Specific Blood Plasma Circulating miRs Are Associated with the Physiological Impact of Total Fish Meal Replacement with Soybean Meal in Diets for Rainbow Trout (Oncorhynchus mykiss)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Diets
2.3. Experimental Design and Rearing Conditions
2.4. Fish Sampling and Growth Performance Assessment
2.5. Chemical Analyses in Diets, Feces, and Fish Fillets
2.6. Digestive Enzymes Activity
2.7. Histopathological Analysis
2.8. Circulating Non-Coding RNAs Analysis
2.8.1. Isolation, Library Preparation, and Sequencing
2.8.2. Bioinformatic Analysis
2.8.3. mRNA Target Prediction
2.9. Statistical Analysis
3. Results
3.1. Fish Growth Performance, Apparent Digestibility, and Fillet Composition
3.2. Digestive Enzyme Activities
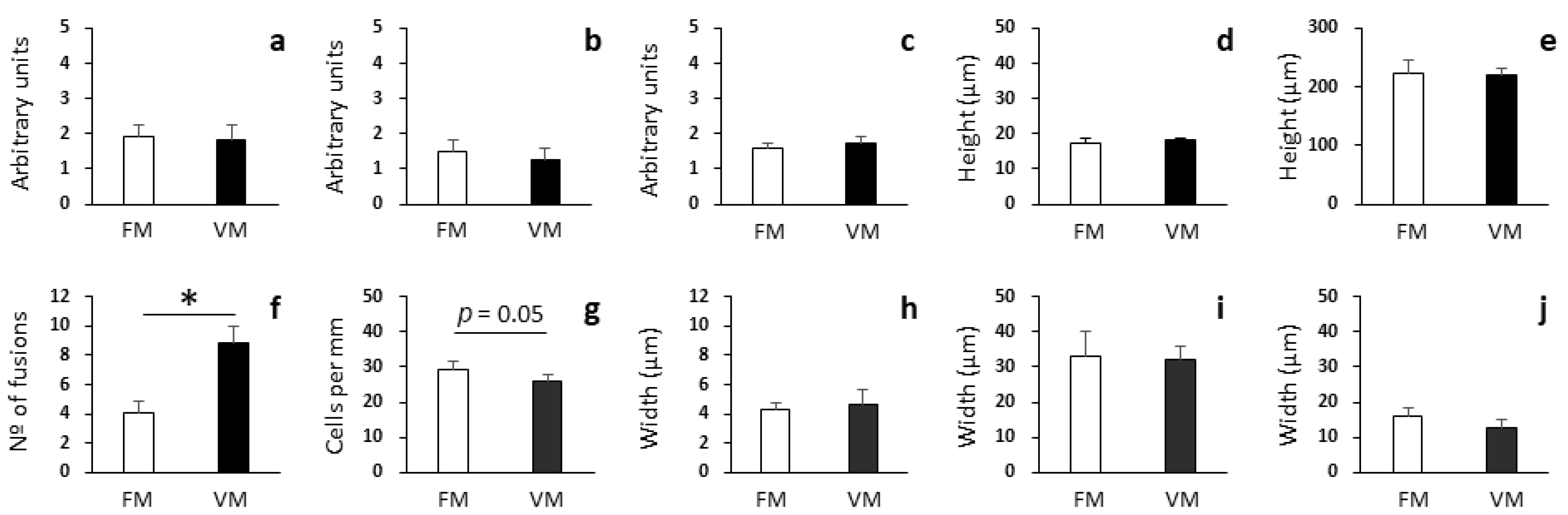
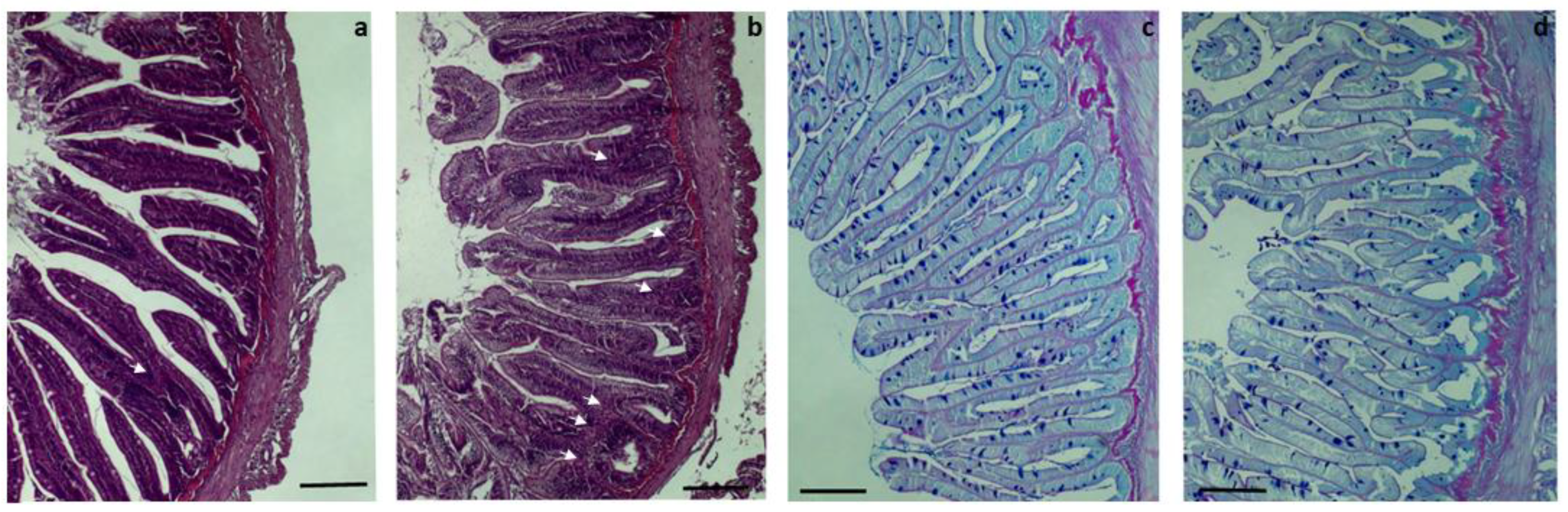
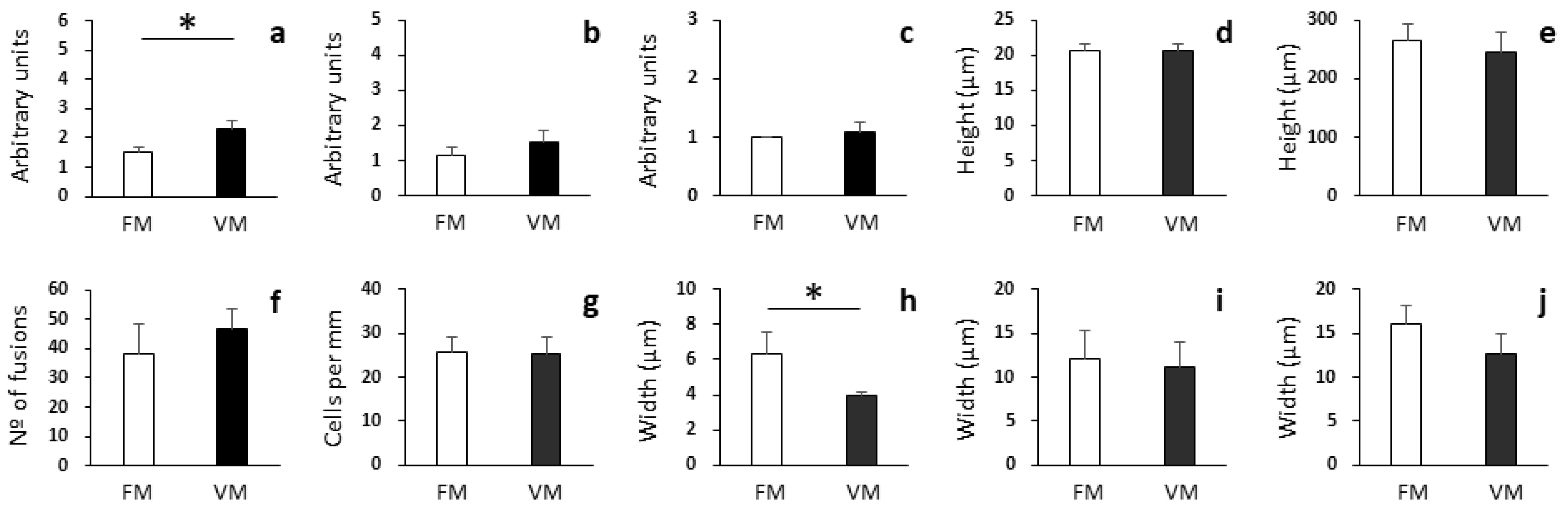
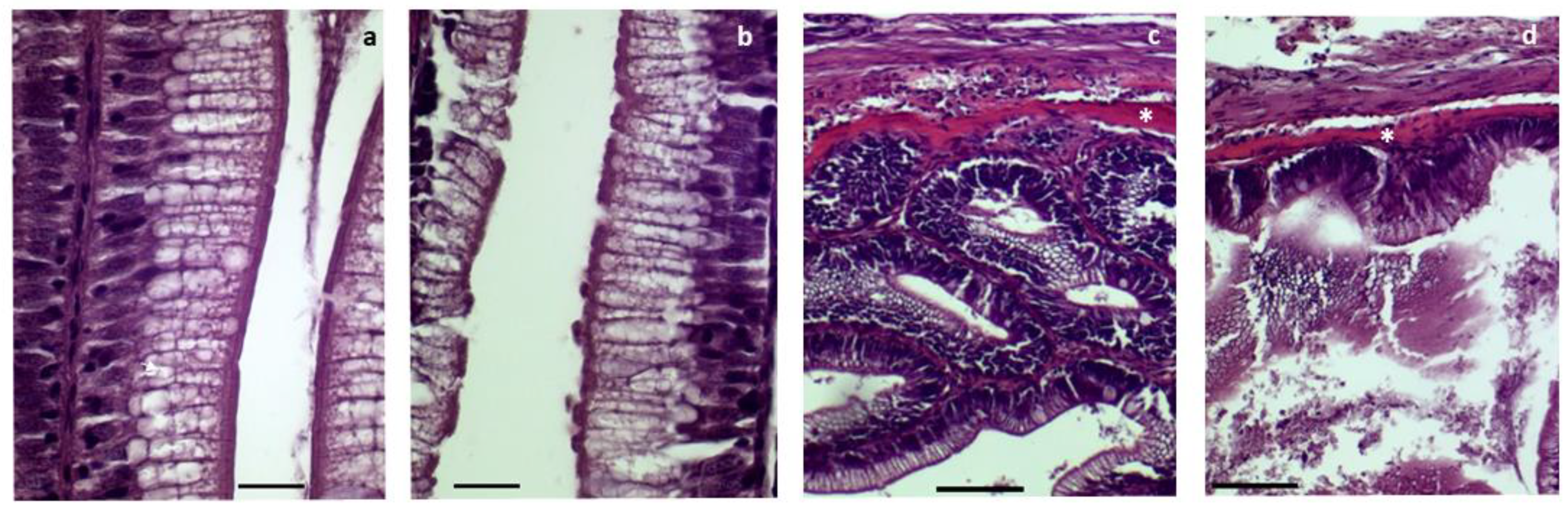
3.3. Histopathological Analysis of Proximal and Distal Intestines
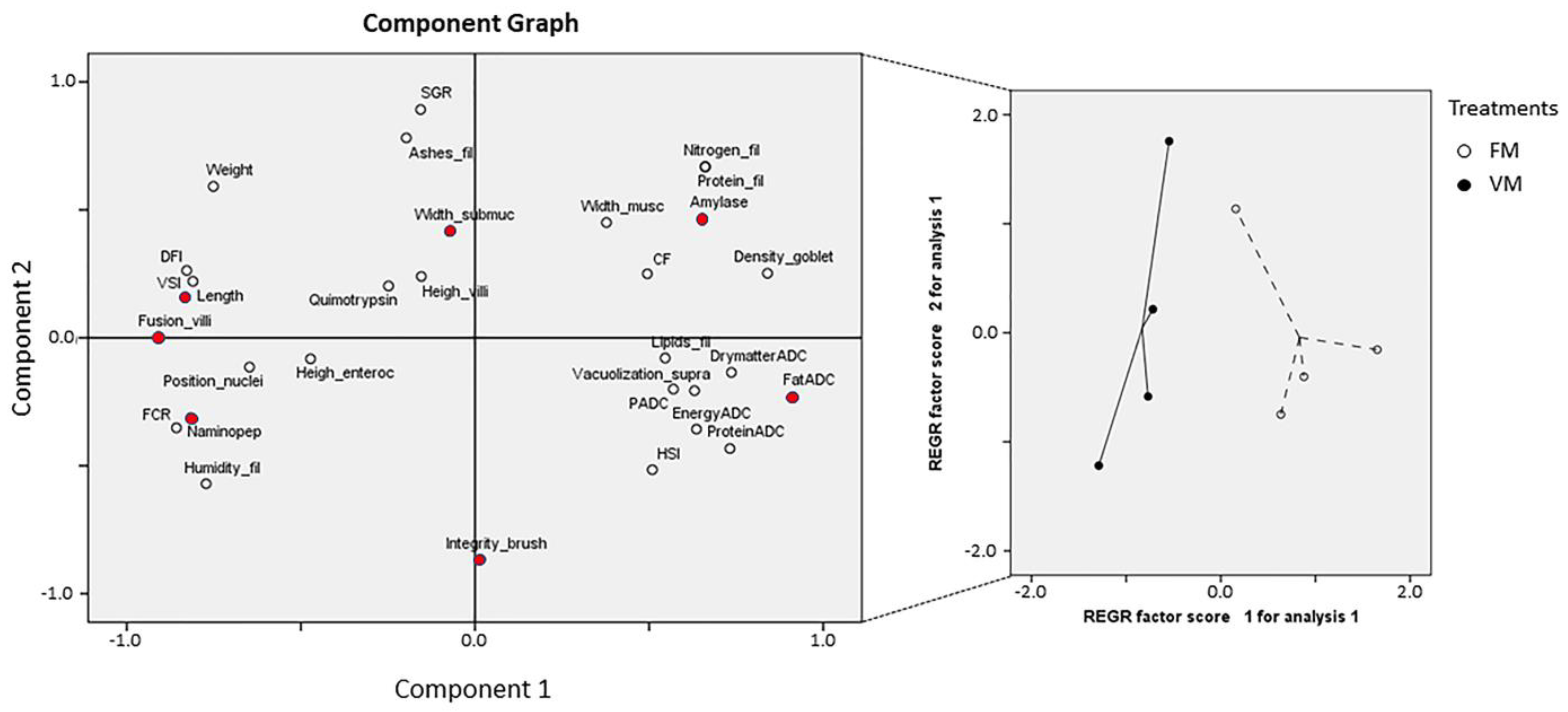
3.4. PCA of Growth Performance, Proximal Composition, Enzyme Activities, and Histopathological Parameters
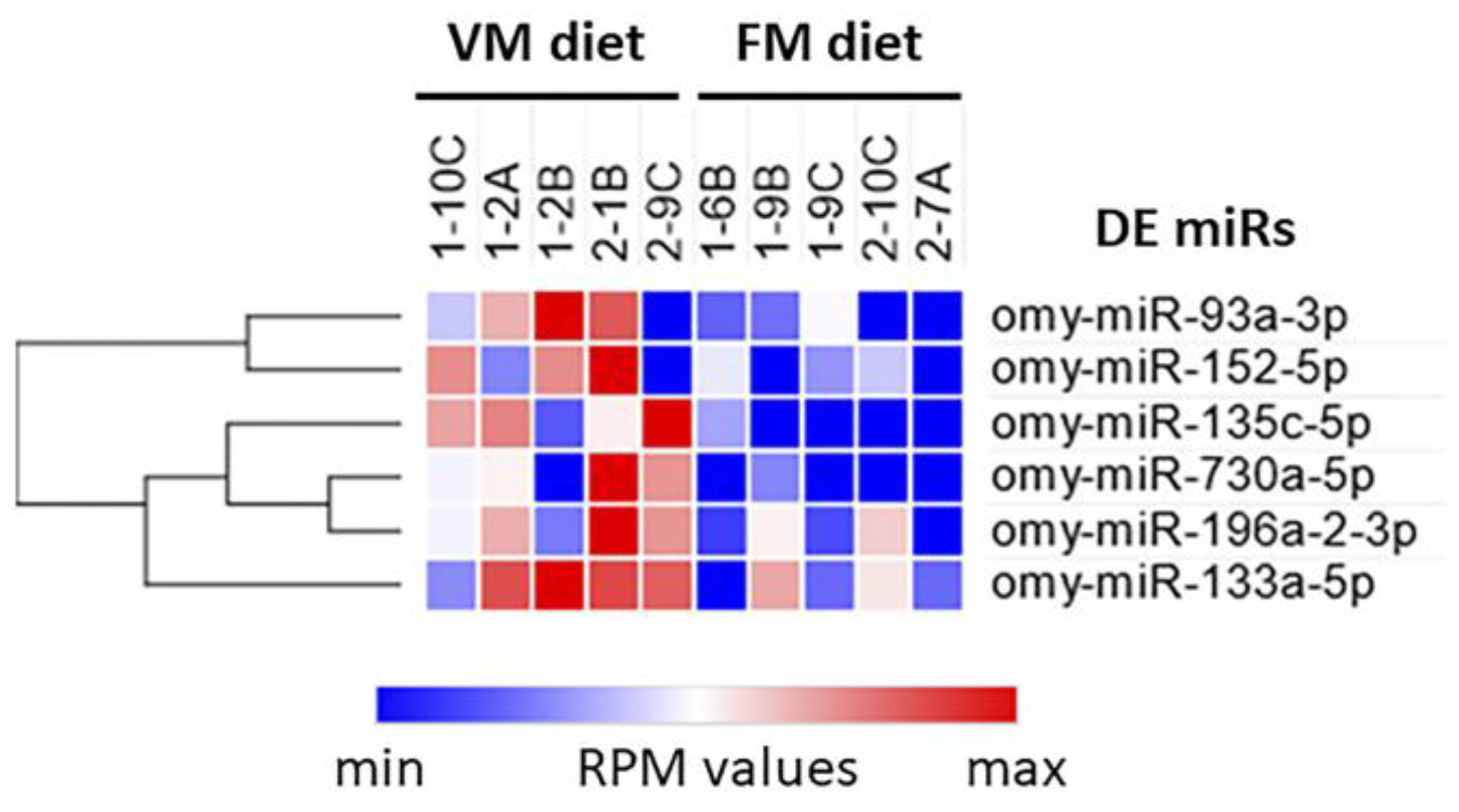
3.5. Circulating Non-Coding RNAs
4. Discussion
4.1. FM Replacement with High SBM Content Impacts Rainbow Trout Physiology
4.2. The Expression of Particular Circulating miRs from Blood Plasma Is Associated with High Dietary SBM Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World, Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat. Food 2020, 1, 301–308. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. N. Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Santigosa, E.; Constant, D.; Prudence, D.; Wahli, T.; Verlhac-Trichet, V. A novel marine algal oil containing both EPA and DHA is an effective source of omega-3 fatty acids for rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2020, 51, 649–665. [Google Scholar] [CrossRef]
- Hemre, G.-I.; Amlund, H.; Aursand, M.; Bakkee, A.M.; Olsen, R.E.; Ringoe, E.; Svihus, B.; Bernhoft, A.; Jenssen, B.M.; Moretro, T.; et al. Criteria for safe use of plant ingredients in diets for aquacultured fish. Eur. J. Nutr. Food Saf. 2018, 8, 240–242. [Google Scholar] [CrossRef]
- Glencross, B.D.; Baily, J.; Berntssen, M.H.G.; Hardy, R.; MacKenzie, S.; Tocher, D.R. Risk assessment of the use of alternative animal and plant raw material resources in Aquaculture feeds. Rev. Aquac. 2020, 12, 703–758. [Google Scholar] [CrossRef]
- Luthada-Raswiswi, R.; Mukaratirwa, S.; O’Brien, G. Animal protein sources as a substitute for fishmeal in aquaculture diets: A systematic review and Meta-Analysis. Appl. Sci. 2021, 11, 3854. [Google Scholar] [CrossRef]
- Kumar, V.; Hossain, M.S.; Ragaza, J.A.; Benito, M.R. The potential impacts of soy protein on fish gut health. In Soybean for Human Consumption and Animal Feed; Intech Open: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Klinger, D.; Naylor, R. Searching for solutions in aquaculture: Charting a sustainable course. Annu. Rev. Environ. Resour. 2012, 37, 247–276. [Google Scholar] [CrossRef]
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Olsen, R.E.; Myklebust, R.; Ringo, E. Dietary effect of soybean (Glycine max) products on gut histology and microbiota of fish. In Soybean and Nutrition; El-Shemy, P.H., Ed.; InTech Open: Rijeka, Croatia, 2011; pp. 231–250. [Google Scholar] [CrossRef]
- Kortner, T.; Gu, J.; Krogdahl, Å.; Bakke, A. Transcriptional regulation of cholesterol and bile acid metabolism after dietary soyabean meal treatment in Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2013, 109, 593–604. [Google Scholar] [CrossRef]
- De Santis, C.; Bartie, K.L.; Olsen, R.E.; Taggart, J.B.; Tocher, D.R. Nutrigenomic profiling of transcriptional processes affected in liver and distal intestine in response to a soybean meal-induced nutritional stress in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Król, E.; Douglas, A.; Tocher, D.R.; Crampton, V.O.; Speakman, J.R.; Secombes, C.J.; Martin, S.A.M. Differential responses of the gut transcriptome to plant protein diets in farmed Atlantic salmon. BMC Genom. 2016, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Treiber, T.; Treiber, N.; Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat. Rev. Mol. Cell Biol. 2019, 20, 5–20. [Google Scholar] [CrossRef]
