Peptide G-Protein-Coupled Receptors and ErbB Receptor Tyrosine Kinases in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
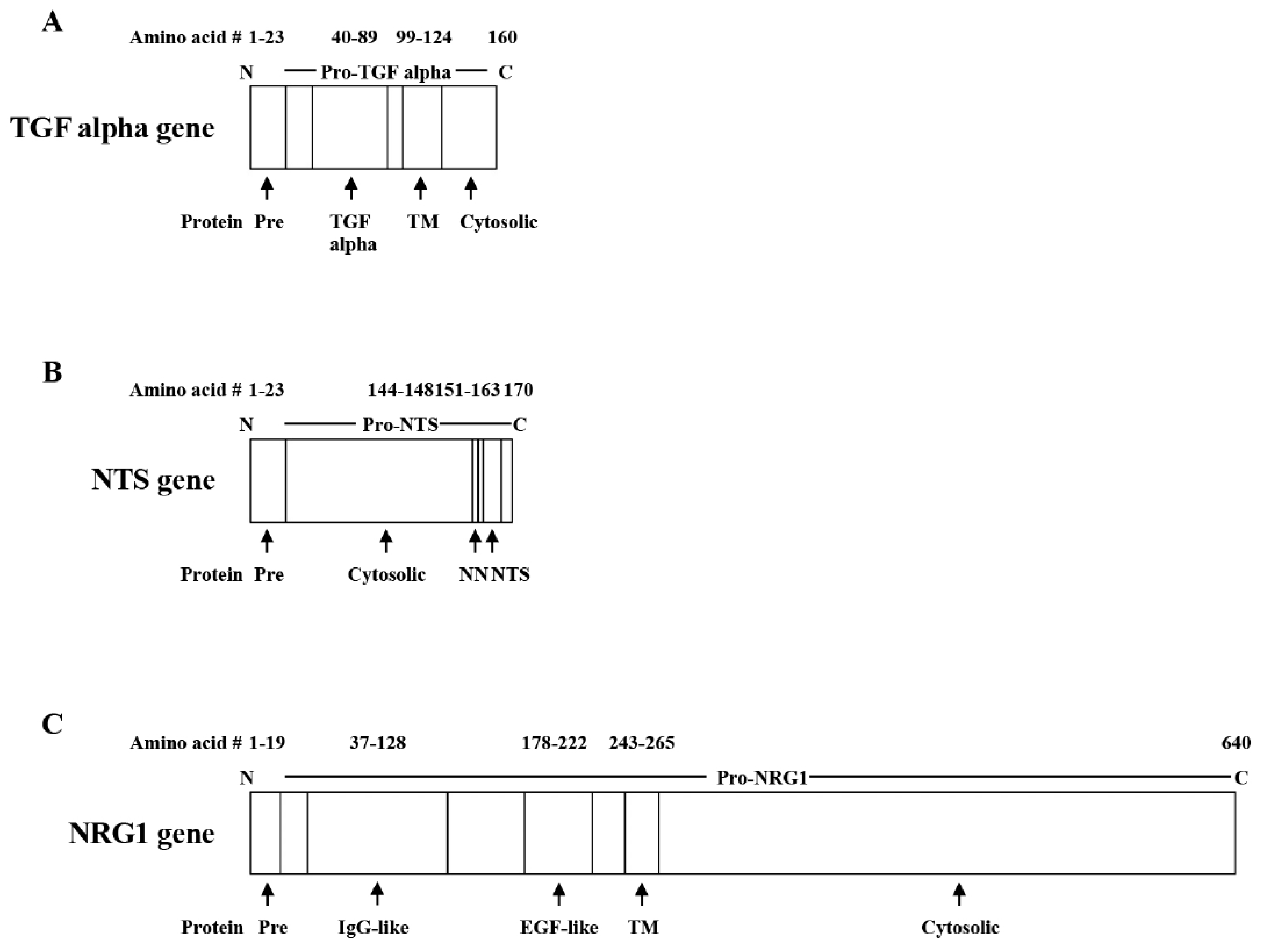
2. RTKs
2.1. EGFR
2.2. HER2
2.3. HER3
2.4. HER4
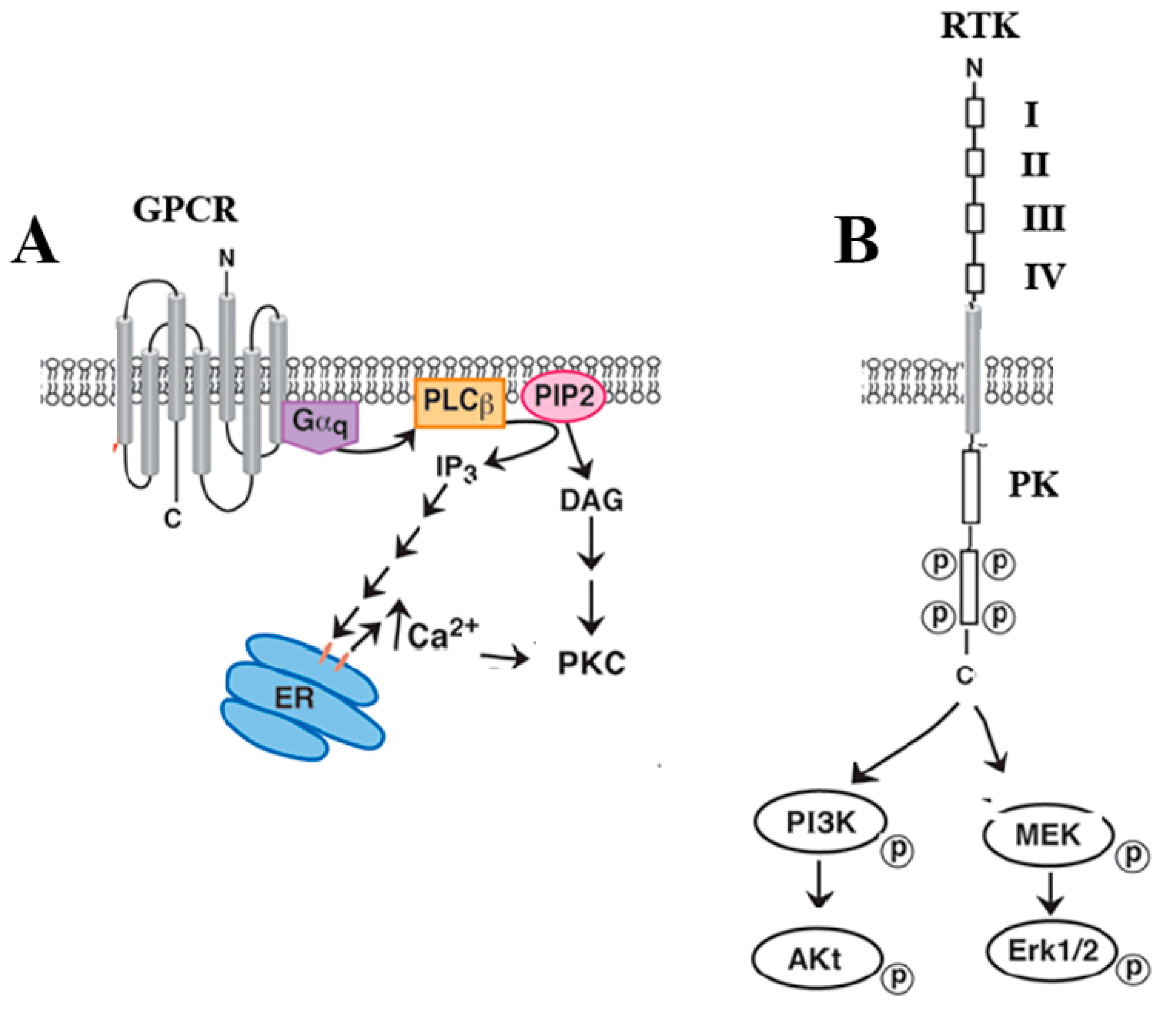
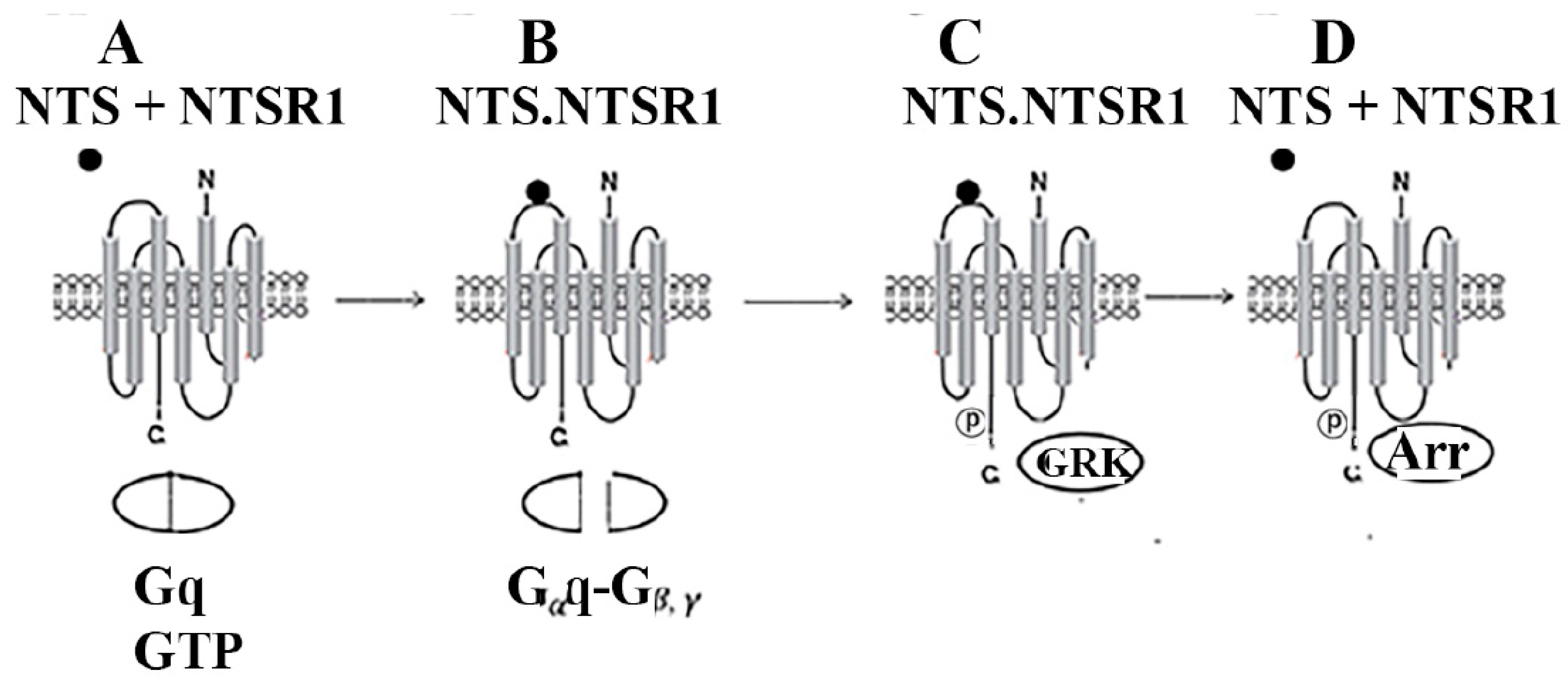
3. GPCRs
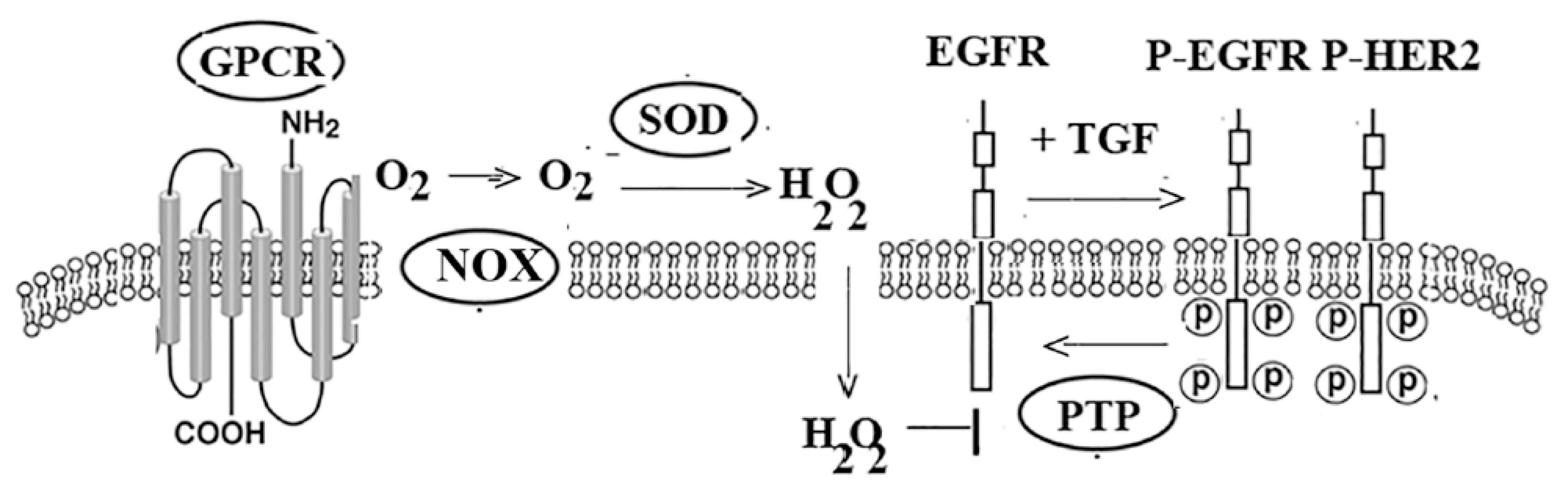
4. Transactivation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z. ErbB receptors and cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [PubMed]
- Yarden, Y.; Pines, G. The ERBB network: At last, cancer therapy meets system biology. Nat. Rev. Cancer 2012, 12, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A.; Schlessinger, J.; Ferguson, K.M. The EGFR family: Not so prototypical receptor tyrosine kinase. Cold Spring Harb. Perspect. Biol. 2014, 6, a020768. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. ErbB/HER protein-tyrosine kinases: Structures and small molecule inhibitors. Pharmacol. Res. 2014, 87, 42–59. [Google Scholar] [CrossRef]
- Herbst, R.S.; Sandler, A. Bevacizumab and erlotinib: A promising new approach to the treatment of advanced NSCLC. Oncologist 2008, 13, 1166–1176. [Google Scholar] [CrossRef]
- Rese, D.M.; Slamon, D.J. HER-2/neu signal transduction in human breast and ovarian cancer. Stem Cells 1997, 15, 1–8. [Google Scholar] [CrossRef]
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The Her2 receptor and breast cancer: Ten years of targeted and anti-Her2 therapy and personalized medicine. Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef]
- Rusnak, D.W.; Affleck, K.; Cockerill, S.G.; Stubberfield, C.; Harris, R.; Page, M.; Smith, K.J.; Guntrip, S.B.; Carter, M.C.; Shaw, R.J.; et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: Potential therapy for cancer. Cancer Res. 2001, 61, 7196–7203. [Google Scholar]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexicity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef]
- Sithanandam, G.; Anderson, L.M. The ERBB3 receptor in cancer and gene therapy. Cancer Gene Ther. 2008, 15, 413–448. [Google Scholar] [CrossRef] [PubMed]
- Reinmuth, N.; Brandt, B.; Kunze, W.P.; Junker, K.; Thomas, M.; Achatzy, R.; Scheld, H.H.; Semik, M. Ploidy, expression of erbB1, ErbB2, p53 and amplification of erbB1, erbB2 and erbB3 in non- small cell lung cancer. Eur. J. Respir. J. 2000, 16, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Hollmen, M.; Liu, P.; Kurppa, K.; Wildiers, H.; Reinvall, I.; Vandorpe, T.; Smeets, A.; Deraedt, K.; Vahlberg, T.; Joensuu, H.; et al. Proteolytic processing of ErbB4 in breast cancer. PLoS ONE 2012, 7, e39413. [Google Scholar] [CrossRef] [PubMed]
- Sartor, C.I.; Zhou, H.; Kozlowska, E.; Guttridge, K.; Kawata, E.; Caskey, L.; Harrelson, J.; Hynes, N.; Ethier, S.; Calvo, B.; et al. HS HER4 mediates ligand-dependent antiproliferative and differentiation responses in human breast cancer cells. Mol. Cell Biol. 2001, 21, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Rauf, F.; Festa, F.; Park, J.G.; Magee, M.; Eaton, S.; Rinaldi, C.; Betanzos, S.M.; Gonzalez-Malerva, L.; LaBaer, J. Ibrutinib inhibition of ERBB4 reduces cell growth in a WNT5A-dependent manner. Oncogene 2018, 37, 2237–2250. [Google Scholar] [CrossRef]
- Mota, J.M.; Collier, K.A.; Costa, R.L.B.; Taxter, T.; Kalyan, A.; Leite, C.A.; Chae, Y.K.; Giles, F.J.; Carneiro, B.A. A comprehensive review of heregulins, HER3 and HER4 as potential therapeutic targets in cancer. Oncotarget 2017, 8, 89284–89306. [Google Scholar] [CrossRef]
- Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Bombesin, endothelin, neurotensin and pituitary adenylate cyclase activating polypeptide cause tyrosine phosphorylation of receptor tyrosine kinases. Peptides 2021, 137, 170480. [Google Scholar] [CrossRef]
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; Waschek, J.A.; Said, S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012, 166, 4–18. [Google Scholar] [CrossRef]
- Forsam, R.T.; Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 2007, 7, 79–94. [Google Scholar]
- Moody, T.W.; Zia, F.; Draoui, M.; Brenneman, D.E.; Fridkin, M.; Davidson, A.; Gozes, I. A vasoactive intestinal peptide antagonist inhibits non-small cell lung cancer growth. Proc. Natl. Acad. Sci. USA 1993, 90, 4345–4349. [Google Scholar] [CrossRef]
- Dupouy, S.; Viardot-Foucault, V.; Alifano, M.; Souazé, F.; Plu-Bureau, G.; Chaouat, M.; Lavaur, A.; Hugol, D.; Gespach, C.; Gompel, A.; et al. The neurotensin receptor-1 pathway contributes to human ductal breast cancer progression. PLoS ONE 2009, 4, e4223. [Google Scholar] [CrossRef]
- Müller, K.M.; Tveteraas, I.H.; Aasrum, M.; Ødegård, J.; Dawood, M.; Dajani, O.; Christoffersen, T.; Sandnes, D.L. Role of protein kinase C and epidermal growth factor receptor signaling in growth stimulation by neurotensin in colon carcinoma cells. BMC Cancer 2011, 11, 421–432. [Google Scholar] [CrossRef]
- Staley, J.; Fiskum, G.; Davis, T.P.; Moody, T.W. Neurotensin elevates cytosolic calcium in small cell lung cancer cells. Peptides 1989, 10, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Chiles, J.; Casibang, M.; Moody, E.; Chan, D.; Davis, T.P. SR48692 is a neurotensin receptor antagonist which inhibits the growth of small cell lung cancer cells. Peptides 2001, 22, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Daub, H.; Weiss, F.U.; Wallasch, C.; Ullrich, A. Role of transactivation of the EGF receptor in signaling by G-protein-coupled receptors. Nature 1996, 379, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Transactivation of epidermal growth factor receptor by G-protein-coupled receptors: Recent Progress, challenges and future research. Int. J. Mol. Sci. 2016, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, C. EGFR receptor activation by GPCRs: A universal pathway reveals different versions. Mol. Cell Endocrinol. 2011, 331, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Sato, J. Cellular functions regulated by phosphorylation of EGFR on Tyr845. Int. J. Mol. Sci. 2013, 14, 10761–10790. [Google Scholar] [CrossRef]
- Catttaneo, F.; Guerra, G.; Parisi, M.; De Marinis, M.; Tafuri, D.; Cinelli, M.; Ammendola, R. Cell-surface receptors transactivation mediated by G protein-coupled receptors. Int. J. Mol. Sci. 2014, 15, 19700–19728. [Google Scholar] [CrossRef]
- Cattaneo, F.; Iaccio, A.; Guerra, G.; Montagnani, S.; Ammendola, R. NADPH-oxidase dependent reactive, oxygen species mediate EGFR transactivation by FPRL1 in WKYMVm-stimulated human lung cancer cells. Free Radic. Biol. Med. 2011, 51, 1126–1136. [Google Scholar] [CrossRef]
- Moody, T.W.; Chan, D.C.; Mantey, S.A.; Moreno, P.; Jensen, R.T. SR48692 inhibits non-small cell lung cancer proliferation in an EGFR receptor-dependent manner. Life Sci. 2014, 100, 25–34. [Google Scholar] [CrossRef]
