35 Years of TFAM Research: Old Protein, New Puzzles
Abstract
Simple Summary
Abstract
1. Introduction
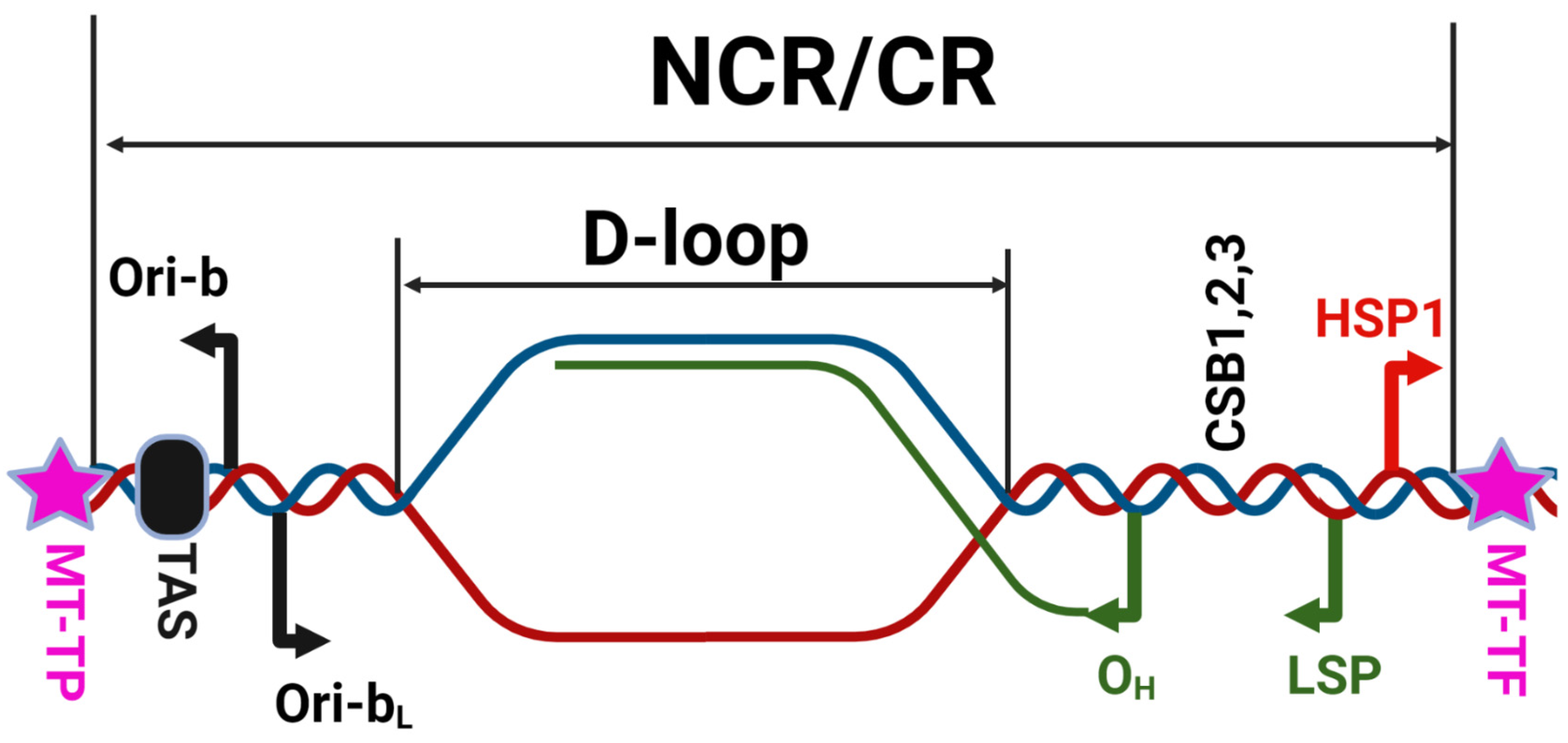
2. Mitochondrial DNA
- (1)
- Circular molecule 11–28 kbp.
- (2)
- Circular molecule 22–1000 kbp.
- (3)
- Circular molecule larger than 22 kbp accompanied by plasmid-like molecules.
- (4)
- Heterogeneous population of circular molecules.
- (5)
- Homogenous population of linear molecules.
- (6)
- Population of heterogeneous linear molecules.
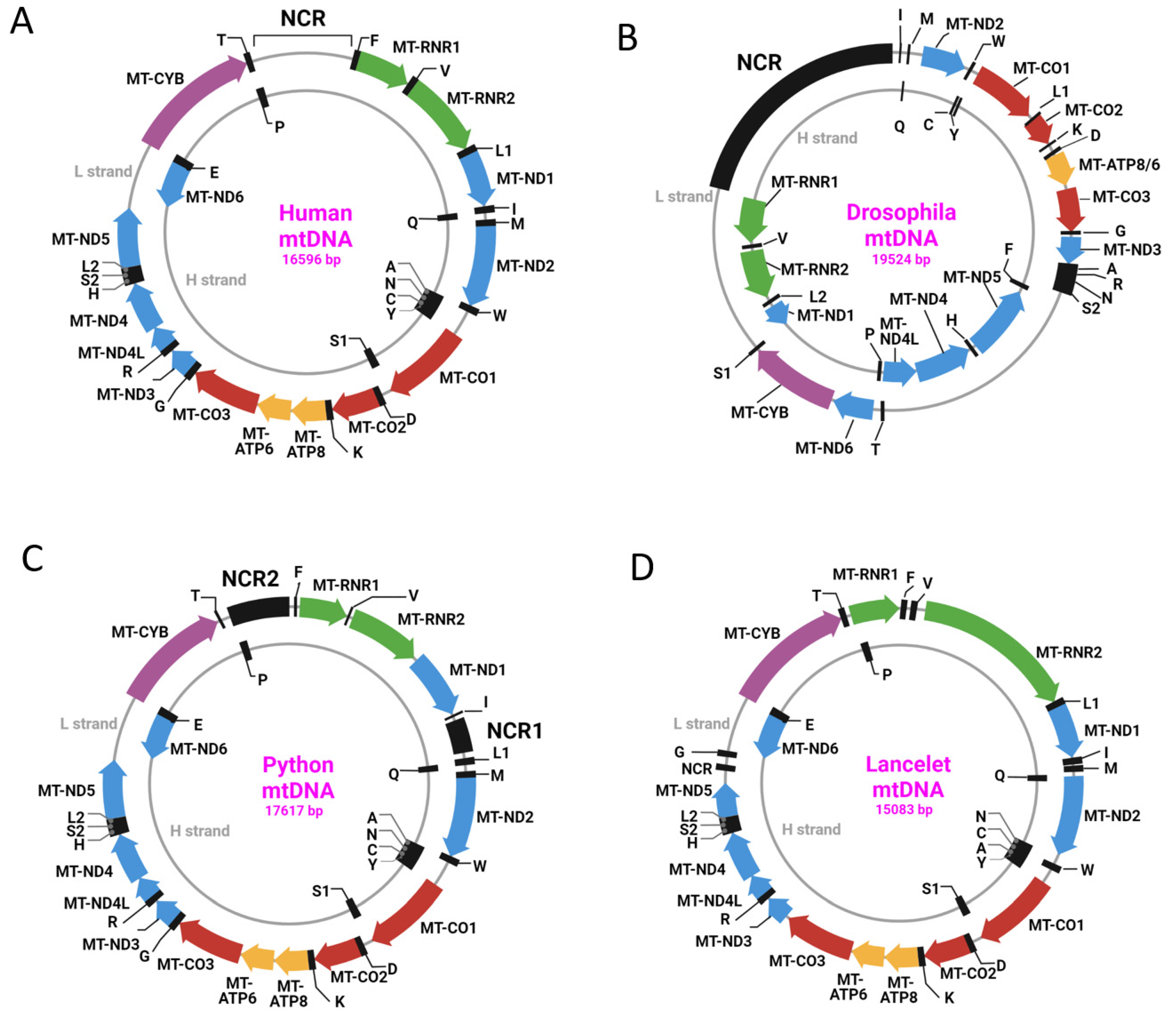
3. TFAM
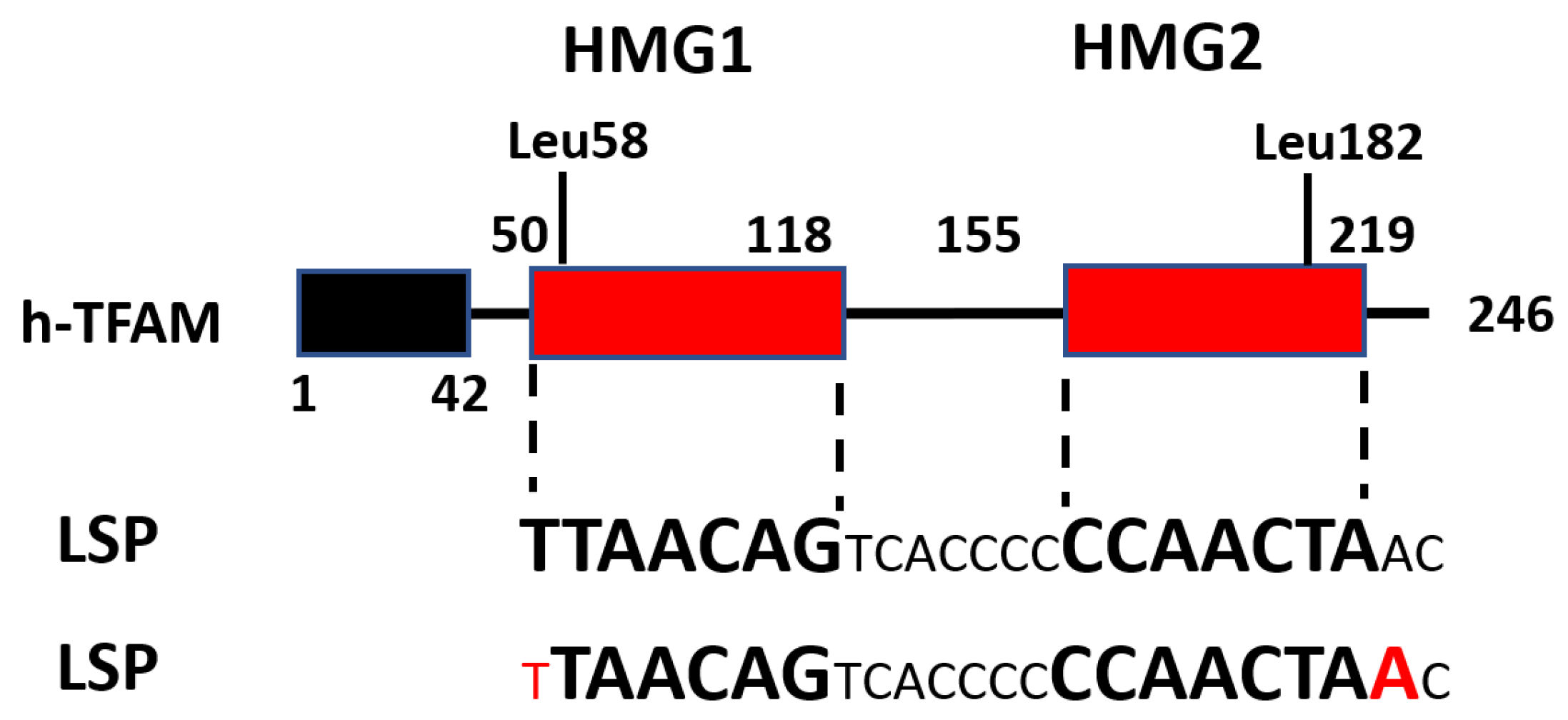
4. TFAM Domain Organization
5. Functional Roles of TFAM Domains
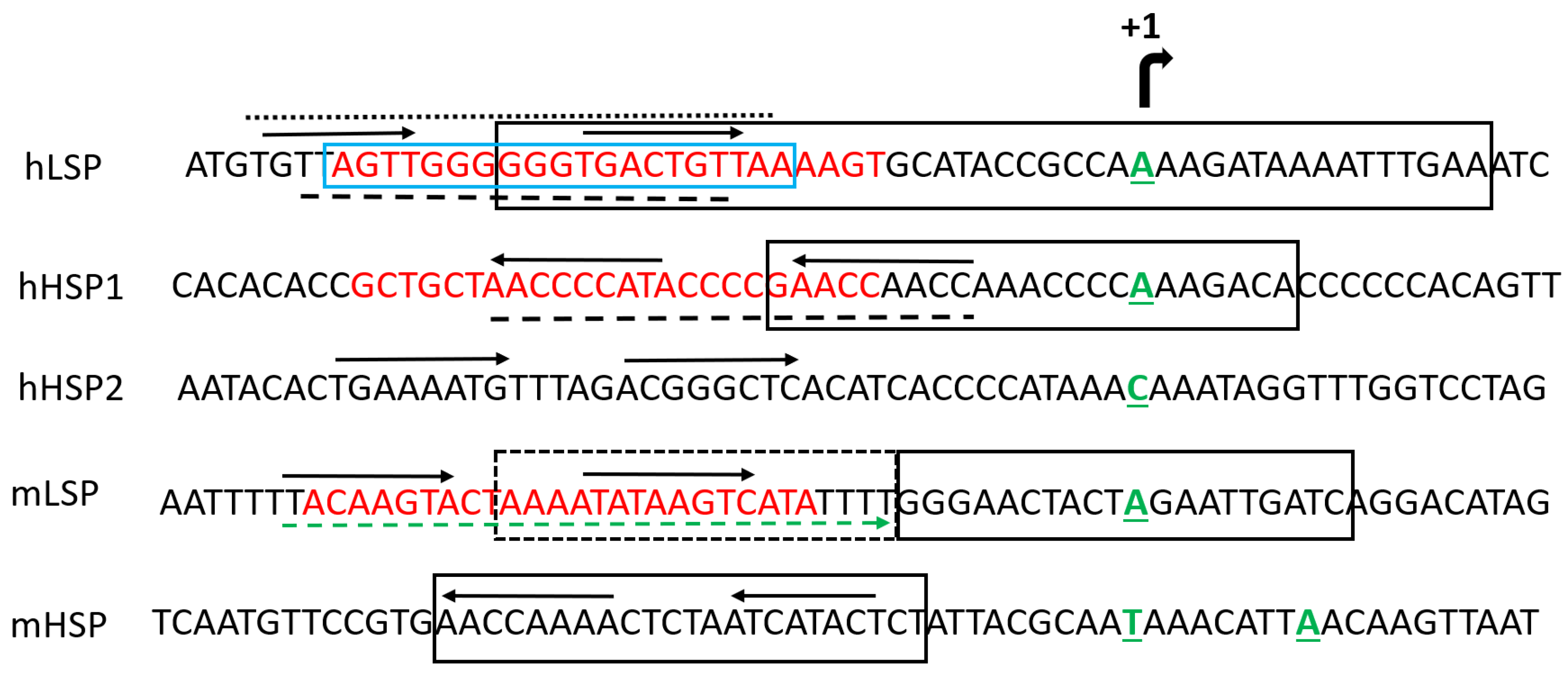
6. TFAM DNA Binding
7. TFAM Residues Interacting with mtDNA
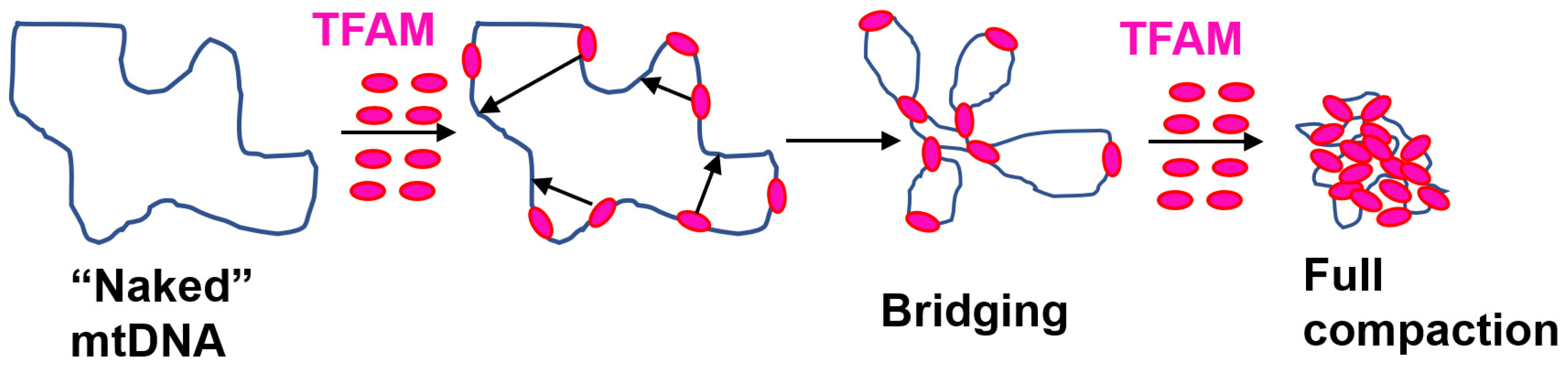
8. TFAM and mtDNA Compaction
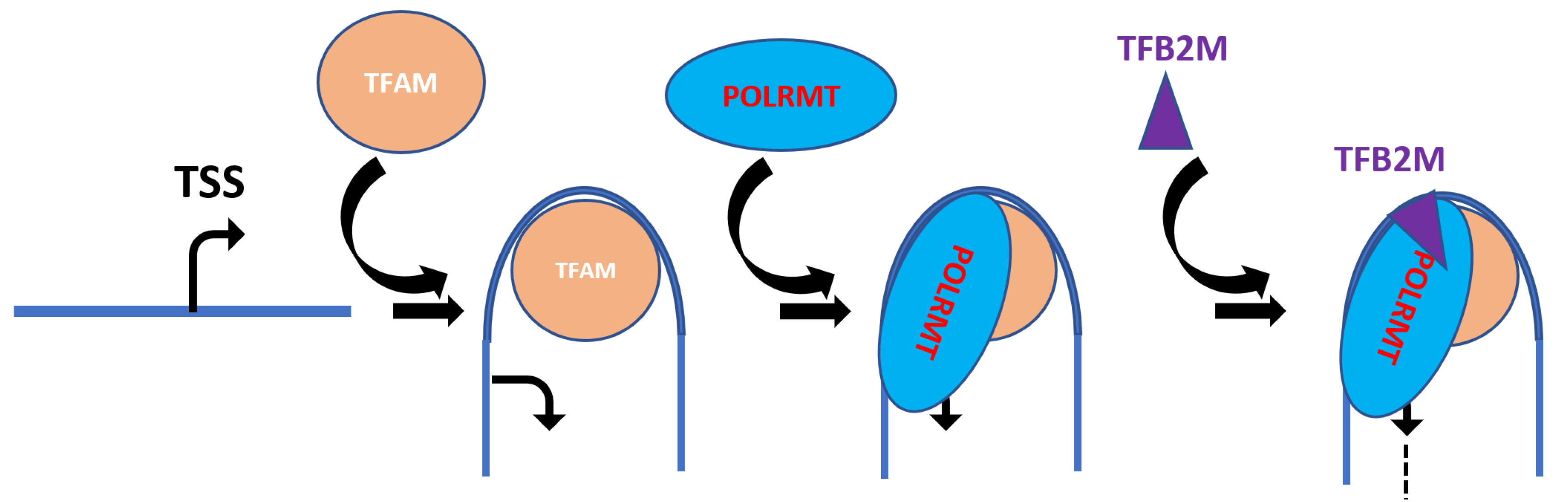
9. TFAM and Mitochondrial Transcription
10. TFAM and mtDNA Replication
11. TFAM and mtDNA Repair
12. TFAM and Mitochondrial Biogenesis
13. TFAM Orthologs
14. Limitations of the Available Experimental Systems
15. TFAM in Disease
16. Concluding Remarks
- What are TFAM’s contributions to mitochondrial transcription beyond the LSP and HSP1? Current models of mitochondrial transcription limit their scope to these two promoters.
- What are the structures of mitochondrial transcription complexes in situ? In vitro studies have suggested that POLRMT and TFB2M and/or partial DNA melting can reverse the orientation of TFAM binding at HSP1. Could other proteins further modify the structure of transcription complexes in situ (e.g., reverse the orientation back)?
- What are the determinants of TFAM sequence-specific DNA binding?
- If sequence-specific TFAM binding upstream of the mitochondrial promoter is an initiating event in the assembly of the transcription complexes, how does the orientation of TFAM binding upstream of the HSP1 promoter invert upon the transition to open complex? Is TFAM dissociation from DNA involved in the process?
- To what extent does the statement that “TFAM ‘coats’ mtDNA” reflect the actual situation in situ?
- What are the mechanistic basis and biological significance of observed uneven TFAM distribution between nucleoids within the same cell?
- What is the mechanism of the resistance of free (not DNA-bound) TFAM to degradation by proteases in POLRMT KO cells [79]?
- What mechanisms govern mtDNA persistence and transcription in tissues of tissue-specific TFAM KO animals?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinna, S.; Kunz, C.; Halpern, A.; Harrison, S.A.; Jordan, S.F.; Ward, J.; Werner, F.; Lane, N. A prebiotic basis for ATP as the universal energy currency. PLoS Biol. 2022, 20, e3001437. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.J.; Rutter, G.A. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim. Biophys. Acta 2009, 1787, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of Mitochondrial ATP Production: Ca(2+) Signaling and Quality Control. Trends Mol. Med. 2020, 26, 21–39. [Google Scholar] [CrossRef]
- Karnkowska, A.; Vacek, V.; Zubacova, Z.; Treitli, S.C.; Petrzelkova, R.; Eme, L.; Novak, L.; Zarsky, V.; Barlow, L.D.; Herman, E.K.; et al. A Eukaryote without a Mitochondrial Organelle. Curr. Biol. 2016, 26, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, A.A.; Gerasimov, E.S. Diversity of mitochondrial genome organization. Biochem. Mosc. 2012, 77, 1424–1435. [Google Scholar] [CrossRef]
- Pohjoismaki, J.L.; Goffart, S. Of circles, forks and humanity: Topological organisation and replication of mammalian mitochondrial DNA. BioEssays 2011, 33, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Pohjoismaki, J.L.; Goffart, S.; Tyynismaa, H.; Willcox, S.; Ide, T.; Kang, D.; Suomalainen, A.; Karhunen, P.J.; Griffith, J.D.; Holt, I.J.; et al. Human Heart Mitochondrial DNA Is Organized in Complex Catenated Networks Containing Abundant Four-way Junctions and Replication Forks. J. Biol. Chem. 2009, 284, 21446–21457. [Google Scholar] [CrossRef] [PubMed]
- Piko, L.; Matsumoto, L. Complex forms and replicative intermediates of mitochondrial DNA in tissues from adult and senescent mice. Nucleic Acids Res. 1977, 4, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Vinograd, J.; Morris, J.; Davidson, N.; Dove, W.F., Jr. The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc. Natl. Acad. Sci. USA 1963, 49, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.D.; Larson, J.E. Buoyant density studies on natural and synthetic deoxyribonucleic acids in neutral and alkaline solutions. J. Biol. Chem. 1972, 247, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M. On separation and coding capacity of mtDNA strands. Protein Sci. 2020, 29, 1070. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M. Mitochondrial DNA: The common confusions. Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2020, 31, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Shokolenko, I.N.; Alexeyev, M.F. Mitochondrial transcription in mammalian cells. Front. Biosci. 2017, 22, 835–853. [Google Scholar] [CrossRef]
