Cepabiflas B and C as Novel Anti-Inflammatory and Anti-Apoptotic Agents against Endotoxin-Induced Acute Kidney and Hepatic Injury in Mice: Impact on Bax/Bcl2 and Nrf2/NF-κB Signalling Pathways
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Plant Materials
2.3. Extraction and Isolation
2.4. Animal Experiments
2.5. Serum Indices of Hepatic and Renal Injury
2.6. Oxidative and Antioxidant Parameters
2.6.1. Malondialdehyde (MDA, Abcam/Cambridge/UK, Cat No: ab233471)
2.6.2. 4-Hydroxynonenal (4-HNE, MyBiosource/USA, Cat No: MBS027502)
2.6.3. Total Antioxidant Capacity (TAC, Sigma-Aldrich/St. Louis/MO/USA, Cat No: MAK187.1KT)
2.6.4. Superoxide Dismutase (SOD, Abcam/Cambridge/UK, Cat No: ab65354)
2.6.5. Reduced Glutathione (GSH, Calbiochem/MERCK Millipore/Darmstadt/Germany, Cat No: 354102,100T)
2.7. Histology
2.8. Immunohistochemistry
2.9. ELISA
2.10. NO (Nitric Oxide) Estimation
2.11. RT-PCR
2.12. Data Analysis
3. Results
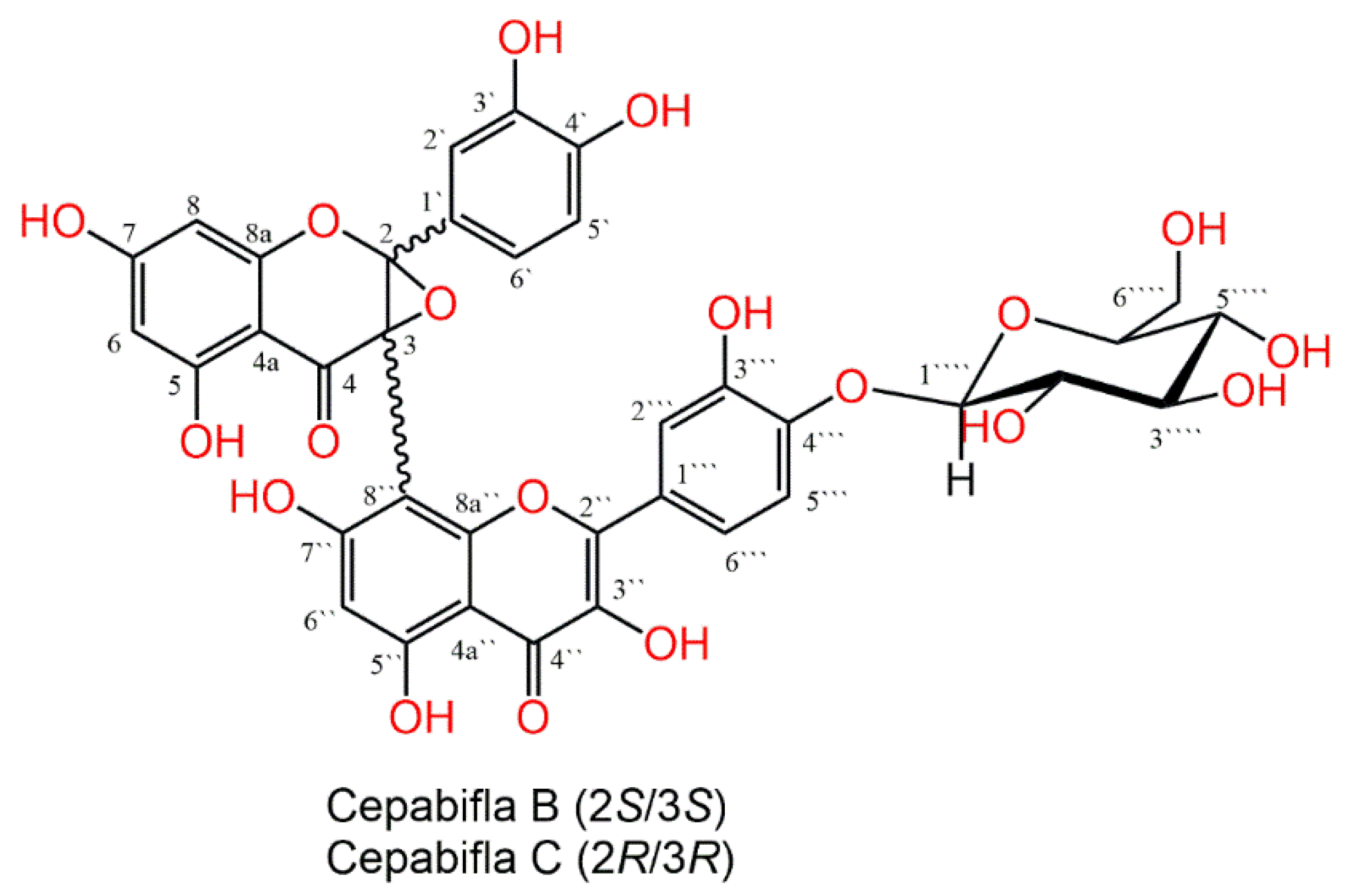