- Moody, T.W.; Lee, L.; Ramos-Alvarez, I.; Jensen, R.T. Neurotensin receptors regulate transactivation of the EGFR and Her2 in a reactive oxygen species-dependent manner. Eur. J. Pharmacol. 2019, 865, 172735. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Adding neurotensin to non-small cell lung cancer cells increases tyrosine phosphorylation of HER3. Peptides 2022, 156, 170858. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Yarden, Y. EGF-ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Chung, E.; Coffey, R.J. EGFR receptor ligands. Exp. Cell Res. 2003, 2–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Shen, Y.; Li, R. BACE1 dependent neuregulin-1 signaling. An implication for schizophrenia. Mol. Neurosci. 2017, 10, 302. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Plenker, D.; Osada, H.; Sun, R.; Menon, R.; Leenders, F.; Ortiz-Cuaran, S.; Peifer, M.; Bos, M.; Daßler, J.; et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014, 4, 415–422. [Google Scholar] [CrossRef]
- Miettinen, P.J.; Berger, J.E.; Meneses, J.; Phung, Y.; Pederson, R.A.; Werb, Z. Epithelial immunity and multi organ failure in mice lacking epidermal growth factor receptor. Nature 1995, 376, 337–341. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Engelman, J.A. ERBB receptors: From oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 2014, 25, 282–303. [Google Scholar] [CrossRef]
- Blobel, C.P. ADAMs: Key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 2005, 6, 32–43. [Google Scholar] [CrossRef]
- Schlessinger, J. Ligand-induced receptor-mediated dimerization and activation of EGF receptor. Cell 2002, 110, 669–672. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jermal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Chen, Z.; Zhang, C.; Zhong, W. Achievements and futures of immune checkpoint inhibitors in non-small cell lung cancer. Exp. Hematol. Oncol. 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Baosheng, L. Pembrolizumab for the treatment of non-small cell lung cancer: Current status and future discussions. J. Cancer Res. Ther. 2019, 15, 743–750. [Google Scholar] [PubMed]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Santoni-Rugiu, E.; Melchior, L.C.; Urbanska, E.M.; Jakobsen, J.N.; Stricker, K.; Grauslund, M.; Sorensen, J.B. Intrinsic resistance to EGFR-tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: Differences and similarities with acquired resistance. Cancers 2019, 11, 923. [Google Scholar] [CrossRef]
- Spellmon, N.; Li, C.; Yang, Z. Allosterically targeting EGFR drug-resistance gatekeeper mutations. J. Thoracic. Dis. 2017, 9, 1756–1758. [Google Scholar] [CrossRef]
- Hatanpaa, K.J.; Burma, S.; Zhao, D.; Habib, A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging and radioresistance. Neoplasia 2010, 12, 675–684. [Google Scholar] [CrossRef]
- Quesnelle, K.M.; Boehm, A.L.; Grandis, J.R. STAT-mediated EGFR signaling in cancer. J. Cell Biochem. 2007, 102, 311–319. [Google Scholar] [CrossRef]