- Toledo-Solís, F.J.; Fernandes, M.O.J.; Sarropoulou, E.; Fernández, I.; Noncoding, R.N. As in fish physiology and development: miRNAs as a cornerstone in gene networks (chapter). In Cellular and Molecular, Approaches in Fish, Biology; Fernández, I., Fernandes, M.O.J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 105–159. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, H.; Li, T.; He, L.; Zong, J.; Shan, H.; Huang, L.; Zhang, Y.; Liu, H.; Jiang, J. Profiling miRNAs of teleost fish in responses to environmental stress: A review. Biology 2023, 12, 388. [Google Scholar] [CrossRef] [PubMed]
- Østbye, T.K.; Berge, G.M.; Lundberg, C.E. Modulation of hepatic miRNA expression in Atlantic salmon (Salmo salar) by family background and dietary fatty acid composition. J. Fish Biol. 2021, 98, 1172–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Corraze, G.; Plagnes-Juan, E.; Montfort, J.; Bobe, J. MicroRNAs related to cholesterol metabolism affected by vegetable diet in rainbow trout (Oncorhynchus mykiss) from control and selected lines. Aquaculture 2019, 498, 132–142. [Google Scholar] [CrossRef]
- Abernathy, J.; Overturf, K. Expression of antisense long noncoding RNAs as potential regulators in rainbow trout with different tolerance to plant-based diets. Anim. Biotechnol. 2019, 30, 87–94. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ye, G.; Chi, S.; Tan, B.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S. Integrative transcriptomic and small RNA sequencing reveals immune-related miRNA–mRNA regulation network for soybean meal-induced enteritis in hybrid grouper, Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂. Front. Immunol. 2020, 11, 1502. [Google Scholar] [CrossRef]
- Turchinovich, A.; Tonevitsky, A.G.; Burwinkel, B. Extracellular miRNA: A collision of two paradigms. Trends Biochem. Sci. 2016, 41, 883–892. [Google Scholar] [CrossRef]
- Fernández, I.; Fernandes, J.M.O.; Roberto, V.P.; Kopp, M.; Oliveira, C.; Riesco, M.F.; Dias, J.; Cox, C.J.; Leonor Cancela, M.; Cabrita, E.; et al. Circulating small non-coding RNAs provide new insights into vitamin K nutrition and reproductive physiology in teleost fish. Biochim. Biophys. Acta-Gen. Subj. 2019, 1863, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.C.V.; Fatsini, E.; Fernández, I.; Anjos, C.; Chauvigné, F.; Cerdá, J.; Mjelle, R.; Fernandes, J.M.O.; Cabrita, E. Kisspeptin influences the reproductive axis and circulating levels of microRNAs in Senegalese, Sole. Int. J. Mol. Sci. 2020, 21, 9051. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; Galaz, M.; Ojeda, N.; Marshall, S.H. Detection of cellular miRNAs in plasma of Salmo salar during an ISAV infection. Aquac. Rep. 2020, 17, 100320. [Google Scholar] [CrossRef]
- Cardona, E.; Guyomar, C.; Desvignes, T.; Montfort, J.; Guendouz, S.; Postlethwait, J.H.; Skiba-Cassy, S.; Bobe, J. Circulating miRNA repertoire as a biomarker of metabolic and reproductive states in rainbow trout. BMC Biol. 2021, 19, 235. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Tomás-Almenar, C.; Toledo-Solís, F.J.; Larrán, A.M.; de Mercado, E.; Alarcón, F.J.; Rico, D.; Martín-Diana, A.B.; Fernández, I. Effects and safe inclusion of narbonne vetch (Vicia narbonensis) in rainbow trout (Oncorhynchus mykiss) diets: Towards a more sustainable aquaculture. Animals 2020, 10, 2175. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Offcial Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- OJEU. Laying down the Methods of Sampling and Analysis for the Offcial Control of Feed, Commision Regulation (EC) No 152/2009. 2009. Available online: http://data.europa.eu/eli/reg/2009/152/oj (accessed on 20 May 2022).
- Brett, J.R.; Groves, T.D.D. Physiological energetics. Fish Physiol. 1979, 8, 279–352. [Google Scholar] [CrossRef]