- Barroso Lima, N.C.; Prosdocimi, F. The heavy strand dilemma of vertebrate mitochondria on genome sequencing age: Number of encoded genes or G + T content? Mitochondrial DNA Part A DNA Mapp. Seq. Anal. 2018, 29, 300–302. [Google Scholar] [CrossRef]
- Kozhukhar, N.; Mitta, S.; Alexeyev, M.F. Unusual mtDNA Control Region Length Heteroplasmy in the COS-7 Cell Line. Genes 2020, 11, 607. [Google Scholar] [CrossRef]
- Clayton, D.A. Replication of animal mitochondrial DNA. Cell 1982, 28, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.A. Transcription and replication of animal mitochondrial DNAs. Int. Rev. Cytol. 1992, 141, 217–232. [Google Scholar]
- Clayton, D.A. Transcription and replication of mitochondrial DNA. Hum. Reprod. 2000, 15 (Suppl. S2), 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Reyes, A.; Cluett, T.J.; Yang, M.Y.; Bowmaker, M.; Jacobs, H.T.; Holt, I.J. Replication of vertebrate mitochondrial DNA entails transient ribonucleotide incorporation throughout the lagging strand. EMBO J. 2006, 25, 5358–5371. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Yang, M.Y.; Jacobs, H.T.; Holt, I.J. A bidirectional origin of replication maps to the major noncoding region of human mitochondrial DNA. Mol. Cell 2005, 18, 651–662. [Google Scholar] [CrossRef]
- Matsushima, Y.; Matsumura, K.; Ishii, S.; Inagaki, H.; Suzuki, T.; Matsuda, Y.; Beck, K.; Kitagawa, Y. Functional domains of chicken mitochondrial transcription factor A for the maintenance of mitochondrial DNA copy number in lymphoma cell line DT40. J. Biol. Chem. 2003, 278, 31149–31158. [Google Scholar] [CrossRef]
- Watanabe, A.; Arai, M.; Koitabashi, N.; Niwano, K.; Ohyama, Y.; Yamada, Y.; Kato, N.; Kurabayashi, M. Mitochondrial transcription factors TFAM and TFB2M regulate Serca2 gene transcription. Cardiovasc. Res. 2011, 90, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kang, Y.C.; Park, W.H.; Jeong, J.H.; Pak, Y.K. Negative transcriptional regulation of mitochondrial transcription factor A (TFAM) by nuclear TFAM. Biochem. Biophys. Res. Commun. 2014, 450, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Kozhukhar, N.; Spadafora, D.; Rodriguez, Y.A.R.; Alexeyev, M.F. A Method for In Situ Reverse Genetic Analysis of Proteins Involved mtDNA Replication. Cells 2022, 11, 2168. [Google Scholar] [CrossRef] [PubMed]
- Larsson, N.G.; Wang, J.; Wilhelmsson, H.; Oldfors, A.; Rustin, P.; Lewandoski, M.; Barsh, G.S.; Clayton, D.A. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 1998, 18, 231–236. [Google Scholar] [CrossRef]
- Baris, O.R.; Klose, A.; Kloepper, J.E.; Weiland, D.; Neuhaus, J.F.; Schauen, M.; Wille, A.; Muller, A.; Merkwirth, C.; Langer, T.; et al. The mitochondrial electron transport chain is dispensable for proliferation and differentiation of epidermal progenitor cells. Stem Cells 2011, 29, 1459–1468. [Google Scholar] [CrossRef]
- Hansson, A.; Hance, N.; Dufour, E.; Rantanen, A.; Hultenby, K.; Clayton, D.A.; Wibom, R.; Larsson, N.G. A switch in metabolism precedes increased mitochondrial biogenesis in respiratory chain-deficient mouse hearts. Proc. Natl. Acad. Sci. USA 2004, 101, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Desdin-Mico, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabande-Rodriguez, E.; Blanco, E.M.; Alfranca, A.; Cusso, L.; Desco, M.; et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef]
- Barth, E.; Stammler, G.; Speiser, B.; Schaper, J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J. Mol. Cell. Cardiol. 1992, 24, 669–681. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wilhelmsson, H.; Hansson, A.; Thoren, P.; Duffy, J.; Rustin, P.; Larsson, N.G. Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc. Natl. Acad. Sci. USA 2000, 97, 3467–3472. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Rajagopalan, S.; Freund, S.M.; Rutherford, T.J.; Andreeva, A.; Townsley, F.M.; Petrovich, M.; Fersht, A.R. Biophysical characterizations of human mitochondrial transcription factor A and its binding to tumor suppressor p53. Nucleic Acids Res. 2009, 37, 6765–6783. [Google Scholar] [CrossRef]
- Rubio-Cosials, A.; Sidow, J.F.; Jimenez-Menendez, N.; Fernandez-Millan, P.; Montoya, J.; Jacobs, H.T.; Coll, M.; Bernado, P.; Sola, M. Human mitochondrial transcription factor A induces a U-turn structure in the light strand promoter. Nat. Struct. Mol. Biol. 2011, 18, 1281–1289. [Google Scholar] [CrossRef]
- Dairaghi, D.J.; Shadel, G.S.; Clayton, D.A. Addition of a 29 residue carboxyl-terminal tail converts a simple HMG box-containing protein into a transcriptional activator. J. Mol. Biol. 1995, 249, 11–28. [Google Scholar] [CrossRef]
- Morozov, Y.I.; Parshin, A.V.; Agaronyan, K.; Cheung, A.C.; Anikin, M.; Cramer, P.; Temiakov, D. A model for transcription initiation in human mitochondria. Nucleic Acids Res. 2015, 43, 3726–3735. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.B.; Kaiser, J.T.; Chan, D.C. The mitochondrial transcription and packaging factor Tfam imposes a U-turn on mitochondrial DNA. Nat. Struct. Mol. Biol. 2011, 18, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Gangelhoff, T.A.; Mungalachetty, P.S.; Nix, J.C.; Churchill, M.E. Structural analysis and DNA binding of the HMG domains of the human mitochondrial transcription factor A. Nucleic Acids Res. 2009, 37, 3153–3164. [Google Scholar] [CrossRef]