- Crone, S.A.; Zhao, Y.Y.; Fan, L.; Gu, Y.; Minamisawa, S.; Liu, Y.; Peterson, K.L.; Chen, J.; Kahn, R.; Condorelli, G.J.R., Jr. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat. Med. 2002, 8, 459–465. [Google Scholar] [CrossRef]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Ponde, N.F.; Zardavas, D.; Piccart, M. Progress in adjuvant systemic therapy for breast cancer. Nat. Rev. Clin. Oncol. 2019, 16, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013, 3, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A. Ligand-induced ErbB receptor dimerization. Exp. Cell Res. 2009, 315, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Erickson, S.L.; O’Shea, K.S.; Ghaboosi, N.; Loverro, L.; Frantz, G.; Bauer, M.; Lu, L.H.; Moore, M.W. ErbB3 is required for normal cerebellar and cardiac development. A comparison with ErbB2- and heregulin-deficient mice. Development 1997, 124, 4999–5011. [Google Scholar] [CrossRef]
- Jackson-Fisher, A.J.; Bellinger, G.; Breindel, J.L.; Tavassoli, F.A.; Booth, C.J.; Duong, J.K.; Stern, D.F. ErbB3 is required for ductal morphogenesis in the mouse mammary gland. Breast Cancer Res. 2008, 10, R96. [Google Scholar] [CrossRef]
- Berger, M.B.; Mendrola, J.M.; Lemmon, M.A. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 2004, 569, 332–336. [Google Scholar] [CrossRef]
- Engelman, J.A.; Jänne, P.A.; Mermel, C.; Pearlberg, J.; Mukohara, T.; Fleet, C.; Cichowski, K.; Johnson, B.E.; Cantley, L.C. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc. Natl. Acad. Sci. USA 2005, 102, 3788–3793. [Google Scholar] [CrossRef]
- Yi, E.S.; Harclerode, D.; Gondo, M.; Stephenson, M.; Brown, R.W.; Younes, M.; Cagle, P.T. High c-erbB-3 protein expression is associated with shorter survival in advanced non-small cell lung carcinomas. Mol. Pathol. 1997, 10, 142–148. [Google Scholar]
- Li, Q.; Zhang, R.; Yan, H.; Zhao, P.; Wu, L.; Wang, H.; Li, T.; Cao, B. Prognostic significance of HER3 in patients with malignant solid tumors. Oncotarget 2017, 8, 67140–67151. [Google Scholar] [CrossRef]
- Jaiswal, B.S.; Kljavin, N.M.; Stawiski, E.W.; Chan, E.; Parikh, C.; Durinck, S.; Chaudhuri, S.; Pujara, K.; Guillory, J.; Edgar, K.A.; et al. Oncogenic ERBB3 mutations in human cancers. Cancer Cell 2013, 23, 603–617. [Google Scholar] [CrossRef]
- Liu, J.F.; Ray-Coquard, I.; Selle, F.; Poveda, A.M.; Cibula, D.; Hirte, H.; Hilpert, F.; Raspagliesi, F.; Gladieff, L.; Harter, P.; et al. Randomized phase II trial of serubantumab in combination with erlotinib in patients with EGFR wild-type non-small cell lung cancer. Oncologist 2019, 34, 1095–1102. [Google Scholar]
- Shimizu, T.; Yonesaka, K.; Hayashi, H.; Iwasa, T.; Haratani, K.; Yamada, H.; Ohwada, S.; Kamiyama, E.; Nakagawa, K. Phase I study of new formulation of patritumab (U3-1287) process 2, a fully human anti-HER3 monoclonal antibody in combination with erlotinib in Japanese patients with advanced non-small cell lung cancer. Cancer Chemother. Pharmacol. 2017, 79, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Haikala, H.M.; Janne, P.A. Thirty years of HER3: From basic biology to therapeutic interventions. Clin. Cancer Res. 2021, 27, 3528–3539. [Google Scholar] [CrossRef]
- Sergina, N.V.; Rausch, M.; Wang, D.; Blair, J.; Hann, B.; Shokat, K.M.; Moasser, M.M. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 2007, 445, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Y.; Shen, E.; Cao, F.; Li, L.; Li, X.; Wang, X.; Kariminia, S.; Chang, B.; Li, H.; et al. NRG1-dependent activation of HER3 induces primary resistance to trastuzumab in HER2-overexpressing breast cancer cells. Int. J. Oncol. 2017, 51, 1553–1562. [Google Scholar] [CrossRef]
- Knuefermann, C.; Lu, Y.; Liu, B.; Jin, W.; Liang, K.; Wu, L.; Schmidt, M.; Mills, G.B.; Mendelsohn, J.; Fan, Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003, 22, 3205–3212. [Google Scholar] [CrossRef]