3.1. Purification and Characterization of Cepabiflas B and C (CBs)
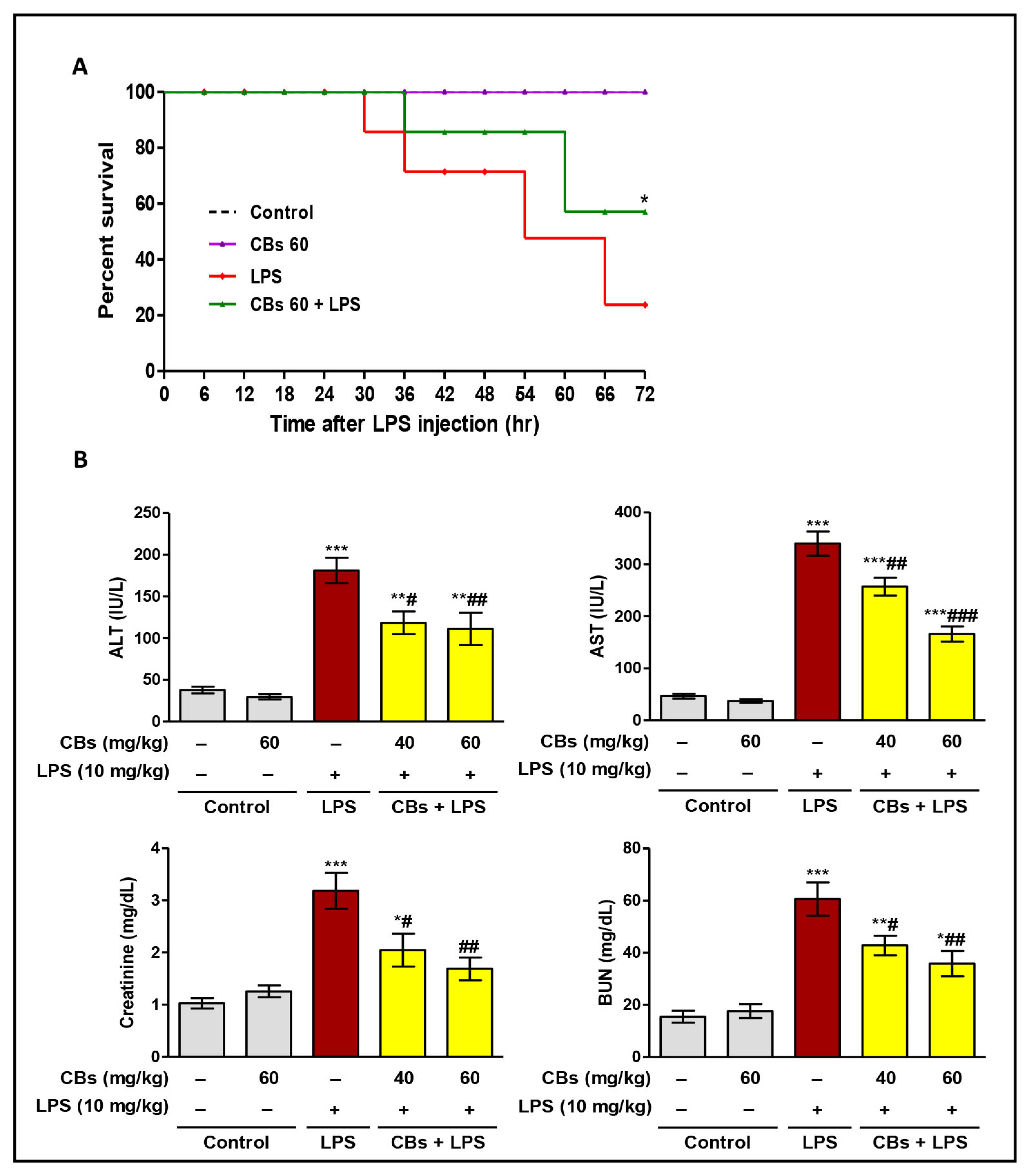
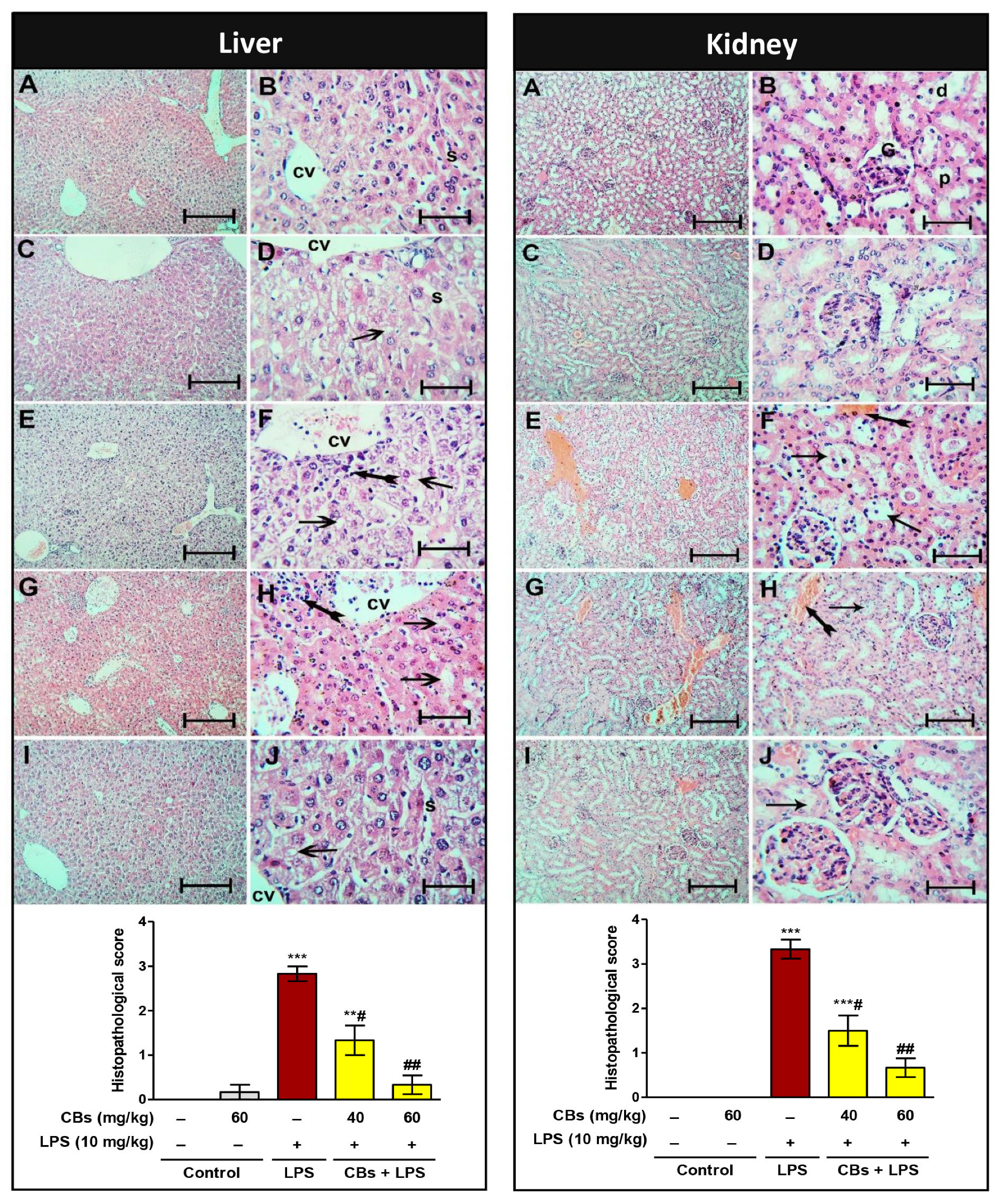
3.2. CBs Increased the Survival Rate and Ameliorated Serum and Histopathological Indices of Hepatorenal Damage in LPS-Intoxicated Mice
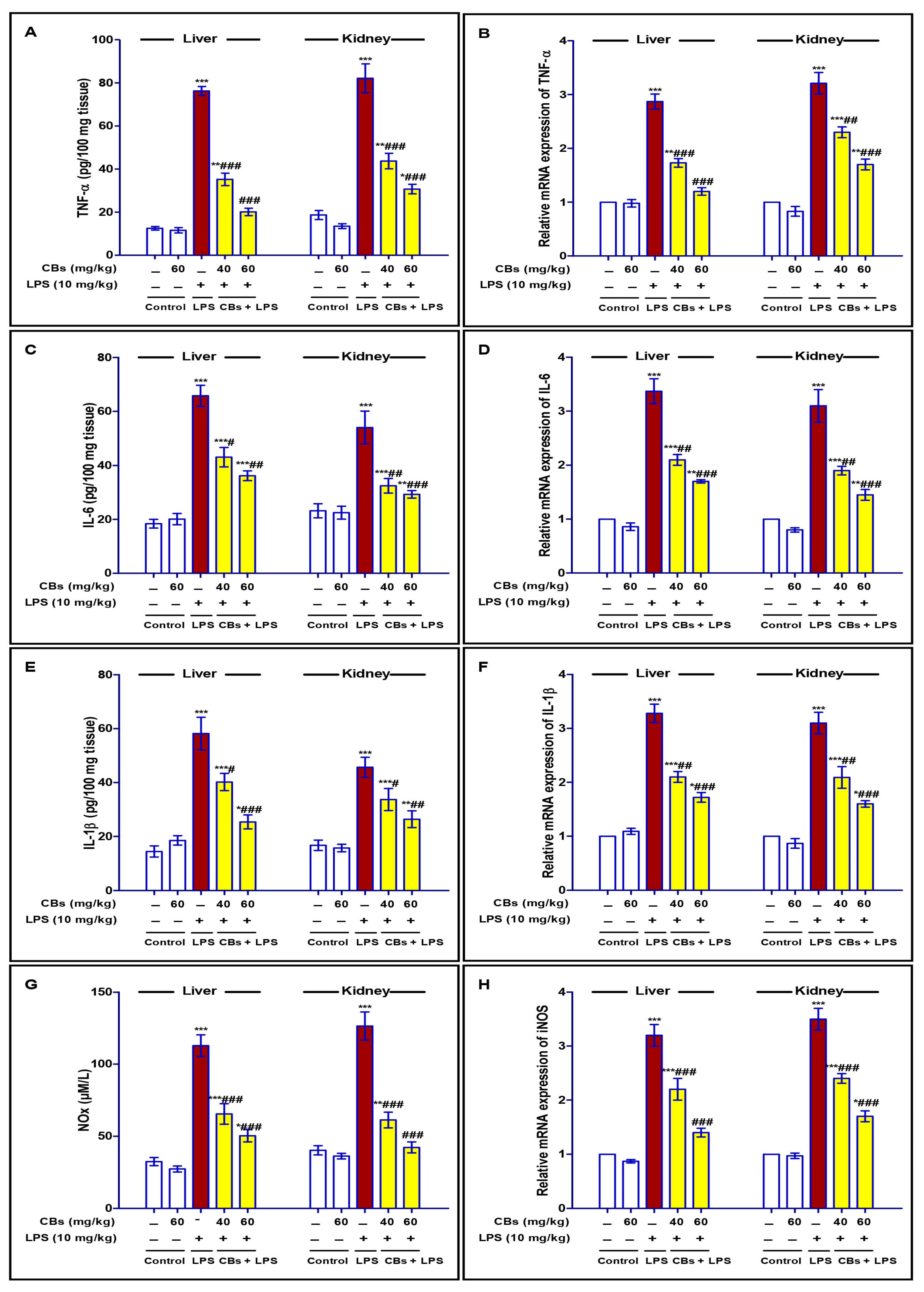
3.3. CBS Repressed LPS-Induced Inflammatory Response in the Liver and Kidney
3.4. CBS Alleviated LPS-Induced Activation of NF-κB in the Liver and Kidney
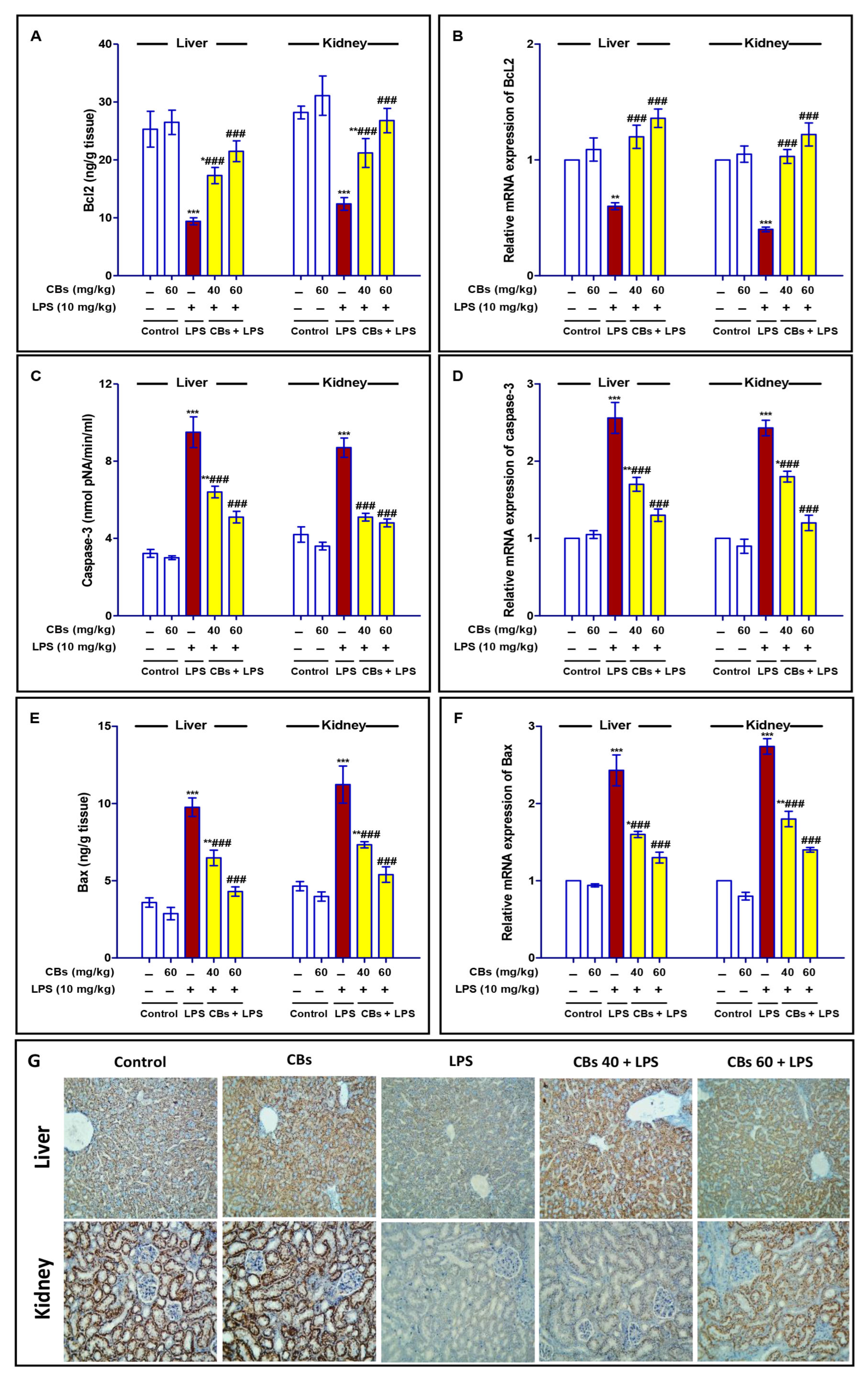
3.5. CBS Inhibited LPS-Induced Apoptosis of the Liver and Kidney
3.6. CBS Reversed LPS-Induced Oxidative Stress and Enhanced the Antioxidants in the Liver and the Kidney
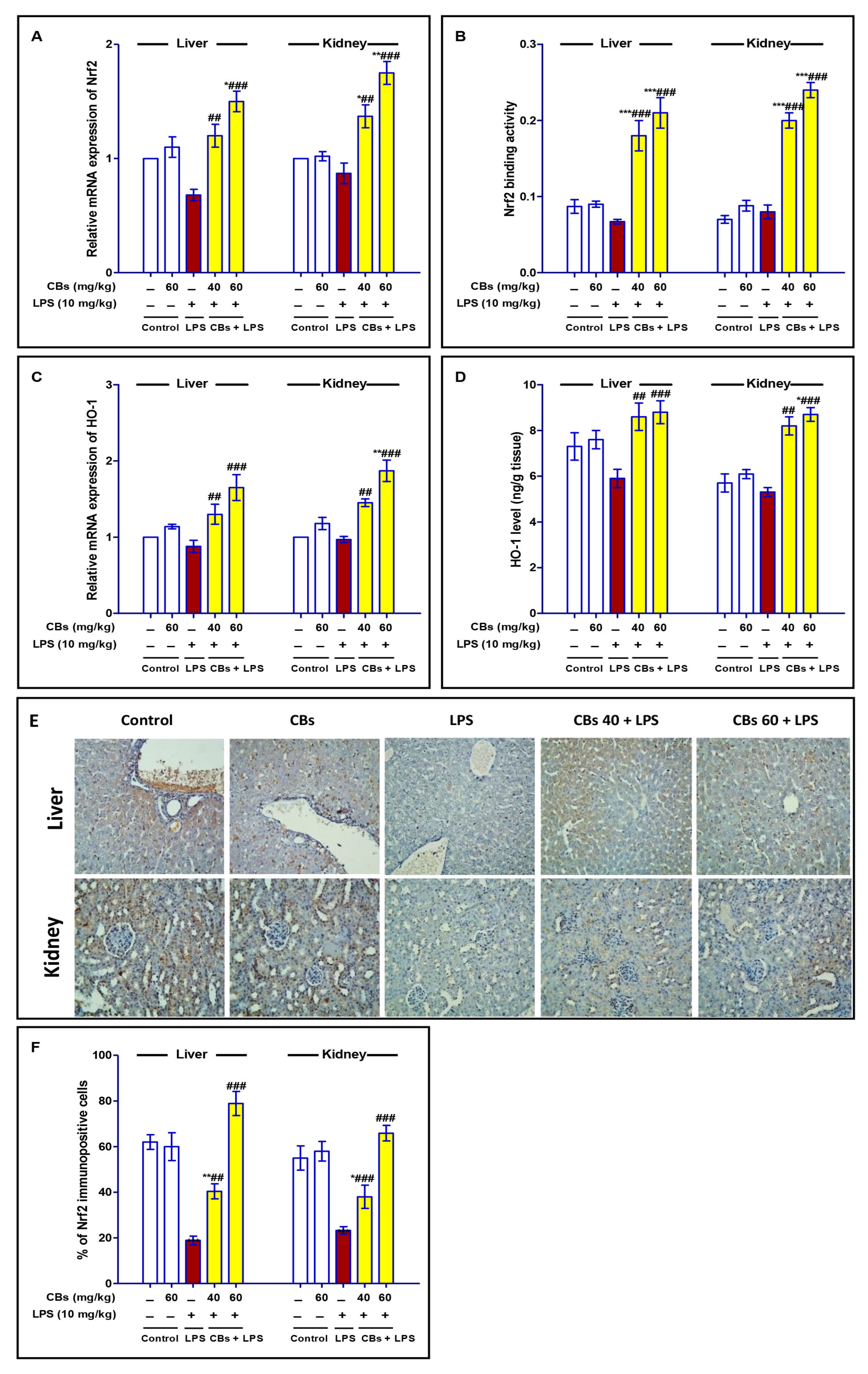
3.7. CBS Enhanced Nrf2 Signalling in the Liver and the Kidney of LPS-Intoxicated Mice
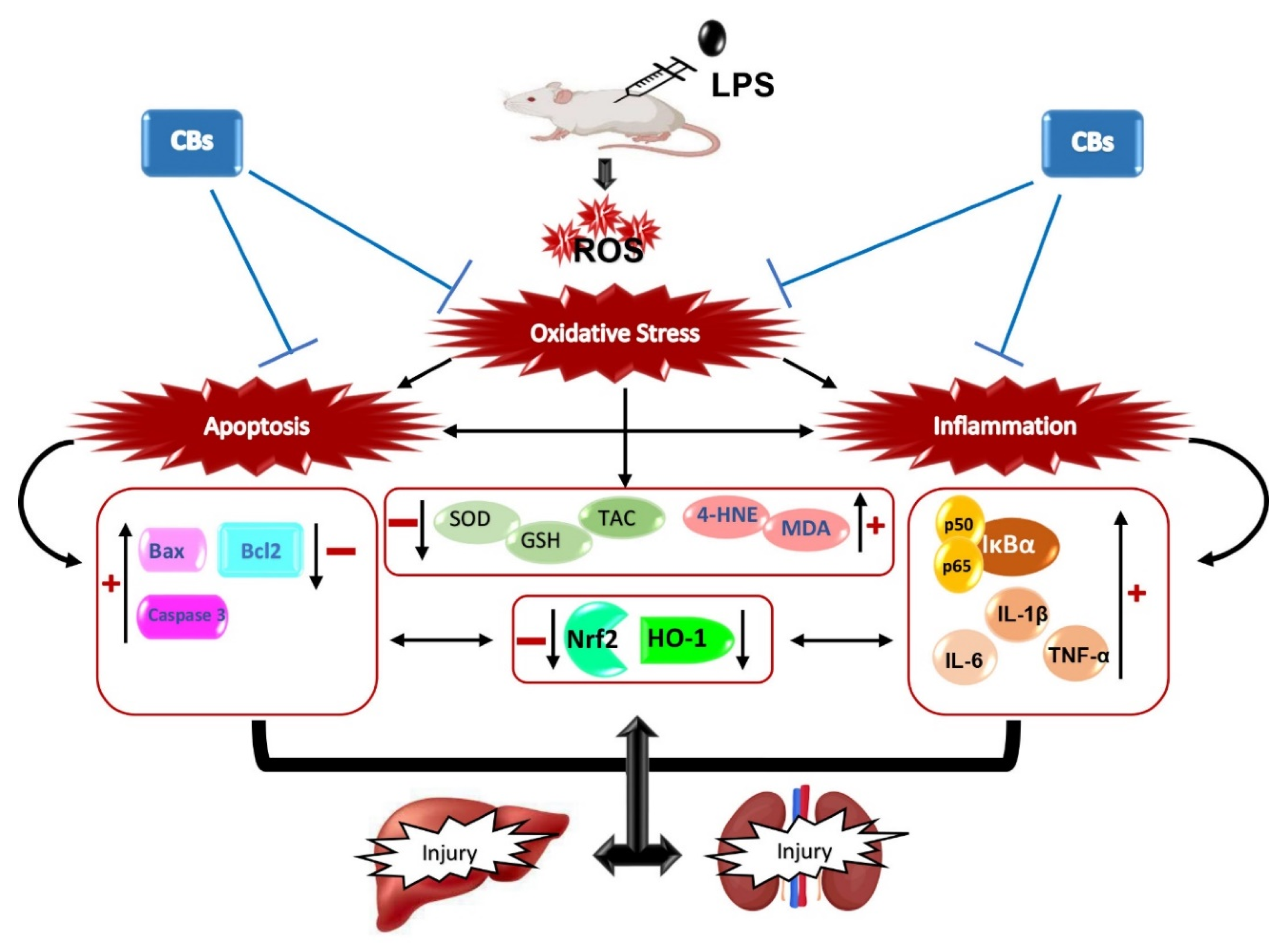