- Morozov, Y.I.; Agaronyan, K.; Cheung, A.C.; Anikin, M.; Cramer, P.; Temiakov, D. A novel intermediate in transcription initiation by human mitochondrial RNA polymerase. Nucleic Acids Res. 2014, 42, 3884–3893. [Google Scholar] [CrossRef]
- Vozarikova, V.; Kunova, N.; Bauer, J.A.; Frankovsky, J.; Kotrasova, V.; Prochazkova, K.; Dzugasova, V.; Kutejova, E.; Pevala, V.; Nosek, J.; et al. Mitochondrial HMG-Box Containing Proteins: From Biochemical Properties to the Roles in Human Diseases. Biomolecules 2020, 10, 1193. [Google Scholar] [CrossRef]
- Lu, B.; Lee, J.; Nie, X.; Li, M.; Morozov, Y.I.; Venkatesh, S.; Bogenhagen, D.F.; Temiakov, D.; Suzuki, C.K. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ Lon protease. Mol. Cell 2013, 49, 121–132. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2022, 51, D523–D531. [Google Scholar] [CrossRef]
- Hillen, H.S.; Morozov, Y.I.; Sarfallah, A.; Temiakov, D.; Cramer, P. Structural Basis of Mitochondrial Transcription Initiation. Cell 2017, 171, 1072–1081.e1010. [Google Scholar] [CrossRef] [PubMed]
- Kozhukhar, N.; Alexeyev, M.F. The C-terminal tail of Mitochondrial Transcription Factor A is Dispensable for Mitochondrial DNA Replication and Transcription in situ. Int. J. Mol. Sci. 2023, 24, 9430. [Google Scholar] [CrossRef]
- Long, Q.; Zhou, Y.; Wu, H.; Du, S.; Hu, M.; Qi, J.; Li, W.; Guo, J.; Wu, Y.; Yang, L.; et al. Phase separation drives the self-assembly of mitochondrial nucleoids for transcriptional modulation. Nat. Struct. Mol. Biol. 2021, 28, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.A.; Clayton, D.A. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science 1991, 252, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef]
- Fisher, R.P.; Clayton, D.A. Purification and characterization of human mitochondrial transcription factor 1. Mol. Cell. Biol. 1988, 8, 3496–3509. [Google Scholar] [PubMed]
- Kaufman, B.A.; Durisic, N.; Mativetsky, J.M.; Costantino, S.; Hancock, M.A.; Grutter, P.; Shoubridge, E.A. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol. Biol. Cell 2007, 18, 3225–3236. [Google Scholar] [CrossRef]
- Ngo, H.B.; Lovely, G.A.; Phillips, R.; Chan, D.C. Distinct structural features of TFAM drive mitochondrial DNA packaging versus transcriptional activation. Nat. Commun. 2014, 5, 3077. [Google Scholar] [CrossRef]
- Rubio-Cosials, A.; Battistini, F.; Gansen, A.; Cuppari, A.; Bernado, P.; Orozco, M.; Langowski, J.; Toth, K.; Sola, M. Protein Flexibility and Synergy of HMG Domains Underlie U-Turn Bending of DNA by TFAM in Solution. Biophys. J. 2018, 114, 2386–2396. [Google Scholar] [CrossRef]
- Malarkey, C.S.; Bestwick, M.; Kuhlwilm, J.E.; Shadel, G.S.; Churchill, M.E. Transcriptional activation by mitochondrial transcription factor A involves preferential distortion of promoter DNA. Nucleic Acids Res. 2012, 40, 614–624. [Google Scholar] [CrossRef]
- Stros, M.; Launholt, D.; Grasser, K.D. The HMG-box: A versatile protein domain occurring in a wide variety of DNA-binding proteins. Cell. Mol. Life Sci. 2007, 64, 2590–2606. [Google Scholar] [CrossRef]
- Uchida, A.; Murugesapillai, D.; Kastner, M.; Wang, Y.; Lodeiro, M.F.; Prabhakar, S.; Oliver, G.V.; Arnold, J.J.; Maher, L.J.; Williams, M.C.; et al. Unexpected sequences and structures of mtDNA required for efficient transcription from the first heavy-strand promoter. eLife 2017, 6, e27283. [Google Scholar] [CrossRef] [PubMed]
- Garstka, H.L.; Schmitt, W.E.; Schultz, J.; Sogl, B.; Silakowski, B.; Perez-Martos, A.; Montoya, J.; Wiesner, R.J. Import of mitochondrial transcription factor A (TFAM) into rat liver mitochondria stimulates transcription of mitochondrial DNA. Nucleic Acids Res. 2003, 31, 5039–5047. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, K.; Kanki, T.; Fukuoh, A.; Kurisaki, H.; Aoki, Y.; Ikeuchi, M.; Kim, S.H.; Hamasaki, N.; Kang, D. The C-terminal tail of mitochondrial transcription factor a markedly strengthens its general binding to DNA. J. Biochem. 2007, 141, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Feric, M.; Demarest, T.G.; Tian, J.; Croteau, D.L.; Bohr, V.A.; Misteli, T. Self-assembly of multi-component mitochondrial nucleoids via phase separation. EMBO J. 2021, 40, e107165. [Google Scholar] [CrossRef] [PubMed]
- Mehmedovic, M.; Martucci, M.; Spahr, H.; Ishak, L.; Mishra, A.; Sanchez-Sandoval, M.E.; Pardo-Hernandez, C.; Peter, B.; van den Wildenberg, S.M.; Falkenberg, M.; et al. Disease causing mutation (P178L) in mitochondrial transcription factor A results in impaired mitochondrial transcription initiation. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166467. [Google Scholar] [CrossRef]