- Jacobsen, H.J.; Poulsen, T.T.; Dahlman, A.; Kjær, I.; Koefoed, K.; Sen, J.W.; Weilguny, D.; Bjerregaard, B.; Andersen, C.R.; Horak, I.D.; et al. Pan-HER, an antibody mixture simultaneously targeting EGFR, HER2 and HER3 effectively overcomes tumor heterogeneity and plasticity. Clin. Cancer Res. 2015, 21, 4110–4122. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Patel, H.; Alanazi, S.; Yuan, L.; Garrett, J.T. HER3 signaling and targeted therapy in cancer. Oncol. Rev. 2018, 12, 45–62. [Google Scholar] [CrossRef]
- Muraoka-Cook, R.S.; Feng, S.M.; Strunk, K.E.; Earp, H.S. ErbB4/HER4: Role in mammary gland development, differentiation and growth inhibition. J. Mammary Gland. Biol. Neoplasia 2008, 13, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.M.; Dugaucquier, L.; Feyen, E.; Shakeri, H.; DeKeulenaer, G.W. The role of ErbB4 in cancer. Cell. Oncol. 2020, 43, 335–352. [Google Scholar] [CrossRef]
- Tidcombe, H.; Jackson-Fisher, A.; Mathers, K.; Stern, D.F.; Gassmann, M.; Golding, J.P. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc. Natl. Acad. Sci. USA 2003, 100, 8281–8286. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Pan, B.; Engel, M.; Huang, X.F. Neuregulin-1 signaling and antipsychotic treatment: Potential therapeutic targets in schizophrenia candidate signalling pathway. Psychopharmacology 2013, 226, 201–215. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Mewafi, N.H.; Abdelmotteleb, N.E.; Emara, M.A.; Tarazi, H.; Sbenati, R.M.; Madkour, M.M.; Zaraei, S.O.; Shahin, A.I.; Anbar, H.S. A review of HER4 (ErbB4) kinase, its impact on cancer and its inhibitors. Molecules 2021, 26, 7376. [Google Scholar] [CrossRef]
- Gilbertson, R.; Hernan, R.; Pietsch, T.; Pinto, L.; Scotting, P.; Allibone, R.; Ellison, D.; Perry, R.; Pearson, A.; Lunec, J. Novel ERBB4 juxtamembrane splice variants are frequently expressed in childhood medulloblastoma. Genes Chromosom. 2001, 31, 288–294. [Google Scholar] [CrossRef]
- Kaiulaoinen, V.; Sundvall, J.A.; Maatta, E.; Santiestevan, M.; Klagsbrun, K.; Elenius, A. A natural ErbB4 isoform that does not activate phosphoinositide 3-kinase mediates proliferation but not survival or chemotaxis. J. Biol. Chem. 2000, 275, 8641–8649. [Google Scholar] [CrossRef] [PubMed]
- Rio, C.; Buxbaum, J.D.; Peschon, J.J.; Corfas, G. Tumor necrosis factor α-converting enzyme is required for cleavage of erbB4/HER4. J. Biol. Chem. 2000, 275, 10379–10387. [Google Scholar] [CrossRef]
- Junttila, T.T.; Sundvall, M.; Lundin, J.; Lundin, M.; Tanner, P.; Harkonen, H.; Joensuu, H.; Isola, J.; Elenius, K. Cleavable ErbB isoform in estrogen-receptor regulated growth of breast cancer cells. Cancer Res. 2005, 65, 1384–1393. [Google Scholar] [CrossRef]
- Sepp-Lorenzino, L.; Eberhard, I.; Ma, Z.; Cho, C.; Serve, H.; Liu, F.; Rosen, N.; Lupu, R. Signal transduction pathways induced by Heregulin in MDA-MB-453 breast cancer cells. Oncogene 1996, 12, 1679–1687. [Google Scholar]
- Farrens, D.L.; Altenbach, C.; Yang, K.; Hubbell, W.L.; Korana, H.G. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science 1996, 274, 768–770. [Google Scholar] [CrossRef]
- Carman, C.V.; Benovic, J.L. G-protein-coupled receptors: Turn-ons and turn-offs. Curr. Opin. Neurobiol. 1998, 8, 335–344. [Google Scholar] [CrossRef]