- Gisbert, E.; Nolasco, H.; Solovyev, M. Towards the standardization of brush border purification and intestinal alkaline phosphatase quantification in fish with notes on other digestive enzymes. Aquaculture 2018, 487, 102–108. [Google Scholar] [CrossRef]
- DelMar, E.G.; Largman, C.; Broderick, J.W.; Geokas, M.C. A sensitive new substrate for chymotrypsin. Anal. Biochem. 1979, 99, 316–320. [Google Scholar] [CrossRef]
- Métais, P.; Bieth, J. Determination of alpha-amylase by a microtechnic. Ann. Biol. Clin. 1968, 26, 133–142. [Google Scholar]
- Maraux, S.; Louvard, D.; Baratti, J. The aminopeptidase from hog-intestinal brush border. Biochem. Biophys. Acta 1973, 321, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Martoja, R.; Leland, C.G.; Martoja-Pierson, M. Técnicas de Histología Animal; S.A. Toray-Masson: Barcelona, Spain, 1970; pp. 1–350. [Google Scholar]
- Pearse, A.G.E. Histochemistry. Theoretical and Applied, 4th ed.; Analytical Technology; Churchill Livingstone: New York, NY, USA, 1985; Volume 2, pp. 1–624. [Google Scholar]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier BV: London, UK, 2019. [Google Scholar]
- Knudsen, D.; Urán, P.; Arnous, A.; Koppe, W.; Frøkiær, H. Saponin-containing subfractions of soybean molasses induce enteritis in the distal intestine of Atlantic salmon. J. Agric. Food Chem. 2007, 55, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, E.; Ortiz-Delgado, J.B.; Sarasquete, C. Nutritional cellular biomarkers in early life stages of fish. Histol. Histopathol. 2008, 23, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef]
- RNAcentral Consortium. RNAcentral 2021: Secondary structure integration, improved sequence search and new member databases. Nucleic Acids Res. 2021, 49, D212–D220. [Google Scholar] [CrossRef]
- Gao, G.; Magadan, S.; Waldbieser, G.C.; Youngblood, R.C.; Wheeler, P.A.; Scheffler, B.E.; Thorgaard, G.H.; Palti, Y. A long reads-based de-novo assembly of the genome of the Arlee homozygous line reveals chromosomal rearrangements in rainbow trout. G3 2021, 11, jkab052. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Blondal, T.; Nielsen, S.J.; Baker, A.; Andreasen, D.; Mouritzen, P.; Teilum, M.W.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Mullany, L.E.; Herrick, J.S.; Wolff, R.K.; Slattery, M.L. MicroRNA seed region length impact on target messenger RNA expression and survival in colorectal cancer. PLoS ONE 2016, 11, e0154177. [Google Scholar] [CrossRef]
- Yartseva, V.; Takacs, C.M.; Vejnar, C.E.; Lee, M.T.; Giraldez, A.J. RESA identifies mRNA-regulatory sequences at high resolution. Nat. Methods 2017, 14, 201–207. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.H.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Blaufuss, P.C.; Bledsoe, J.W.; Gaylord, T.G.; Sealey, W.M.; Overturf, K.E.; Powell, M.S. Selection on a plant-based diet reveals changes in oral tolerance, microbiota and growth in rainbow trout (Oncorhynchus mykiss) when fed a high soy diet. Aquaculture 2020, 525, 735287. [Google Scholar] [CrossRef]
- Vélez-Calabria, G.; Peñaranda, D.S.; Jover-Cerdá, M.; Llorens, S.M.; Tomás-Vidal, A. Successful inclusion of high vegetable protein sources in feed for rainbow trout without decrement in intestinal health. Animals 2021, 11, 3577. [Google Scholar] [CrossRef]
- Refstie, S.; Korsøen, Ø.J.; Storebakken, T.; Baeverfjord, G.; Lein, I.; Roem, A.J. Differing nutritional responses to dietary soybean meal in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Aquaculture 2000, 190, 49–63. [Google Scholar] [CrossRef]
- Chikwati, E.M.; Sahlmann, C.; Holm, H.; Penn, M.H.; Krogdahl, Å.; Bakke, A.M. Alterations in digestive enzyme activities during the development of diet-induced enteritis in Atlantic salmon, Salmo salar L. Aquaculture 2013, 402–403, 28–37. [Google Scholar] [CrossRef]