- Kasashima, K.; Sumitani, M.; Endo, H. Human mitochondrial transcription factor A is required for the segregation of mitochondrial DNA in cultured cells. Exp. Cell Res. 2010, 37, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Kanki, T.; Ohgaki, K.; Gaspari, M.; Gustafsson, C.M.; Fukuoh, A.; Sasaki, N.; Hamasaki, N.; Kang, D. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol. Cell. Biol. 2004, 24, 9823–9834. [Google Scholar] [CrossRef]
- McCulloch, V.; Shadel, G.S. Human mitochondrial transcription factor B1 interacts with the C-terminal activation region of h-mtTFA and stimulates transcription independently of its RNA methyltransferase activity. Mol. Cell. Biol. 2003, 23, 5816–5824. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.P.; Topper, J.N.; Clayton, D.A. Promoter selection in human mitochondria involves binding of a transcription factor to orientation-independent upstream regulatory elements. Cell 1987, 50, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.D.; Clayton, D.A. Precise identification of individual promoters for transcription of each strand of human mitochondrial DNA. Cell 1984, 36, 635–643. [Google Scholar] [CrossRef]
- Chang, D.D.; Clayton, D.A. Identification of primary transcriptional start sites of mouse mitochondrial DNA: Accurate in vitro initiation of both heavy- and light-strand transcripts. Mol. Cell. Biol. 1986, 6, 1446–1453. [Google Scholar] [PubMed]
- Martin, M.; Cho, J.; Cesare, A.J.; Griffith, J.D.; Attardi, G. Termination factor-mediated DNA loop between termination and initiation sites drives mitochondrial rRNA synthesis. Cell 2005, 123, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.D.; Clayton, D.A. Precise assignment of the light-strand promoter of mouse mitochondrial DNA: A functional promoter consists of multiple upstream domains. Mol. Cell. Biol. 1986, 6, 3253–3261. [Google Scholar] [PubMed]
- Dairaghi, D.J.; Shadel, G.S.; Clayton, D.A. Human mitochondrial transcription factor A and promoter spacing integrity are required for transcription initiation. Biochim. Biophys. Acta 1995, 1271, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lodeiro, M.F.; Uchida, A.U.; Arnold, J.J.; Reynolds, S.L.; Moustafa, I.M.; Cameron, C.E. Identification of multiple rate-limiting steps during the human mitochondrial transcription cycle in vitro. J. Biol. Chem. 2010, 285, 16387–16402. [Google Scholar] [CrossRef]
- Gaspari, M.; Falkenberg, M.; Larsson, N.G.; Gustafsson, C.M. The mitochondrial RNA polymerase contributes critically to promoter specificity in mammalian cells. EMBO J. 2004, 23, 4606–4614. [Google Scholar] [CrossRef]
- Fisher, R.P.; Parisi, M.A.; Clayton, D.A. Flexible recognition of rapidly evolving promoter sequences by mitochondrial transcription factor 1. Genes Dev. 1989, 3, 2202–2217. [Google Scholar] [CrossRef]
- Campbell, C.T.; Kolesar, J.E.; Kaufman, B.A. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta 2012, 1819, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Ghivizzani, S.C.; Madsen, C.S.; Nelen, M.R.; Ammini, C.V.; Hauswirth, W.W. In organello footprint analysis of human mitochondrial DNA: Human mitochondrial transcription factor A interactions at the origin of replication. Mol. Cell. Biol. 1994, 14, 7717–7730. [Google Scholar] [PubMed]
- Takamatsu, C.; Umeda, S.; Ohsato, T.; Ohno, T.; Abe, Y.; Fukuoh, A.; Shinagawa, H.; Hamasaki, N.; Kang, D. Regulation of mitochondrial D-loops by transcription factor A and single-stranded DNA-binding protein. EMBO Rep. 2002, 3, 451–456. [Google Scholar] [CrossRef]
- Kukat, C.; Wurm, C.A.; Spahr, H.; Falkenberg, M.; Larsson, N.G.; Jakobs, S. Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proc. Natl. Acad. Sci. USA 2011, 108, 13534–13539. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.E.; Marinov, G.K.; Wold, B.J.; Chan, D.C. Genome-wide analysis reveals coating of the mitochondrial genome by TFAM. PLoS ONE 2013, 8, e74513. [Google Scholar] [CrossRef]
- Kanki, T.; Nakayama, H.; Sasaki, N.; Takio, K.; Alam, T.I.; Hamasaki, N.; Kang, D. Mitochondrial nucleoid and transcription factor A. Ann. N. Y. Acad. Sci. 2004, 1011, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.I.; Kanki, T.; Muta, T.; Ukaji, K.; Abe, Y.; Nakayama, H.; Takio, K.; Hamasaki, N.; Kang, D. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 2003, 31, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, Y.; Goto, Y.I.; Kaguni, L.S. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM). Proc. Natl. Acad. Sci. USA 2010, 107, 18410–18415. [Google Scholar] [CrossRef]
- Kuhl, I.; Miranda, M.; Posse, V.; Milenkovic, D.; Mourier, A.; Siira, S.J.; Bonekamp, N.A.; Neumann, U.; Filipovska, A.; Polosa, P.L.; et al. POLRMT regulates the switch between replication primer formation and gene expression of mammalian mtDNA. Sci. Adv. 2016, 2, e1600963. [Google Scholar] [CrossRef] [PubMed]
- Rohs, R.; West, S.M.; Sosinsky, A.; Liu, P.; Mann, R.S.; Honig, B. The role of DNA shape in protein-DNA recognition. Nature 2009, 461, 1248–1253. [Google Scholar] [CrossRef]
