrossRef]
- Karry, R.; Klein, E.; Ben Shachar, D. Mitochondrial complex I subunits expression is altered in schizophrenia: A postmortem study. Biol. Psychiatry 2004, 55, 676–684. [Google Scholar] [CrossRef]
- Coker, S.B.; Melnyk, A.R. Rett syndrome and mitochondrial enzyme deficiencies. J. Child Neurol. 1991, 6, 164–166. [Google Scholar] [CrossRef]
- Matsuura, K.; Otani, M.; Takano, M.; Kadoyama, K.; Matsuyama, S. The influence of chronic nicotine treatment on proteins expressed in the mouse hippocampus and cortex. Eur. J. Pharmacol. 2016, 780, 16–25. [Google Scholar] [CrossRef]
- Kohutiar, M.; Eckhardt, A.; Miksik, I.; Santorova, P.; Wilhelm, J. Proteomic analysis of peroxynitrite-induced protein nitration in isolated beef heart mitochondria. Physiol. Res. 2018, 67, 239–250. [Google Scholar] [CrossRef]
- Lee, K.; Haddad, A.; Osme, A.; Kim, C.; Borzou, A.; Ilchenko, S.; Allende, D.; Dasarathy, S.; McCullough, A.; Sadygov, R.G.; et al. Hepatic mitochondrial defects in a nonalcoholic fatty liver disease mouse model are associated with increased degradation of oxidative phosphorylation subunits. Mol. Cell. Proteom. 2018, 17, 2371–2386. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.B.; Cadete, V.J.; Sawicka, J.; Wozniak, M.; Sawicki, G. Effect of the myosin light chain kinase inhibitor ML-7 on the proteome of hearts subjected to ischemia-reperfusion injury. J. Proteom. 2012, 75, 5386–5395. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.S.; Rahman, M.S.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Increased male fertility using fertility-related biomarkers. Sci. Rep. 2015, 5, 15654. [Google Scholar] [CrossRef] [PubMed]
- Cruciat, C.M.; Brunner, S.; Baumann, F.; Neupert, W.; Stuart, R.A. The cytochrome bc1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J. Biol. Chem. 2000, 275, 18093–18098. [Google Scholar] [CrossRef]
- Fernández-Vizarra, E.; Ugalde, C. Cooperative assembly of the mitochondrial respiratory chain. Trends Biochem. Sci. 2022, 47, 999–1008. [Google Scholar] [CrossRef]
- Fernández-Vizarra, E.; López-Calcerrada, S.; Sierra-Magro, A.; Pérez-Pérez, R.; Formosa, L.E.; Hock, D.H.; Illescas, M.; Penas, A.; Brischigliaro, M.; Ding, S.; et al. Two independent respiratory chains adapt OXPHOS performance to glycolytic switch. Cell Metab. 2022, 34, 1792–1808. [Google Scholar] [CrossRef]
- Toloe, J.; Mollajew, R.; Kügler, S.; Mironov, S.L. Metabolic differences in hippocampal ‘Rett’ neurons revealed by ATP imaging. Mol. Cell. Neurosci. 2014, 59C, 47–56. [Google Scholar] [CrossRef]
- Fischer, M.; Reuter, J.; Gerich, F.J.; Hildebrandt, B.; Hägele, S.; Katschinski, D.; Müller, M. Enhanced hypoxia susceptibility in hippocampal slices from a mouse model of Rett syndrome. J. Neurophysiol. 2009, 101, 1016–1032. [Google Scholar] [CrossRef]
- Mironov, S.L.; Skorova, E.Y.; Kügler, S. Epac-mediated cAMP-signalling in the mouse model of Rett Syndrome. Neuropharmacology 2011, 60, 869–877. [Google Scholar] [CrossRef]