- Murashita, K.; Yoshiura, Y.; Chisada, S.; Furuita, H.; Sugita, T.; Matsunari, H.; Iwashita, Y.; Yamamoto, Y. Homologue gene of bile acid trans- porters ntcp, asbt, and ost-alpha in rainbow trout Oncorhynchus mykiss: Tissue expression, effect of fasting, and response to bile acid administration. Fish Physiol. Biochem. 2014, 40, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Perera, E.; Yúfera, M. Effects of soybean meal on digestive enzymes activity, expression of inflammation-related genes, and chromatin modifications in marine fish (Sparus aurata L.) larvae. Fish Physiol. Biochem. 2017, 43, 563–578. [Google Scholar] [CrossRef]
- Silva, F.C.P.; Nicoli, J.R.; Zambonino-infante, J.L.; Le Gall, M.; Kaushik, S.; Gatesoupe, F. Influence of partial substitution of dietary fish meal on the activity of digestive enzymes in the intestinal brush border membrane of gilthead sea bream, Sparus aurata and goldfish, Carassius auratus. Aquaculture 2010, 306, 233–237. [Google Scholar] [CrossRef]
- Norén, K.; Hansen, G.H.; Clausen, H.; Norén, O.; Sjöström, H.; Vogel, L.K. Defectively N-glycosylated and non-O-glycosylated aminopeptidase N (CD13) is normally expressed at the cell surface and has full enzymatic activity. Exp. Cell Res. 1997, 231, 112–118. [Google Scholar] [CrossRef]
- Santigosa, E.; Sánchez, J.; Médale, F.; Kaushik, S.; Pérez-Sánchez, J.; Gallardo, M.A. Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fish meal replacement by plant protein sources. Aquaculture 2008, 282, 68–74. [Google Scholar] [CrossRef]
- Murashita, K.; Matsunari, H.; Furuita, H.; Rønnestad, I.; Oku, H.; Yamamoto, T. Effects of dietary soybean meal on the digestive physiology of red seabream Pagrus major. Aquaculture 2018, 493, 219–228. [Google Scholar] [CrossRef]
- Liu, H.; Jin, J.; Zhu, X.; Han, D.; Yang, Y.; Xie, S. Effect of substitution of dietary fish meal by soybean meal on different sizes of gibel carp (Carassius auratus gibelio): Digestive enzyme gene expressions and activities, and intestinal and hepatic histology. Aquac. Nutr. 2015, 23, 129–147. [Google Scholar] [CrossRef]
- Jahan, H.; Tumpa, I.J.; Qasem, W.A.; Moniruzzaman, M.; Pervin, M.A.; Akter, R.; Omri, A.; Min, T.; Hossain, Z. Evaluation of the partial replacement of dietary fish meal with fermented or untreated soybean meal in juvenile Silver barb, Barbonymus gonionotus. Front. Nutr. 2021, 8, 733402. [Google Scholar] [CrossRef]
- Barnes, M.E.; Brown, M.L.; Bruce, T.; Sindelar, S.; Neiger, R. Rainbow trout rearing performance, intestinal morphology, and immune response after long-term feeding of high levels of fermented soybean meal. N. Am. J. Aquac. 2014, 76, 333–345. [Google Scholar] [CrossRef]
- Mosberian-Tanha, P.; Landsverk, T.; Press, C.M.; Mydland, L.T.; Schrama, J.W.; Øverland, M. Granulomatous enteritis in rainbow trout (Oncorhynchus mykiss) associated with soya bean meal regardless of water dissolved oxygen level. J. Fish Dis. 2018, 41, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Venold, F.F.; Penn, M.H.; Krogdahl, Å.; Overturf, K. Severity of soybean meal induced distal intestinal inflammation, enterocyte proliferation rate, and fatty acid binding protein (Fabp2) level differ between strains of rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 364–365, 281–292. [Google Scholar] [CrossRef]
- Romano, N.; Kumar, V.; Yang, G.; Kajbaf, K.; Rubio, M.B.; Overturf, K.; Brezas, A.; Hardy, R. Bile acid metabolism in fish: Disturbances caused by fishmeal alternatives and some mitigating effects from dietary bile inclusions. Rev. Aquac. 2020, 12, 1792–1817. [Google Scholar] [CrossRef]
- Kiron, V.; Park, Y.; Siriyappagouder, P.; Dahle, D.; Dias, J.; Fernandes, J.M.O.; Sørensen, M.; Trichet, V.V. Intestinal transcriptome analysis reveals soy derivative-linked changes in Atlantic salmon. Front. Immunol. 2020, 11, 596514. [Google Scholar] [CrossRef]
- Kumar, V.; Fawole, F.J.; Romano, N.; Hossain, M.S.; Labh, S.N.; Overturf, K.; Small, B.C. Insect (Black soldier fly, Hermetia illucens) meal supplementation prevents the soybean meal-induced intestinal enteritis in rainbow trout and health benefits of using insect oil. Fish Shellfish Immunol. 2021, 109, 116–124. [Google Scholar] [CrossRef]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef]