- Seeman, N.C.; Rosenberg, J.M.; Rich, A. Sequence-specific recognition of double helical nucleic acids by proteins. Proc. Natl. Acad. Sci. USA 1976, 73, 804–808. [Google Scholar] [CrossRef]
- Joshi, R.; Passner, J.M.; Rohs, R.; Jain, R.; Sosinsky, A.; Crickmore, M.A.; Jacob, V.; Aggarwal, A.K.; Honig, B.; Mann, R.S. Functional specificity of a Hox protein mediated by the recognition of minor groove structure. Cell 2007, 131, 530–543. [Google Scholar] [CrossRef]
- Rohs, R.; Jin, X.; West, S.M.; Joshi, R.; Honig, B.; Mann, R.S. Origins of specificity in protein-DNA recognition. Annu. Rev. Biochem. 2010, 79, 233–269. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kuroiwa, T. Organization of multiple nucleoids and DNA molecules in mitochondria of a human cell. Exp. Cell Res. 1991, 196, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Iborra, F.J.; Kimura, H.; Cook, P.R. The functional organization of mitochondrial genomes in human cells. BMC Biol. 2004, 2, 9. [Google Scholar] [CrossRef]
- Alan, L.; Spacek, T.; Jezek, P. Delaunay algorithm and principal component analysis for 3D visualization of mitochondrial DNA nucleoids by Biplane FPALM/dSTORM. Eur. Biophys. J. 2016, 45, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Bogenhagen, D.F.; Rousseau, D.; Burke, S. The layered structure of human mitochondrial DNA nucleoids. J. Biol. Chem. 2008, 283, 3665–3675. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cooper, H.M.; Reyes, A.; Di Re, M.; Sembongi, H.; Litwin, T.R.; Gao, J.; Neuman, K.C.; Fearnley, I.M.; Spinazzola, A.; et al. Mitochondrial nucleoid interacting proteins support mitochondrial protein synthesis. Nucleic Acids Res. 2012, 40, 6109–6121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bogenhagen, D.F. Human mitochondrial DNA nucleoids are linked to protein folding machinery and metabolic enzymes at the mitochondrial inner membrane. J. Biol. Chem. 2006, 281, 25791–25802. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, B.A.; Newman, S.M.; Hallberg, R.L.; Slaughter, C.A.; Perlman, P.S.; Butow, R.A. In organello formaldehyde crosslinking of proteins to mtDNA: Identification of bifunctional proteins. Proc. Natl. Acad. Sci. USA 2000, 97, 7772–7777. [Google Scholar] [CrossRef] [PubMed]
- Rajala, N.; Hensen, F.; Wessels, H.J.; Ives, D.; Gloerich, J.; Spelbrink, J.N. Whole cell formaldehyde cross-linking simplifies purification of mitochondrial nucleoids and associated proteins involved in mitochondrial gene expression. PLoS ONE 2015, 10, e0116726. [Google Scholar] [CrossRef]
- Hensen, F.; Cansiz, S.; Gerhold, J.M.; Spelbrink, J.N. To be or not to be a nucleoid protein: A comparison of mass-spectrometry based approaches in the identification of potential mtDNA-nucleoid associated proteins. Biochimie 2014, 100, 219–226. [Google Scholar] [CrossRef]
- Wai, T.; Teoli, D.; Shoubridge, E.A. The mitochondrial DNA genetic bottleneck results from replication of a subpopulation of genomes. Nat. Genet. 2008, 40, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Tkachuk, A.N.; Shtengel, G.; Kopek, B.G.; Bogenhagen, D.F.; Hess, H.F.; Clayton, D.A. Superresolution fluorescence imaging of mitochondrial nucleoids reveals their spatial range, limits, and membrane interaction. Mol. Cell. Biol. 2011, 31, 4994–5010. [Google Scholar] [CrossRef] [PubMed]
- Legros, F.; Malka, F.; Frachon, P.; Lombes, A.; Rojo, M. Organization and dynamics of human mitochondrial DNA. J. Cell Sci. 2004, 117, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.P.; Lisowsky, T.; Parisi, M.A.; Clayton, D.A. DNA wrapping and bending by a mitochondrial high mobility group-like transcriptional activator protein. J. Biol. Chem. 1992, 267, 3358–3367. [Google Scholar] [CrossRef] [PubMed]
- Cotney, J.; Wang, Z.; Shadel, G.S. Relative abundance of the human mitochondrial transcription system and distinct roles for h-mtTFB1 and h-mtTFB2 in mitochondrial biogenesis and gene expression. Nucleic Acids Res. 2007, 35, 4042–4054. [Google Scholar] [CrossRef] [PubMed]
- Maniura-Weber, K.; Goffart, S.; Garstka, H.L.; Montoya, J.; Wiesner, R.J. Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res. 2004, 32, 6015–6027. [Google Scholar] [CrossRef] [PubMed]
- Farge, G.; Mehmedovic, M.; Baclayon, M.; van den Wildenberg, S.M.; Roos, W.H.; Gustafsson, C.M.; Wuite, G.J.; Falkenberg, M. In vitro-reconstituted nucleoids can block mitochondrial DNA replication and transcription. Cell Rep. 2014, 8, 66–74. [Google Scholar] [CrossRef]
- Bonekamp, N.A.; Jiang, M.; Motori, E.; Garcia Villegas, R.; Koolmeister, C.; Atanassov, I.; Mesaros, A.; Park, C.B.; Larsson, N.G. High levels of TFAM repress mammalian mitochondrial DNA transcription in vivo. Life Sci. Alliance 2021, 4, e202101034. [Google Scholar] [CrossRef] [PubMed]
- Farge, G.; Laurens, N.; Broekmans, O.D.; van den Wildenberg, S.M.; Dekker, L.C.; Gaspari, M.; Gustafsson, C.M.; Peterman, E.J.; Falkenberg, M.; Wuite, G.J. Protein sliding and DNA denaturation are essential for DNA organization by human mitochondrial transcription factor A. Nat. Commun. 2012, 3, 1013. [Google Scholar] [CrossRef] [PubMed]