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleischmann, C.; Scherag, A.; Adhikari, N.; Hartog, C.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of global incidence and mortality of hospital treated sepsis. Current estimates and limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Ma, S.; Evans, R.G.; Iguchi, N.; Tare, M.; Parkington, H.C.; Bellomo, R.; May, C.N.; Lankadeva, Y.R. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation 2019, 26, e12483. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, L.; Ai, Y.; Zhang, L. Epidemiology of acute kidney injury in intensive care septic patients based on the KDIGO guidelines. Chin. Med. J. 2014, 127, 1820–1826. [Google Scholar]
- Yan, J.; Li, S.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef]
- Fiorentino, M.; Tohme, F.A.; Wang, S.; Murugan, R.; Angus, D.C.; Kellum, J.A. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS ONE 2018, 13, e0198269. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Islam, M.S.; Miao, L.; Yu, H.; Han, Z.; Sun, H. Ethanol Extract of Illicium henryi Attenuates LPS-Induced Acute Kidney Injury in Mice via Regulating Inflammation and Oxidative Stress. Nutrients 2019, 11, 1412. [Google Scholar] [CrossRef]
- Flannery, A.H.; Li, X.; Delozier, N.L.; Toto, R.D.; Moe, O.W.; Yee, J.; Neyra, J.A. Sepsis-Associated Acute Kidney Disease and Long-term Kidney Outcomes. Kidney Med. 2021, 3, 507–514.e1. [Google Scholar] [CrossRef]
- Zarbock, A.; Nadim, M.K.; Pickkers, P.; Gomez, H.; Bell, S.; Joannidis, M.; Kashani, K.; Koyner, J.L.; Pannu, N.; Meersch, M.; et al. Sepsis-associated acute kidney injury: Consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat. Rev. Nephrol. 2023, 19, 401–417. [Google Scholar] [CrossRef]
- Doi, K. Role of kidney injury in sepsis. J. Intensive Care 2016, 4, 17. [Google Scholar] [CrossRef]
- Remick, G.D.; Newcomb, D.E.; Bolgos, G.L.; Call, D.R. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation and puncture. Shock 2000, 13, 110–116. [Google Scholar] [CrossRef]