- Orom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a binds the 5’ UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenet. 2018, 10, 59. [Google Scholar] [CrossRef]
- Sun, Z.; Hao, T.; Tian, J. Identification of exosomes and its signature miRNAs of male and female Cynoglossus semilaevis. Sci. Rep. 2017, 7, 860. [Google Scholar] [CrossRef]
- Zhu, T.; Plagnes-Juan, E.; Skiba-Cassy, S. Circulating miRNA measurements are reflective of cholesterol-based changes in rainbow trout (Oncorhynchus mykiss). PLoS ONE 2018, 13, e0206727. [Google Scholar] [CrossRef]
- Zhu, T.; Corraze, G.; Plagnes-Juan, E.; Skiba-Cassy, S. Cholesterol metabolism regulation mediated by SREBP-2, L.X.R α and miR-33a in rainbow trout (Oncorhynchus mykiss) both in vivo and in vitro. PLoS ONE 2020, 15, e0223813. [Google Scholar] [CrossRef] [PubMed]
- Bizuayehu, T.T.; Babiak, I. Heterogenic origin of micro RNAs in Atlantic Salmon (Salmo salar) seminal plasma. Int. J. Mol. Sci. 2020, 21, 2723. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Bao, W.J.; Tao, F.Y.; He, J.; Li, X.H.; Xu, P.; Sun, L.Y. The expression profiles of miRNA–mRNA of early response in genetically improved farmed tilapia (Oreochromis niloticus) liver by acute heat stress. Sci. Rep. 2017, 7, 8705. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.L.; Wei, W.; Yu, Z.P.; Qin, C.K. miR-152-5p inhibits proliferation and induces apoptosis of liver cancer cells by up-regulating FOXO expression. Pharmazie 2017, 72, 338–343. [Google Scholar] [CrossRef]
- You, W.; Zhang, X.; Ji, M.; Yu, Y.; Chen, C.; Xiong, Y.; Liu, Y.; Sun, Y.; Tan, C.; Zhang, H.; et al. MiR-152-5p as a microRNA passenger strand special functions in human gastric cancer cells. Int. J. Biol. Sci. 2018, 14, 644–653. [Google Scholar] [CrossRef]
- Pang, Q.; Wang, Y.; Xu, M.; Xu, J.; Xu, S.; Shen, Y.; Xu, J.; Lei, R. MicroRNA-152-5p inhibits proliferation and migration and promotes apoptosis by regulating expression of Smad3 in human keloid fibroblasts. BMB Rep. 2019, 52, 202–207. [Google Scholar] [CrossRef]
- Zheng, L.; Kang, Y.; Zhang, L.; Zou, W. MiR-133a-5p inhibits androgen receptor (AR)-induced proliferation in prostate cancer cells via targeting FUsed in Sarcoma (FUS) and AR. Cancer Biol. Ther. 2019, 21, 34–42. [Google Scholar] [CrossRef]
- Woldemariam, N.T.; Agafonov, O.; Høyheim, B.; Houston, R.D. Expanding the miRNA repertoire in Atlantic salmon; discovery of IsomiRs and miRNAs highly expressed. Cells 2019, 8, 24. [Google Scholar] [CrossRef]
- Mori, M.; Nakagami, H.; Rodriguez-Araujo, G.; Nimura, K.; Kaneda, Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012, 10, e1001314. [Google Scholar] [CrossRef]
- Karabegovic, I.; Maas, S.; Medina-Gomez, C.; Zrimšek, M.; Reppe, S.; Gautvik, K.M.; Uitterlinden, A.G.; Rivadeneira, F.; Ghanbari, M. Genetic polymorphism of miR-196a-2 is associated with bone mineral density (BMD). Int. J. Mol. Sci. 2017, 18, 2529. [Google Scholar] [CrossRef]
- Ranjha, R.; Meena, N.K.; Singh, A.; Ahuja, V.; Paul, J. Association of miR-196a-2 and miR-499 variants with ulcerative colitis and their correlation with expression of respective miRNAs. PLoS ONE 2017, 12, e0173447. [Google Scholar] [CrossRef]
- Liu, W.; Bi, P.; Shan, T.; Yang, X.; Yin, H.; Wang, Y.; Liu, N.; Rudnicki, M.A.; Kuang, S. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013, 9, e1003626. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, C. Adipogenesis in fish. J. Exp. Biol. 2018, 221, jeb161588. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, B.; Cui, Z.W.; Zhang, X.Y.; Cheng, Y.Y.; Xu, X.; Li, X.M.; Wang, Z.X.; Chen, D.D.; Zhang, Y.A. Integrative, Transcriptomic and microRNAomic profiling reveals immune mechanism for the resilience to soybean meal stress in fish gut and liver. Front. Physiol. 2018, 110, 1154. [Google Scholar] [CrossRef]