- Vogelgesang, S.; Niebert, M.; Bischoff, A.M.; Hülsmann, S.; Manzke, T. Persistent expression of serotonin receptor 5b alters breathing behavior in male MeCP2 knockout mice. Front. Mol. Neurosci. 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Niebert, M.; Richter, D.W. Rescue of cyclic AMP mediated long term potentiation impairment in the hippocampus of Mecp2 knockout (Mecp2−/y) mice by rolipram. Front. Cell. Neurosci. 2016, 10, 15. [Google Scholar] [CrossRef]
- Signorile, A.; Sgaramella, G.; Bellomo, F.; De Rasmo, D. Prohibitins: A critical role in mitochondrial functions and implication in diseases. Cells 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, J.D.; Wang, H.D.; Shi, Y.M.; Yuan, Y.L.; Hou, S.X. Prohibitin 1 gene delivery promotes functional recovery in rats with spinal cord injury. Neuroscience 2015, 286, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Supale, S.; Thorel, F.; Merkwirth, C.; Gjinovci, A.; Herrera, P.L.; Scorrano, L.; Meda, P.; Langer, T.; Maechler, P. Loss of prohibitin induces mitochondrial damages altering β-cell function and survival and is responsible for gradual diabetes development. Diabetes 2013, 62, 3488–3499. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Yamada, J.; Ueno, S.; Fukuda, A. Brain-type creatine kinase activates neuron-specific K+-Cl- co-transporter KCC2. J. Neurochem. 2006, 96, 598–608. [Google Scholar] [CrossRef]
- Miyake, K.; Yang, C.; Minakuchi, Y.; Ohori, K.; Soutome, M.; Hirasawa, T.; Kazuki, Y.; Adachi, N.; Suzuki, S.; Itoh, M.; et al. Comparison of genomic and epigenomic expression in monozygotic twins discordant for Rett syndrome. PLoS ONE 2013, 8, e66729. [Google Scholar]
- Obsilova, V.; Obsil, T. Structural insights into the functional roles of 14-3-3 proteins. Front. Mol. Biosci. 2022, 9, 1016071. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Hu, T.; Yang, B.; Li, H.; Chen, T.; Yin, D.; He, H.; He, M. Capsaicin alleviates the deteriorative mitochondrial function by upregulating 14-3-3η in anoxic or anoxic/reoxygenated cardiomyocytes. Oxid. Med. Cell. Longev. 2020, 2020, 1750289. [Google Scholar] [CrossRef]
- Isgrò, M.A.; Bottoni, P.; Scatena, R. Neuron-specific enolase as a biomarker: Biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015, 867, 125–143. [Google Scholar]
- Butterfield, D.A.; Lange, M.L. Multifunctional roles of enolase in Alzheimer’s disease brain: Beyond altered glucose metabolism. J. Neurochem. 2009, 111, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Giegé, P.; Heazlewood, J.L.; Roessner-Tunali, U.; Millar, A.H.; Fernie, A.R.; Leaver, C.J.; Sweetlove, L.J. Enzymes of glycolysis are functionally associated with the mitochondrion in Arabidopsis cells. Plant Cell 2003, 15, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Baleva, M.; Gowher, A.; Kamenski, P.; Tarassov, I.; Entelis, N.; Masquida, B. A moonlighting human protein is involved in mitochondrial import of tRNA. Int. J. Mol. Sci. 2015, 16, 9354–9367. [Google Scholar] [CrossRef]