- Kukat, C.; Davies, K.M.; Wurm, C.A.; Spahr, H.; Bonekamp, N.A.; Kuhl, I.; Joos, F.; Polosa, P.L.; Park, C.B.; Posse, V.; et al. Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid. Proc. Natl. Acad. Sci. USA 2015, 112, 11288–11293. [Google Scholar] [CrossRef] [PubMed]
- Shutt, T.E.; Bestwick, M.; Shadel, G.S. The core human mitochondrial transcription initiation complex: It only takes two to tango. Transcription 2011, 2, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Shutt, T.E.; Lodeiro, M.F.; Cotney, J.; Cameron, C.E.; Shadel, G.S. Core human mitochondrial transcription apparatus is a regulated two-component system in vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 12133–12138. [Google Scholar] [CrossRef] [PubMed]
- Zollo, O.; Tiranti, V.; Sondheimer, N. Transcriptional requirements of the distal heavy-strand promoter of mtDNA. Proc. Natl. Acad. Sci. USA 2012, 109, 6508–6512. [Google Scholar] [CrossRef] [PubMed]
- Lodeiro, M.F.; Uchida, A.; Bestwick, M.; Moustafa, I.M.; Arnold, J.J.; Shadel, G.S.; Cameron, C.E. Transcription from the second heavy-strand promoter of human mtDNA is repressed by transcription factor A in vitro. Proc. Natl. Acad. Sci. USA 2012, 109, 6513–6518. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Dierckx, A.; Wanrooij, P.H.; Wanrooij, S.; Larsson, N.G.; Wilhelmsson, L.M.; Falkenberg, M.; Gustafsson, C.M. Mammalian transcription factor A is a core component of the mitochondrial transcription machinery. Proc. Natl. Acad. Sci. USA 2012, 109, 16510–16515. [Google Scholar] [CrossRef]
- Zamudio-Ochoa, A.; Morozov, Y.I.; Sarfallah, A.; Anikin, M.; Temiakov, D. Mechanisms of mitochondrial promoter recognition in humans and other mammalian species. Nucleic Acids Res. 2022, 50, 2765–2781. [Google Scholar] [CrossRef]
- Posse, V.; Hoberg, E.; Dierckx, A.; Shahzad, S.; Koolmeister, C.; Larsson, N.G.; Wilhelmsson, L.M.; Hallberg, B.M.; Gustafsson, C.M. The amino terminal extension of mammalian mitochondrial RNA polymerase ensures promoter specific transcription initiation. Nucleic Acids Res. 2014, 42, 3638–3647. [Google Scholar] [CrossRef]
- Ekstrand, M.I.; Falkenberg, M.; Rantanen, A.; Park, C.B.; Gaspari, M.; Hultenby, K.; Rustin, P.; Gustafsson, C.M.; Larsson, N.G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004, 13, 935–944. [Google Scholar] [CrossRef]
- Freyer, C.; Park, C.B.; Ekstrand, M.I.; Shi, Y.; Khvorostova, J.; Wibom, R.; Falkenberg, M.; Gustafsson, C.M.; Larsson, N.G. Maintenance of respiratory chain function in mouse hearts with severely impaired mtDNA transcription. Nucleic Acids Res. 2010, 38, 6577–6588. [Google Scholar] [CrossRef]
- Fuste, J.M.; Wanrooij, S.; Jemt, E.; Granycome, C.E.; Cluett, T.J.; Shi, Y.; Atanassova, N.; Holt, I.J.; Gustafsson, C.M.; Falkenberg, M. Mitochondrial RNA polymerase is needed for activation of the origin of light-strand DNA replication. Mol. Cell 2010, 37, 67–78. [Google Scholar] [CrossRef]
- Wanrooij, S.; Fuste, J.M.; Farge, G.; Shi, Y.; Gustafsson, C.M.; Falkenberg, M. Human mitochondrial RNA polymerase primes lagging-strand DNA synthesis in vitro. Proc. Natl. Acad. Sci. USA 2008, 105, 11122–11127. [Google Scholar] [CrossRef]
- Montoya, J.; Christianson, T.; Levens, D.; Rabinowitz, M.; Attardi, G. Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1982, 79, 7195–7199. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, P.; Attardi, G. Mapping of nascent light and heavy strand transcripts on the physical map of HeLa cell mitochondrial DNA. Nucleic Acids Res. 1980, 8, 2605–2625. [Google Scholar] [CrossRef]
- Blumberg, A.; Rice, E.J.; Kundaje, A.; Danko, C.G.; Mishmar, D. Initiation of mtDNA transcription is followed by pausing, and diverge across human cell types and during evolution. Genome Res. 2017, 27, 362–373. [Google Scholar] [CrossRef]
- Bouda, E.; Stapon, A.; Garcia-Diaz, M. Mechanisms of mammalian mitochondrial transcription. Protein Sci. 2019, 28, 1594–1605. [Google Scholar] [CrossRef]
- Kozhukhar, N.; Alexeyev, M.F. TFAM’s Contributions to mtDNA Replication and OXPHOS Biogenesis Are Genetically Separable. Cells 2022, 11, 3754. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, E.; Clayton, D.A. Transcription-dependent DNA transactions in the mitochondrial genome of a yeast hypersuppressive petite mutant. Mol. Cell. Biol. 1998, 18, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wilhelmsson, H.; Graff, C.; Li, H.; Oldfors, A.; Rustin, P.; Bruning, J.C.; Kahn, C.R.; Clayton, D.A.; Barsh, G.S.; et al. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat. Genet. 1999, 21, 133–137. [Google Scholar] [CrossRef]
- Matsuda, T.; Kanki, T.; Tanimura, T.; Kang, D.; Matsuura, E.T. Effects of overexpression of mitochondrial transcription factor A on lifespan and oxidative stress response in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2013, 430, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Franko, A.; Mayer, S.; Thiel, G.; Mercy, L.; Arnould, T.; Hornig-Do, H.T.; Wiesner, R.J.; Goffart, S. CREB-1alpha is recruited to and mediates upregulation of the cytochrome c promoter during enhanced mitochondrial biogenesis accompanying skeletal muscle differentiation. Mol. Cell. Biol. 2008, 28, 2446–2459. [Google Scholar] [CrossRef] [PubMed]