- Rehman, S.; Gora, A.H.; Siriyappagouder, P.; Brugman, S.; Fernandes, J.M.O.; Dias, J.; Kiron, V. Zebrafish intestinal transcriptome highlights subdued inflammatory responses to dietary soya bean and efficacy of yeast β-glucan. J. Fish Dis. 2021, 44, 1619–1737. [Google Scholar] [CrossRef] [PubMed]
- Nersisyan, L.; Hopp, L.; Loeffler-Wirth, H.; Galle, J.; Loeffler, M.; Binder, H. Telomere length maintenance and its transcriptional regulation in lynch syndrome and sporadic colorectal carcinoma. Front. Oncol. 2019, 9, 1172. [Google Scholar] [CrossRef] [PubMed]
- Jonassaint, N.L.; Guo, N.; Califano, J.A.; Montgomery, E.A.; Armanios, M. The gastrointestinal manifestations of telomere-mediated disease. Aging Cell 2013, 12, 319–323. [Google Scholar] [CrossRef]
- Chakravarti, D.; Lee, R.; Multani, A.S.; Santoni, A.; Keith, Z.; Hsu, W. Telomere dysfunction instigates inflammation in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2024853118. [Google Scholar] [CrossRef]
- Ilan-Ber, T.; Ilan, Y.; Israel, O.S. The role of microtubules in the immune system and as potential targets for gut-based immunotherapy. Mol. Immunol. 2019, 111, 73–82. [Google Scholar] [CrossRef]
- Marcil, M.; Brooks-Wilson, A.; Clee, S.M.; Roomp, K.; Zhang, L.H.; Yu, L.; Collins, J.A.; Dam, M.V.; Molhuizen, H.O.; Loubster, O.; et al. Mutations in the ABC 1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet 1999, 354, 1341–1346. [Google Scholar] [CrossRef]
- Hirohashi, T.; Suzuki, H.; Takikawa, H.; Sugiyama, Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J. Biol. Chem. 2000, 275, 2905–2910. [Google Scholar] [CrossRef]
- Hayashi, H.; Takada, T.; Suzuki, H.; Onuki, R.; Hofmann, A.F.; Sugiyama, Y. Transport by vesicles of glycine- and taurine-conjugated bile salts and taurolithocholate 3-sulfate: A comparison of human BSEP with rat Bsep. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2005, 1738, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hayashi, H.; Sugiyama, Y. Short- and medium-chain fatty acids enhance the cell surface expression and transport capacity of the bile salt export pump (BSEP/ABCB11). Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2010, 1801, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Telbisz, Á.; Özvegy-Laczka, C.; Hegedűs, T.; Váradi, A.; Sarkadi, B. Effects of the lipid environment, cholesterol and bile acids on the function of the purified and reconstituted human ABCG2 protein. Biochem. J. 2013, 450, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Lu, Y.; Wu, T. The impact of ATP-binding cassette transporters on metabolic diseases. Nutr. Metab. 2020, 17, 61. [Google Scholar] [CrossRef]
- Romarheim, O.H.; Skrede, A.; Penn, M.; Mydland, L.T.; Krogdahl, Å.; Storebakken, T. Lipid digestibility, bile drainage and development of morphological intestinal changes in rainbow trout (Oncorhynchus mykiss) fed diets containing defatted soybean meal. Aquaculture 2008, 274, 329–338. [Google Scholar] [CrossRef]
- Murashita, K.; Rønnestad, I.; Furuita, H.; Matsunari, H.; Oku, H.; Yamamoto, T. Effects of dietary soybean meal on the bile physiology in rainbow trout, Oncorhynchus mykiss. Aquaculture 2018, 490, 303–310. [Google Scholar] [CrossRef]
- Fontagné-Dicharry, S.; Lataillade, E.; Surget, A.; Brèque, J.; Zambonino-Infante, J.; Kaushik, S.J. Effects of dietary vitamin A on broodstock performance, egg quality, early growth and retinoid nuclear receptor expression in rainbow trout (Oncorhynchus mykiss). Aquaculture 2010, 303, 40–49. [Google Scholar] [CrossRef]
- Kern, F.; Backes, C.; Hirsch, P.; Fehlmann, T.; Hart, M.; Meese, E.; Keller, A. What’s the target: Understanding two decades of in silico microRNA-target prediction. Brief. Bioinform. 2020, 21, 1999–2010. [Google Scholar] [CrossRef]
- Desvignes, T.; Sydes, J.; Montfort, J.; Bobe, J.; Postlethwait, J.H. Evolution after whole-genome duplication: Teleost microRNAs. Mol. Biol. Evol. 2021, 38, 3308–3331. [Google Scholar] [CrossRef]

| Ingredients (g/100 g, on Wet Basis) | FM | VM |
|---|---|---|
| Fishmeal Super Prime (Diamante) | 12.00 | 0.00 |
| Soy protein concentrate (Soycomil) | 20.00 | 20.00 |
| Wheat gluten | 8.50 | 8.50 |
| Corn gluten | 5.00 | 5.00 |