- Pecorelli, A.; Leoni, G.; Cervellati, F.; Canali, R.; Signorini, C.; Leoncini, S.; Cortelazzo, A.; De Felice, C.; Ciccoli, L.; Hayek, J.; et al. Genes related to mitochondrial functions, protein degradation, and chromatin folding are differentially expressed in lymphomonocytes of Rett syndrome patients. Mediat. Inflamm. 2013, 2013, 137629. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, S.; Tropea, D. Transcriptome level analysis in Rett syndrome using human samples from different tissues. Orphanet J. Rare Dis. 2018, 13, 113. [Google Scholar] [CrossRef]
- Cicaloni, V.; Pecorelli, A.; Tinti, L.; Rossi, M.; Benedusi, M.; Cervellati, C.; Spiga, O.; Santucci, A.; Hayek, J.; Salvini, L.; et al. Proteomic profiling reveals mitochondrial alterations in Rett syndrome. Free Radic. Biol. Med. 2020, 155, 37–48. [Google Scholar] [CrossRef]
- Kappler, L.; Hoene, M.; Hu, C.; von Toerne, C.; Li, J.; Bleher, D.; Hoffmann, C.; Böhm, A.; Kollipara, L.; Zischka, H.; et al. Linking bioenergetic function of mitochondria to tissue-specific molecular fingerprints. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E374–E387. [Google Scholar] [CrossRef]








| Protein | Hippocampus | Neocortex | ||||
|---|---|---|---|---|---|---|
| Relative Units (Mean ± SD) | p-Value | Relative Units (Mean ± SD) | p-Value | |||
| WT | Mecp2−/y | WT | Mecp2−/y | |||
| NADH: ubiquinone oxidoreductase subunit B8 (NDUFB8), complex I | 1.35 ± 0.11 | 1.17 ± 0.23 | 0.12 | 0.78 ± 0.15 | 0.85 ± 0.21 | 0.51 |
| Succinate dehydrogenase complex iron sulfur subunit B (SDHB), complex II | 0.44 ± 0.07 | 0.50 ± 0.17 | 0.42 | 0.87 ± 0.11 | 0.92 ± 0.16 | 0.7 |
| Ubiquinol-cytochrome C reductase core protein 2 (UQCRC2, alternate name cytochrome B-C1 complex subunit 2), complex III | 0.44 ± 0.16 | 0.53 ± 0.16 | 0.32 | 1.05 ± 0.11 | 1.06 ± 0.13 | 0.9 |
| Cytochrome c oxidase subunit 1 (MTCO1), complex IV | 0.27 ± 0.04 | 0.29 ± 0.05 | 0.43 | 0.87 ± 0.13 | 0.85 ± 0.16 | 0.84 |
| ATP synthase F1 subunit alpha (ATP5A), FoF1 ATP synthase complex | 0.61 ± 0.12 | 0.68 ± 0.20 | 0.73 | 1.11 ± 0.08 | 1.09 ± 0.13 | 0.74 |
| N | Protein | Direction of Changes as Compared to WT | Gene | Uniprot ID | % Vol Wt Sample, Mecp2-/Y Sample, p-Value | Molecular/Biological Functions of Proteins |
|---|---|---|---|---|---|---|
| HIPPOCAMPUS | ||||||
| 1 | Pyruvate dehydrogenase E1 component subunit beta, mitochondrial | upregulated | Pdhb | Q9D051 ODPB_MOUSE | 0.24 ± 0.23 (n = 6) 0.68 ± 0.17 (n = 6) p = 0.004 | pyruvate dehydrogenase (acetyl-transferring) activity/glucose metabolic process |
| 2 | NADH-ubiquinone oxidoreductase 75 kDa subunit, mitochondrial | upregulated | Ndufs1 | Q91VD9 NDUS1_MOUSE | 0.20 ± 0.14 (n = 6) 0.53 ± 0.14 (n = 6) p = 0.002 | 2 iron, 2 sulfur cluster binding/electron transfer activity |
| 3 | NADH dehydrogenase [ubiquinone] iron- sulfur protein 8, mitochondrial | upregulated | Ndufs8 | Q8K3J1 NDUS8_MOUSE | 0.46 ± 0.41 (n = 6) 1.24 ± 0.66 (n = 6) p = 0.034 | NADH dehydrogenase (ubiquinone) activity/mitochondrial electron transfer, NADH to ubiquinone |
| 4 | NADH dehydrogenase [ubiquinone] flavoprotein 2, mitochondrial | upregulated | Ndufv2 | Q9D6J6 NDUV2_MOUSE | 0.066 ± 0.075 (n = 4) 0.36 ± 0.12 (n = 4) p = 0.006 | NADH dehydrogenase (ubiquinone) activity/mitochondrial electron transfer, NADH to ubiquinone |