- Seidel-Rogol, B.L.; Shadel, G.S. Modulation of mitochondrial transcription in response to mtDNA depletion and repletion in HeLa cells. Nucleic Acids Res. 2002, 30, 1929–1934. [Google Scholar] [CrossRef]
- Brinckmann, A.; Weiss, C.; Wilbert, F.; von Moers, A.; Zwirner, A.; Stoltenburg-Didinger, G.; Wilichowski, E.; Schuelke, M. Regionalized pathology correlates with augmentation of mtDNA copy numbers in a patient with myoclonic epilepsy with ragged-red fibers (MERRF-syndrome). PLoS ONE 2010, 5, e13513. [Google Scholar] [CrossRef]
- Jiang, S.; Koolmeister, C.; Misic, J.; Siira, S.; Kuhl, I.; Silva Ramos, E.; Miranda, M.; Jiang, M.; Posse, V.; Lytovchenko, O.; et al. TEFM regulates both transcription elongation and RNA processing in mitochondria. EMBO Rep. 2019, 20, e48101. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Cecconi, C.; Tkachuk, A.N.; Bustamante, C.; Clayton, D.A. Replication of mitochondrial DNA occurs by strand displacement with alternative light-strand origins, not via a strand-coupled mechanism. Genes Dev. 2005, 19, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Zelenaya-Troitskaya, O.; Newman, S.M.; Okamoto, K.; Perlman, P.S.; Butow, R.A. Functions of the high mobility group protein, Abf2p, in mitochondrial DNA segregation, recombination and copy number in Saccharomyces cerevisiae. Genetics 1998, 148, 1763–1776. [Google Scholar] [CrossRef]
- Zelenaya-Troitskaya, O.; Perlman, P.S.; Butow, R.A. An enzyme in yeast mitochondria that catalyzes a step in branched-chain amino acid biosynthesis also functions in mitochondrial DNA stability. EMBO J. 1995, 14, 3268–3276. [Google Scholar] [CrossRef]
- Diffley, J.F.; Stillman, B. DNA binding properties of an HMG1-related protein from yeast mitochondria. J. Biol. Chem. 1992, 267, 3368–3374. [Google Scholar] [CrossRef]
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The maintenance of mitochondrial DNA integrity--critical analysis and update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641. [Google Scholar] [CrossRef]
- Xu, W.; Boyd, R.M.; Tree, M.O.; Samkari, F.; Zhao, L. Mitochondrial transcription factor A promotes DNA strand cleavage at abasic sites. Proc. Natl. Acad. Sci. USA 2019, 116, 17792–17799. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, W.; Tang, J.; Kaushik, S.; Chang, C.A.; Zhao, L. Key Amino Acid Residues of Mitochondrial Transcription Factor A Synergize with Abasic (AP) Site Dynamics To Facilitate AP-Lyase Reactions. ACS Chem. Biol. 2023, 18, 1168–1179. [Google Scholar] [CrossRef]
- Xu, W.; Tang, J.; Zhao, L. DNA-protein cross-links between abasic DNA damage and mitochondrial transcription factor A (TFAM). Nucleic Acids Res. 2023, 51, 41–53. [Google Scholar] [CrossRef]
- Chew, K.; Zhao, L. Interactions of Mitochondrial Transcription Factor A with DNA Damage: Mechanistic Insights and Functional Implications. Genes 2021, 12, 1246. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhao, W.; Hendricks, N.G.; Zhao, L. High-Resolution Mapping of Amino Acid Residues in DNA-Protein Cross-Links Enabled by Ribonucleotide-Containing DNA. Anal. Chem. 2021, 93, 13398–13406. [Google Scholar] [CrossRef]
- Canugovi, C.; Maynard, S.; Bayne, A.C.; Sykora, P.; Tian, J.; de Souza-Pinto, N.C.; Croteau, D.L.; Bohr, V.A. The mitochondrial transcription factor A functions in mitochondrial base excision repair. DNA Repair 2010, 9, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Tkachuk, A.N.; Clayton, D.A. Mitochondrial Transcription Factor A (TFAM) Binds to RNA Containing 4-Way Junctions and Mitochondrial tRNA. PLoS ONE 2015, 10, e0142436. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, E.; Tonello, C.; Cardile, A.; Cozzi, V.; Bracale, R.; Tedesco, L.; Falcone, S.; Valerio, A.; Cantoni, O.; Clementi, E.; et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 2005, 310, 314–317. [Google Scholar] [CrossRef]
- Matsushima, Y.; Garesse, R.; Kaguni, L.S. Drosophila mitochondrial transcription factor B2 regulates mitochondrial DNA copy number and transcription in schneider cells. J. Biol. Chem. 2004, 279, 26900–26905. [Google Scholar] [CrossRef]
- Pellegrini, M.; Asin-Cayuela, J.; Erdjument-Bromage, H.; Tempst, P.; Larsson, N.G.; Gustafsson, C.M. MTERF2 is a nucleoid component in mammalian mitochondria. Biochim. Biophys. Acta 2009, 1787, 296–302. [Google Scholar] [CrossRef]
- Pohjoismaki, J.L.; Wanrooij, S.; Hyvarinen, A.K.; Goffart, S.; Holt, I.J.; Spelbrink, J.N.; Jacobs, H.T. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res. 2006, 34, 5815–5828. [Google Scholar] [CrossRef] [PubMed]
- Kozhukhar, N.; Alexeyev, M.F. Limited predictive value of TFAM in mitochondrial biogenesis. Mitochondrion 2019, 49, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, G.L.; Oliveira, M.T.; Kaguni, L.S. Animal Mitochondrial DNA Replication. Enzymes 2016, 39, 255–292. [Google Scholar] [CrossRef]