| Soybean meal 48 | 8.00 | 26.20 |
| Rapeseed meal | 5.00 | 5.00 |
| Wheat meal | 16.38 | 8.37 |
| Fish oil | 6.72 | 7.05 |
| Rapeseed oil | 14.56 | 15.28 |
| Linseed oil | 1.12 | 1.18 |
| Vit & Min Premix INVIVO 1% | 1.00 | 1.00 |
| Antioxidant powder (Verdilox) | 0.20 | 0.20 |
| Sodium propionate | 0.10 | 0.10 |
| MAP (monoammonium phosphate) | 0.70 | 1.40 |
| L-lysine | 0.20 | 0.20 |
| DL-methionine | 0.50 | 0.50 |
| Yttrium oxide | 0.02 | 0.02 |
| Total | 100.00 | 100.00 |
| Proximate Composition (Dry Matter Basis) | FM | VM |
| Crude protein, % feed | 38.00 | 38.00 |
| Crude fat, % feed | 25.00 | 25.00 |
| Fiber, % feed | 2.30 | 2.70 |
| Starch, % feed | 11.40 | 7.40 |
| Ash, % feed | 6.10 | 5.50 |
| Gross energy, MJ/kg feed | 23.40 | 23.40 |
| Total P, % feed | 0.80 | 0.80 |
| Days | Parameter | FM | VM |
|---|---|---|---|
| 0 | IBW (g) | 23.24 ± 0.20 | 23.31 ± 0.22 |
| ITL (cm) | 12.44 ± 0.10 | 12.51 ± 0.04 | |
| 63 | FBW (g) | 148.84 ± 6.46 | 155.96 ± 3.41 |
| FTL (cm) | 21.87 ± 0.35 | 22.34 ± 0.39 | |
| CF | 1.34 ± 0.02 | 1.30 ± 0.06 | |
| WG (%) | 540.39 ± 23.32 | 569.18 ± 16.94 | |
| DFI (%) | 2.06 ± 0.06 | 2.16 ± 0.02 | |
| SGR (%/day) | 2.95 ± 0.06 | 3.02 ± 0.04 | |
| FCR | 0.77 ± 0.01 | 0.79 ± 0.02 | |
| HSI (%) | 1.62 ± 0.11 | 1.49 ± 0.04 | |
| VSI (%) | 13.84 ± 0.61 | 18.13 ± 0.95 * | |
| Apparent digestibility coefficients (%) | |||
| Dry matter | 82.94 ± 1.78 | 80.56 ± 0.97 | |
| Protein | 93.70 ± 0.82 | 92.70 ± 0.67 | |
| Fat | 98.03 ± 0.48 | 96.56 ± 0.29 * | |
| Phosphorus | 61.44 ± 3.34 | 58.50 ± 2.66 | |
| Energy | 87.36 ± 1.19 | 86.03 ± 0.92 | |
| Proximate analysis of muscle (% on wet weight basis) | |||
| Moisture | 72.09 ± 0.05 | 73.16 ± 1.02 | |
| Protein | 18.25 ± 0.32 | 17.29 ± 0.73 | |
| Fat | 8.20 ± 0.65 | 8.03 ± 0.33 | |
| Ash | 1.46 ± 0.07 | 1.52 ± 0.15 | |
| Enzyme | Units | FM | VM |
|---|---|---|---|
| Chymotrypsin | mU/µg | 22.05 ± 8.29 | 23.32 ± 4.99 |
| Aminopeptidase N | U/mg | 21.60 ± 4.07 | 32.02 ± 2.28 * |
| α-amylase | U/mg | 51.72 ± 9.40 | 30.81 ± 8.07 * |
| Experimental Group | Fish Code | Total Raw Reads | After Trimming | GC (%) | Q30 (%) | miRNAs | ||
|---|---|---|---|---|---|---|---|---|
| # of Reads | % | # of Reads | % | |||||
| FM | 1-6B | 20,732,616 | 11,067,387 | 53.38 | 57.82 | 95.51 | 157,593 | 1.42 |
| 1-9B | 15,395,251 | 8,972,934 | 58.28 | 56.71 | 95.37 | 136,808 | 1.52 | |
| 1-9C | 18,470,158 | 9,318,040 | 50.45 | 56.07 | 95.45 | 122,959 | 1.32 | |
| 2-7A | 15,098,313 | 6,595,979 | 43.69 | 52.30 | 95.71 | 40,643 | 0.62 | |
| 2-10C | 21,119,667 | 11,611,974 | 54.98 | 57.82 | 95.74 | 183,007 | 1.58 | |
| VM | 1-2A | 22,114,004 | 12,143,219 | 54.91 | 56.77 | 95.38 | 138,485 | 1.14 |
| 1-2B | 18,349,426 | 11,755,667 | 64.07 | 59.33 | 95.43 | 149,491 | 1.27 | |
| 1-10C | 21,806,977 | 12,516,351 | 57.40 | 59.01 | 95.38 | 151,753 | 1.21 | |
| 2-1B | 19,896,356 | 12,452,179 | 62.59 | 60.17 | 95.47 | 144,622 | 1.16 | |
| 2-9C | 20,865,121 | 8,480,471 | 40.64 | 52,52 | 95.53 | 50,970 | 0.60 | |
| Name | Sequence | Mean RPM in VM Group | Mean RPM in FM Group | Log2 (Fold Change) | q-Value |
|---|---|---|---|---|---|
| omy-miR-730a-5p | uccucauugugcaugcugugug | 14.45 | 1.41 | +3.36 | 0.047 |
| omy-miR-135c-5p | uauggcuuuuuauuccuacguga | 23.53 | 2.45 | +3.26 | 0.018 |
| omy-miR-93a-3p | acugcaaaaccagcacuuccug | 18.52 | 5.77 | +1.68 | 0.047 |
| omy-miR-152-5p | caaguucugugauacacuuaggcu | 14.42 | 6.13 | +1.23 | 0.036 |
| omy-miR-133a-5p | agcugguaaaauggaaccaaa | 87.99 | 37.60 | +1.22 | 0.019 |
| omy-miR-196a-2-3p | cuacaacacgaaacugucuga | 32.88 | 14.76 | +1.15 | 0.037 |
| GO Slim Biological Process | Nº of Genes | Fold Enrichment | FDR |
|---|---|---|---|
| Telomere maintenance (GO:0000723) | 9 | +6.93 | 8.65 × 10−3 |
| Telomere organization (GO:0032200) | 9 | +6.77 | 8.49 × 10−3 |
| Chromosome organization (GO:0051276) | 37 | +2.42 | 2.42 × 10−3 |
| Organelle organization (GO:0006996) | 124 | +1.70 | 6.98 × 10−5 |
| Cellular component organization (GO:0016043) | 178 | +1.49 | 9.83 × 10−5 |