| 5 | Cytochrome b-c1 complex subunit 1, mitochondrial | upregulated | Uqcrc1 | Q9CZ13 QCR1_MOUSE | 0.22 ± 0.20 (n = 6) 0.61 ± 0.23 (n = 6) p = 0.01 | metal ion binding/mitochondrial electron transfer, ubiquinol to cytochrome c |
| 6 | ATP synthase subunit d | upregulated | Atp5pd | Q9DCX2 ATP5H_MOUSE | 0.16 ± 0.12 (n = 6) 0.61 ± 0.25 (n = 6) p = 0.002 | proton-transporting ATP synthase activity, rotational mechanism/proton motive force-driven mitochondrial ATP synthesis |
| 7 | Creatine kinase B-type | upregulated | Ckb | Q04447 KCRB_MOUSE | 0.23 ± 0.17 (n = 6) 0.92 ± 0.70 (n = 6) p = 0.038 | Kinase activity/phosphocreatine biosynthetic process |
| 8 | Prohibitin 1 | upregulated | Phb | P67778 PHB1_MOUSE | 0.036 ± 0.031 (n = 4) 0.23 ± 0.10 (n = 4) p = 0.011 | protein heterodimerization activity/mitochondrial organization |
| 9 | Gamma-enolase | downregulated | Eno2 | P17183 ENOG_MOUSE | 0.92 ± 0.27 (n = 6) 0.36 ± 0.26 (n = 6) p = 0.004 | Lyase/glycolysis |
| 10 | cAMP-dependent protein kinase catalytic subunit alpha | downregulated | Prkaca | P05132 KAPCA_MOUSE | 0.17 ± 0.08 (n = 3) 0.037 ± 0.008 (n = 3) p = 0.046 | Serine/threonine protein kinase activity |
| NEOCORTEX | ||||||
| 11 | Cytochrome b-c1 complex subunit 1, mitochondrial | upregulated | Uqcrc1 | Q9CZ13 QCR1_MOUSE | 0.07 ± 0.04 (n = 5) 0.20± 0.10 (n = 5) p = 0.039 | metal ion binding/mitochondrial electron transfer, ubiquinol to cytochrome c |
| 12 | Guanine nucleotide-binding protein G(o) subunit alpha | upregulated | Gnao1 | P18872 GNAO_MOUSE | 0.20 ± 0.22 (n = 6) 0.70 ±0.47 (n = 6) p = 0.043 | G protein-coupled receptor binding/G protein coupled receptor signaling pathway |
| 13 | Prohibitin 1 | upregulated | Phb | P67778 PHB1_MOUSE | 0.13 ± 0.09 (n = 6) 0.25 ± 0.05 (n = 6) p = 0.024 | protein heterodimerization activity/mitochondria lorganization |
| 14 | Gamma-enolase | downregulated | Eno2 | P17183 ENOG_MOUSE | 0.48 ± 0.13 (n = 3) 0.11 ± 0.03 (n = 3) p = 0.009 | Lyase/glycolysis |
| 15 | cAMP-dependent protein kinase catalytic subunit alpha | downregulated | Prkaca | P05132 KAPCA_MOUSE | 0.28 ± 0.03 (n = 3) 0.11 ± 0.09 (n = 3) p = 0.031 | Serine/threonine protein kinase activity |
| 16 | 14-3-3 protein theta | downregulated | Ywhag | P61982 1433G_MOUSE | 0.58 ± 0.15 (n = 4) 0.26 ± 0.14 (n = 4) p = 0.022 | protein domain specific binding/protein targeting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubiani, G.; van Agen, L.; Tsverava, L.; Solomonia, R.; Müller, M. Mitochondrial Proteome Changes in Rett Syndrome. Biology 2023, 12, 956. https://doi.org/10.3390/biology12070956
Golubiani G, van Agen L, Tsverava L, Solomonia R, Müller M. Mitochondrial Proteome Changes in Rett Syndrome. Biology. 2023; 12(7):956. https://doi.org/10.3390/biology12070956
Chicago/Turabian StyleGolubiani, Gocha, Laura van Agen, Lia Tsverava, Revaz Solomonia, and Michael Müller. 2023. "Mitochondrial Proteome Changes in Rett Syndrome" Biology 12, no. 7: 956. https://doi.org/10.3390/biology12070956
APA StyleGolubiani, G., van Agen, L., Tsverava, L., Solomonia, R., & Müller, M. (2023). Mitochondrial Proteome Changes in Rett Syndrome. Biology, 12(7), 956. https://doi.org/10.3390/biology12070956