- Inagaki, S.; Ghirlando, R.; Vishnivetskiy, S.A.; Homan, K.T.; White, J.F.; Tesmer, J.J.; Gurevich, V.V.; Grisshammer, R. G protein-coupled receptor kinase 2 (GRK2) and (GRK5) exhibit selective phosphorylation of the neurotensin receptor in vitro. Biochemistry 2015, 54, 4320–4329. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Masureel, M.; Qu, Q.; Janetzko, J.; Inoue, A.; Kato, H.E.; Robertson, M.J.; Nguyen, K.C.; Glenn, J.S.; Skiniotis, G.; et al. Structure of neurotensin receptor 1 complex with β-arrestin 1. Nature 2020, 579, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Shenoy, S.K. GPCR desensitization. Cell Signal 2018, 41, 9–16. [Google Scholar] [CrossRef]
- Kitabgi, P. Neurotensin and neuromedin N are differentially processed from a common precursor by prohormone convertases in tissues and cell lines. Results Prob. Cell Differ. 2010, 50, 85–96. [Google Scholar]
- Wu, Z.; Fournel, L.; Stadler, N.; Liu, J.; Boullier, A.; Hoyeau, N.; Fléjou, J.F.; Duchatelle, V.; Djebrani-Oussedik, N.; Agopiantz, M.; et al. Modulation of lung cancer cell plasticity and heterogeneity with restoration of cisplatin sensitivity by neurotensin antibody. Cancer Lett. 2017, 388, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Carraway, R.; Leeman, S. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalamus. J. Biol. Chem. 1973, 248, 6854–6861. [Google Scholar] [CrossRef] [PubMed]
- Kitabgi, P. Functional domains of the subtype neurotensin receptor (NTR1). Peptides 2006, 37, 2461–2468. [Google Scholar] [CrossRef]
- White, J.F.; Noinaj, N.; Shibata, Y.; Love, J.; Kloss, B.; Xu, F.; Gvozdenovic-Jeremic, J.; Shah, P.; Shiloach, J.; Tate, C.G.; et al. Structure of the agonist bound neurotensin receptor. Nature 2012, 490, 508–513. [Google Scholar] [CrossRef]
- Kisfalvi, K.; Guha, S.; Rozengurt, E. Neurotensin and EGF induce synergistic simulation of DNA synthesis by increasing the duration of ERK signaling to ductal pancreatic cancer cells. J. Cell Physiol. 2005, 202, 880–890. [Google Scholar] [CrossRef]
- Ye, Y.; Long, X.; Zhang, L.; Chen, J.; Lin, P.; Li, H.; Wei, F.; Yu, W.; Ren, X.; Yu, J. NTS/NTSR1 over-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/b-catenin signaling pathway in hepatocellular carcinoma. Oncotarget 2016, 7, 70303–70322. [Google Scholar] [CrossRef]
- Evers, B.M. Neurotensin and growth of normal and neoplastic tissues. Peptides 2006, 27, 2424–2433. [Google Scholar] [CrossRef] [PubMed]
- Alifano, M.; Souazé, F.; Dupouy, S.; Camilleri-Broët, S.; Younes, M.; Ahmed-Zaïd, S.-M.; Takahashi, T.; Cancellieri, A.; Damiani, S.; Boaron, M.; et al. Neurotensin receptor 1 determines the outcome of non-small cell lung cancer. Clin. Cancer Res. 2010, 16, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Tsukada, J.; Sugimoto, T.; Kikkawa, N.; Sasaki, K.; Chazono, H.; Hanazawa, T.; Okamoto, Y.; Seki, N. Identification of a novel therapeutic targets for head and neck squamous cell carcinomas: A role for the neurotensin-neurotensin receptor 1 oncogenic signaling pathway. Int. J. Cancer 2008, 123, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Dobner, P.R.; Carraway, R.E. Involvement of MAP kinase, PI-3-kinase and EGF-receptor in stimulatory effect of neurotensin on DNA synthesis in PC3 cells. Regul. Pept. 2004, 120, 155–166. [Google Scholar] [CrossRef]
- Ostrom, R.S.; Insel, P.A. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: Implications for molecular pharmacology. Br. J. Pharmacol. 2004, 143, 235–245. [Google Scholar] [CrossRef]