- Moore, G.W.; Hutchins, G.M.; Bulkley, B.H.; Tseng, J.S.; Ki, P.F. Constituents of the human ventricular myocardium: Connective tissue hyperplasia accompanying muscular hypertrophy. Am. Heart J. 1980, 100, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Grove, D.; Zak, R.; Nair, K.G.; Aschenbrenner, V. Biochemical correlates of cardiac hypertrophy. IV. Observations on the cellular organization of growth during myocardial hypertrophy in the rat. Circ. Res. 1969, 25, 473–485. [Google Scholar] [CrossRef]
- Schmidt-Supprian, M.; Rajewsky, K. Vagaries of conditional gene targeting. Nat. Immunol. 2007, 8, 665–668. [Google Scholar] [CrossRef]
- Becher, B.; Waisman, A.; Lu, L.F. Conditional Gene-Targeting in Mice: Problems and Solutions. Immunity 2018, 48, 835–836. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Shimizu, A. Highly sensitive enzyme immunoassay for human creatine kinase MM and MB isozymes. Clin. Chim. Acta 1986, 158, 99–108. [Google Scholar] [CrossRef]
- Stiles, A.R.; Simon, M.T.; Stover, A.; Eftekharian, S.; Khanlou, N.; Wang, H.L.; Magaki, S.; Lee, H.; Partynski, K.; Dorrani, N.; et al. Mutations in TFAM, encoding mitochondrial transcription factor A, cause neonatal liver failure associated with mtDNA depletion. Mol. Genet. Metab. 2016, 119, 91–99. [Google Scholar] [CrossRef]
- Shokolenko, I.N.; Alexeyev, M.F. Mitochondrial DNA: A disposable genome? Biochim. Biophys. Acta 2015, 1852, 1805–1809. [Google Scholar] [CrossRef]
- Ullah, F.; Rauf, W.; Khan, K.; Khan, S.; Bell, K.M.; de Oliveira, V.C.; Tariq, M.; Bakhshalizadeh, S.; Touraine, P.; Katsanis, N.; et al. A recessive variant in TFAM causes mtDNA depletion associated with primary ovarian insufficiency, seizures, intellectual disability and hearing loss. Hum. Genet. 2021, 140, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
| h-TFAM Domains Expanse/Length (aa) | Reference | |||||
|---|---|---|---|---|---|---|
| MTS | Leader | HMG1 | Linker | HMG2 | Tail | |
| 1–42/42 | - | 43–121/79 | 122–151/30 | 152–221/70 | 222–246/25 | [37] |
| 1–42/42 | - | 43–125/83 | 127–153/27 * | 154–221/68 | 222–246/25 | [34] |
| 1–42/42 | - | 43–122/80 ** | 122–152/31 ** | 152–222/71 ** | 222–246/25 ** | [36,42,44] |
| 1–49/49 | - | 50–118/69 | 119–154/36 | 155–219/65 | 220/246/27 | [32] |
| 1–42/42 | 1 | 44–120/77 | 124(123)–152/29–30 *** | 153–225/73 | 226–246/21 | [33] |
| 1–42/42 | 7 | 50–122/73 | 123–152/30 | 153–223/71 | 224–246/23 | [45] |
| 1–42/42 | 7 | 50–115/66 | 116–154/39 | 155–234/80 | 235–246/12 | [46,47] |
| 1–42/42 | 7 | 50–118/69 | 119–154/36 | 155–219/65 | 220–246/27 | [25,41] |
| LSP [29] | LSP [32] | LSP [42] ** | HSP1 [46] | HSP1 [42] ** | Nonspecific DNA [46] | |
|---|---|---|---|---|---|---|
| HMG1 | Lys51 | Lys51 | Lys51 | |||
| Lys52 | Lys52 | Lys52 | Lys52 | |||
| Ser55 | Ser55 | Ser55 | ||||
| Ser56 | Ser56 | |||||
| Tyr57 | Tyr57 | Tyr57 | Tyr57 | Tyr57 | Tyr57 | |
| Leu58 | Leu58 | Leu58 | Leu58 | Leu58 | ||
| Ser61 | Ser61 | Ser61 | ||||
| Leu65 | Leu65 | |||||
| Lys69 | Lys69 | |||||
| Thr77 | Thr77 | Thr77 | Thr77 | |||
| Thr78 | Thr78 | Thr78 | Thr78 | Thr78 | ||
| Ile81 | Ile81 | Ile81 | Ile81 | |||
| Arg82 | Arg82 | Arg82 | Arg82 | |||
| Trp88 | Trp88 | Trp88 | Trp88 | |||
| Arg89 | Arg89 | Arg89 | Arg89 | |||
| Gln100 | Gln100 | Gln100 | Gln100 | |||
| Tyr103 | Tyr103 | Tyr103 | Tyr103 | |||
| Trp107 | Trp107 | |||||
| Linker | Lys136 | |||||
| His137 | His137 | |||||
| Lys139 | Lys139 | |||||
| Arg140 | Arg140 | Arg140 | Arg140 | Arg140 | ||
| Met143 | Met143 | |||||
| Lys145 | ||||||
| Lys146 | Lys146 | Lys146 | ||||
| Lys147 | Lys147 | Lys147 | ||||
| HMG2 | Thr150 | Thr150 | ||||
| Lys156 | Lys156 | Lys156 | Lys156 | |||
| Arg157 | Arg157 | Arg157 | Arg157 | Arg157 | Arg157 | |
| Arg159 | Arg159 | Arg159 | Arg159 | Arg159 | ||
| Tyr162 | Tyr162 | Tyr162 | Tyr162 | Tyr162 | Tyr162 | |
| Asn163 | Asn163 | Asn163 | Asn163 | Asn163 | Asn163 | |
| Val166 | ||||||
| Ala167 | Ala167 | Ala167 | ||||
| Phe170 | ||||||
| Pro178 | Pro178 | Pro178 | Pro178 | |||
| Gln179 | Gln179 | Gln179 | Gln179 | Gln179 | ||
| Leu182 | *** | Leu182 | *** | ** | ||
| Lys186 | Lys186 | |||||
| C-Ter | Trp189 | Trp189 | Trp189 | Trp189 | ||
| Glu208 | Glu208 | Glu208 | ||||
| Tyr211 | Tyr211 | |||||
| Arg232 | Arg232 | Arg232 | ||||
| Arg233 | Arg233 | Arg233 | Arg233 | |||
| Thr234 | Thr234 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozhukhar, N.; Alexeyev, M.F. 35 Years of TFAM Research: Old Protein, New Puzzles. Biology 2023, 12, 823. https://doi.org/10.3390/biology12060823
Kozhukhar N, Alexeyev MF. 35 Years of TFAM Research: Old Protein, New Puzzles. Biology. 2023; 12(6):823. https://doi.org/10.3390/biology12060823
Chicago/Turabian StyleKozhukhar, Natalya, and Mikhail F. Alexeyev. 2023. "35 Years of TFAM Research: Old Protein, New Puzzles" Biology 12, no. 6: 823. https://doi.org/10.3390/biology12060823
APA StyleKozhukhar, N., & Alexeyev, M. F. (2023). 35 Years of TFAM Research: Old Protein, New Puzzles. Biology, 12(6), 823. https://doi.org/10.3390/biology12060823