| Cellular component organization or biogenesis (GO:0071840) | 180 | +1.44 | 4.32 × 10−4 |
| Cellular process (GO:0009987) | 551 | +1.17 | 8.81 × 10−5 |
| Macromolecule metabolic process (GO:0043170) | 207 | +1.29 | 2.65 × 10−2 |
| Nucleic acid metabolic process (GO:0090304) | 71 | +1.61 | 4.76 × 10−2 |
| Nitrogen compound metabolic process (GO:0006807) | 228 | +1.26 | 3.49 × 10−2 |
| Regulation of microtubule-based process (GO:0032886) | 14 | +3.77 | 1.89 × 10−2 |
| Morphogenesis of an epithelium (GO:0002009) | 31 | +2.12 | 4.80 × 10−2 |
| Tissue development (GO:0009888) | 76 | +1.68 | 8.60 × 10−3 |
| Anatomical structure development (GO:0048856) | 201 | +1.43 | 1.92 × 10−4 |
| Developmental process (GO:0032502) | 203 | +1.39 | 6.48 × 10−4 |
| Anatomical structure morphogenesis (GO:0009653) | 111 | +1.69 | 1.67 × 10−4 |
| Plasma membrane bounded cell projection organiz. (GO:0120036) | 53 | +1.85 | 1.28 × 10−2 |
| Cell projection organization (GO:0030030) | 56 | +1.89 | 6.32 × 10−3 |
| Intracellular transport (GO:0046907) | 51 | +1.77 | 4.85 × 10−2 |
| Localization (GO:0051179) | 182 | +1.46 | 2.23 × 10−4 |
| Establishment of localization (GO:0051234) | 138 | +1.44 | 8.85 × 10−3 |
| Transport (GO:0006810) | 138 | +1.47 | 3.88 × 10−3 |
| Cell development (GO:0048468) | 71 | +1.63 | 3.44 × 10−2 |
| Cell differentiation (GO:0030154) | 114 | +1.54 | 4.78 × 10−3 |
| Cellular developmental process (GO:0048869) | 116 | +1.55 | 2.50 × 10−3 |
| Nervous system development (GO:0007399) | 88 | +1.61 | 9.00 × 10−3 |
| System development (GO:0048731) | 170 | +1.49 | 1.47 × 10−4 |
| Multicellular organism development (GO:0007275) | 185 | +1.43 | 4.36 × 10−4 |
| Multicellular organismal process (GO:0032501) | 213 | +1.37 | 8.97 × 10−4 |
| Animal organ development (GO:0048513) | 122 | +1.48 | 8.38 × 10−3 |
| Negative regulation of apoptotic process (GO:0043066) | 1 | −0.08 | 2.41 × 10−2 |
| Regulation of apoptotic process (GO:0042981) | 5 | −0.25 | 4.76 × 10−2 |
| GO Slim Biological Process | Nº of Genes | Fold Enrichment | FDR |
|---|---|---|---|
| Microtubule binding motor protein (PC00156) | 12 | +5.62 | 1.02 × 10−3 |
| Microtubule or microtubule-binding cytoskeletal protein (PC00157) | 19 | +2.49 | 2.48 × 10−2 |
| ATP-binding cassette (ABC) transporter (PC00003) | 9 | +5.49 | 4.53 × 10−3 |
| Membrane traffic protein (PC00150) | 36 | +2.23 | 2.16 × 10−3 |
| Immunoglobulin receptor superfamily (PC00124) | 0 | −0.01 | 1.24 × 10−2 |
| Defense/immunity protein (PC00090) | 2 | −0.13 | 3.46 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo-Solís, F.J.; Larrán, A.M.; Ortiz-Delgado, J.B.; Sarasquete, C.; Dias, J.; Morais, S.; Fernández, I. Specific Blood Plasma Circulating miRs Are Associated with the Physiological Impact of Total Fish Meal Replacement with Soybean Meal in Diets for Rainbow Trout (Oncorhynchus mykiss). Biology 2023, 12, 937. https://doi.org/10.3390/biology12070937
Toledo-Solís FJ, Larrán AM, Ortiz-Delgado JB, Sarasquete C, Dias J, Morais S, Fernández I. Specific Blood Plasma Circulating miRs Are Associated with the Physiological Impact of Total Fish Meal Replacement with Soybean Meal in Diets for Rainbow Trout (Oncorhynchus mykiss). Biology. 2023; 12(7):937. https://doi.org/10.3390/biology12070937
Chicago/Turabian StyleToledo-Solís, Francisco Javier, Ana M. Larrán, Juan B. Ortiz-Delgado, Carmen Sarasquete, Jorge Dias, Sofia Morais, and Ignacio Fernández. 2023. "Specific Blood Plasma Circulating miRs Are Associated with the Physiological Impact of Total Fish Meal Replacement with Soybean Meal in Diets for Rainbow Trout (Oncorhynchus mykiss)" Biology 12, no. 7: 937. https://doi.org/10.3390/biology12070937
APA StyleToledo-Solís, F. J., Larrán, A. M., Ortiz-Delgado, J. B., Sarasquete, C., Dias, J., Morais, S., & Fernández, I. (2023). Specific Blood Plasma Circulating miRs Are Associated with the Physiological Impact of Total Fish Meal Replacement with Soybean Meal in Diets for Rainbow Trout (Oncorhynchus mykiss). Biology, 12(7), 937. https://doi.org/10.3390/biology12070937