- Burke, P.; Schooler, K.; Wiley, H.S. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol. Biol. Cell 2001, 12, 1897–1910. [Google Scholar] [CrossRef]
- Kilpatrick, L.E.; Hill, S.J. Transactivation of G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs): Recent insights using luminescence and fluorescence technologies. Curr. Opin. Endocr. Metab. Res. 2021, 16, 102–112. [Google Scholar] [CrossRef]
- Almendro, V.; Garcia-Recio, S.; Gascon, P. Tyrosine kinase receptor transactivation associated to G protein-coupled receptors. Curr. Drug Targets 2010, 11, 1169–1180. [Google Scholar] [CrossRef]
- Zhou, Z.; Xie, J.; Cai, Y.; Yang, S.; Chen, Y.; Wu, H. The significance of NTR1 expression and its correlation with β-catenin and EGFR in gastric cancer. Diagn. Pathol. 2015, 10, 128. [Google Scholar] [CrossRef]
- DiFlorio, A.; Sancho, V.; Moreno, P.; Delle Fave, G.; Jensen, R.T. Gastrointestinal hormones stimulate the growth of foregut neuroendocrine tumors by transactivating the EGF receptor. Biochem. Biophys. Acta 2013, 1833, 573–582. [Google Scholar] [CrossRef]
- Younes, M.; Wu, Z.; Dupouy, S.; Lupo, A.M.; Mourra, N.; Takahashi, T.; Fléjou, J.F.; Trédaniel, J.; Régnard, J.F.; Damotte, D.; et al. Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 overexpression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib. Oncotarget 2014, 5, 8252–8269. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Hsien, C.; Yung-Ho, H.; Yuh-Mou, S.; Chun-Cheng, H.; Horng-Mo, L.; Huei-Mei, H.; Tso-Hsiao, C. Src homology 2-containing phosphotyrosine phosphatase regulates endothelin-1-induced epidermal growth factor receptor transactivation in rat renal tubular cell NRK-52E. Pflug. Arch. 2006, 452, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Heppner, D.E.; Van der Vliet, A. Redox-dependent regulation of epidermal growth factor receptor signaling. Redox Biol. 2016, 8, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Ramso-Alvarez, I.; Mantey, S.A.; Jensen, R.T. Bombesin Receptors Regulate Transactivation of HER4. Cancer Res. 2022, 82 (Suppl. 12), 2688. [Google Scholar] [CrossRef]
- Lee, L.; Ramos-Alvarez, I.; Moody, T.W.; Mantey, S.A.; Jensen, R.T. Neuropeptide bombesin receptor activation stimulates growth of lung cancer cells through HER3 with a MAPK-dependent mechanism. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118625. [Google Scholar] [CrossRef]
- Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Neuropeptide G protein-coupled receptors as oncotargets. Front. Endocrinol. 2018, 9, 345. [Google Scholar] [CrossRef]
- Valdehita, A.; Bajo, A.M.; Schally, A.V.; Varga, J.L.; Carmena, M.J.; Prieto, J.C. Vasoactive intestinal peptide (VIP) induces transactivation of EGFR and HER2 in human breast cancer cells. Mol. Cell Endocrinol. 2009, 302, 41–48. [Google Scholar] [CrossRef]
- Moody, T.W.; Lee, L.; Jensen, R.T. The G protein-coupled receptor PAC1 regulates transactivation of the receptor tyrosine kinase HER3. J. Mol. Neurosci. 2021, 71, 1589–1597. [Google Scholar] [CrossRef]
- Moody, T.W.; Osefo, N.; Nuche-Berengeur, B.; Ridnour, L.; Wink, D.; Jensen, R.T. Pituitary adenylate cyclase activating polypeptide causes tyrosine phosphorylation of the epidermal growth factor receptor in lung cancer cells. J. Pharmacol. Exp. Ther. 2012, 341, 873–881. [Google Scholar] [CrossRef]
- Moody, T.W.; Osefo, N.; Nuche-Berenguer, B.; Ridnour, L.; Wink, D.; Jensen, R.T. PAC1 regulates receptor tyrosine kinase transactivation in a reactive oxygen species-dependent manner. Peptides 2019, 120, 170017. [Google Scholar] [CrossRef]
- Cattaneo, F.; Parisi, M.; Ammendola, R. WKYMVm-induced cross-talk between FRP2 and HGF receptor in human prostate epithelial cell line PNT1A. FEBS Lett. 2013, 587, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Castaido, M.; Parisi, M.; Faratonio, R.; Esposito, G.; Ammendola, R. Formyl peptide receptor 1 modulates endothelial cell functions by NADPH Oxidase-dependent VEGFR2 transactivation. Oxid. Med. Cell Longev. 2018, 2018, 2609847. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, M.; Zollo, C.; Esposito, G.; Amendola, R.; Cattaneo, F. Nox2-dependent reactive oxygen species regulate formyl-peptide receptor 1-mediated TrkA transactivation in SH-SY5Y cells. Oxid. Med. Cell Longev. 2019, 2019, 2051235. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative stress and cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Whitehouse, S.; Jaquet, V. Challenges, progress and promises for developing future NADPH oxidase therapeutics. Antioxid Redox Signal 2015, 23, 355–357. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant therapy in cancer: Rationale and progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef]
- Augsburger, F.; Filippova, A.; Rasti, D.; Seredenina, T.; Lam, M.; Maghzal, G.; Mahiout, Z.; Jansen-Dürr, P.; Knaus, U.G.; Doroshow, J.; et al. Pharmacological characterization of the seven human NOX isoforms and their inhibitors. Redox Biol. 2019, 26, 101272. [Google Scholar] [CrossRef]
- Cifuentes-Pagano, E.; Csanyi, G.; Pagano, P.J. NADPH oxidase inhibitors: A decade of discovery from Nox2ds to HTS. Cell Mol. Life Sci. 2012, 69, 2315–2325. [Google Scholar] [CrossRef]
| RTK Activated | Mechanism | Cancer Type | Reference |
|---|---|---|---|
| EGFR | MMP | Head and neck cancer | [93] |
| EGFR | Arachidonic acid release | Prostate cancer | [94] |
| EGFR | TKI inhibit | Neuroendocrine | [100] |
| EGFR | β–χατενιν | Gastric | [99] |
| EGFR | ERK | NSCLC | [30] |
| HER2 | ROS | NSCLC | [31] |
| HER3 | ERK + AKT | NSCLC | [32] |
| EGFR, HER2, HER3 | TKI inhibit | NSCLC | [101] |
| Addition | % ROS | % PY1068-EGFR | %PY1248-HER2 |
|---|---|---|---|
| None | 100 + 7 | 100 + 8 | 100 + 6 |
| NTS | 228 + 9 ** | 355 + 26 ** | 285 + 19 ** |
| NTS + DPI | 117 + 15 | 112 + 6 | 107 + 7 |
| NTS + SR48692 | 108 + 6 | 106 + 7 | 111 + 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moody, T.W.; Ramos-Alvarez, I.; Jensen, R.T. Peptide G-Protein-Coupled Receptors and ErbB Receptor Tyrosine Kinases in Cancer. Biology 2023, 12, 957. https://doi.org/10.3390/biology12070957
Moody TW, Ramos-Alvarez I, Jensen RT. Peptide G-Protein-Coupled Receptors and ErbB Receptor Tyrosine Kinases in Cancer. Biology. 2023; 12(7):957. https://doi.org/10.3390/biology12070957
Chicago/Turabian StyleMoody, Terry W., Irene Ramos-Alvarez, and Robert T. Jensen. 2023. "Peptide G-Protein-Coupled Receptors and ErbB Receptor Tyrosine Kinases in Cancer" Biology 12, no. 7: 957. https://doi.org/10.3390/biology12070957
APA StyleMoody, T. W., Ramos-Alvarez, I., & Jensen, R. T. (2023). Peptide G-Protein-Coupled Receptors and ErbB Receptor Tyrosine Kinases in Cancer. Biology, 12(7), 957. https://doi.org/10.3390/biology12070957