- Dai, J.M.; Guo, W.N.; Tan, Y.Z.; Niu, K.W.; Zhang, J.J.; Liu, C.L.; Yang, X.M.; Tao, K.S.; Chen, Z.N.; Dai, J.Y. Wogonin alleviates liver injury in sepsis through Nrf2-mediated NF-κB signalling suppression. J. Cell Mol. Med. 2021, 25, 5782. [Google Scholar] [CrossRef]
- Yuan, R.; Huang, L.; Du, L.J.; Feng, J.F.; Li, J.; Luo, Y.Y.; Xu, Q.M.; Yang, S.L.; Gao, H.; Feng, Y.L. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization. Pharmacol. Res. 2019, 142, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, C.; Zhao, S.; Li, J.; Yu, F. Combination therapy of ulinastatin with thrombomodulin alleviates endotoxin (LPS)—Induced liver and kidney injury via inhibiting apoptosis, oxidative stress and HMGB1/TLR4/NF-κB pathway. Bioengineered 2022, 13, 2951. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.U.; Kim, M.O. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef] [PubMed]
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. 1), S39. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Fossen, T.; Vågen, I.M. Onions: A source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W. Garlic and onion: Production, biochemistry, and processing. In Handbook of Vegetables and Vegetable Processing; Wiley Online Library: Hoboken, NJ, USA, 2011; Volume 1, pp. 625–642. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Chen, S. Spice plant Allium cepa: Dietary supplement for treatment of type 2 diabetes mellitus. Nutrition 2014, 30, 1128. [Google Scholar] [CrossRef]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological properties of allium cepa, preclinical and clinical evidences; a review. Iran. J. Pharm. Res. 2021, 20, 107. [Google Scholar] [PubMed]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; López-Andréu, F.J.; Cools, K.; Terry, L.A.; Esteban, R.M. Characterization of industrial onion wastes (Allium cepa L.): Dietary fibre and bioactive compounds. Plant Foods Hum. Nutr. 2011, 66, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A. Alliuocide A: A new antioxidant flavonoid from Allium cepa L. Phytopharmacology 2013, 4, 220. [Google Scholar]
- Mohamed, G.A. Alliuocide G, a new flavonoid with potent α-amylase inhibitory activity from Allium cepa L. Arkivoc 2008, xi, 202. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Mai, Y.; Li, H.; Wang, Z.; Xu, J.; He, X. Health Benefits of the Flavonoids from Onion: Constituents and Their Pronounced Antioxidant and Anti-neuroinflammatory Capacities. J. Agric. Food Chem. 2020, 68, 799–807. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Nutraceutical, pharmaceutical and therapeutic uses of Allium cepa: A review. Int. J. Green Pharm. 2016, 10, S46. [Google Scholar]
- Yao, L.H.; Jiang, Y.M.; Shi, J.; Tomás-Barberán, F.A.; Datta, N.; Singanusong, R.; Chen, S.S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113. [Google Scholar] [CrossRef]
- Elsheekh, K.M.; Kamel, R.R.; Elsherif, D.M.; Shalaby, A.M. Achieving sustainable development goals from the perspective of solid waste management plans. J. Eng. Appl. Sci. 2021, 68, 9. [Google Scholar] [CrossRef]
- Provin, A.P.; de Aguiar Dutra, A.R. Circular economy for fashion industry: Use of waste from the food industry for the production of biotextiles. Technol. Forecast. Soc. Chang. 2021, 169, 120858. [Google Scholar] [CrossRef]
- Vu, N.K.; Kim, C.S.; Ha, M.T.; Ngo, Q.T.; Park, S.E.; Kwon, H.; Lee, D.; Choi, J.S.; Kim, J.A.; Min, B.S. Antioxidant and antidiabetic activities of flavonoid derivatives from the outer skins of Allium cepa L. J. Agric. Food Chem. 2020, 68, 8797–8811. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Wang, L.; Jiang, L.; Qin, Z.; Zhao, Y.; Su, B. Maresin 1 attenuates lipopolysaccharide-induced acute kidney injury via inhibiting NOX4/ROS/NF-κB pathway. Front. Pharmacol. 2021, 12, 782660. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.Y.P.; Hung, M.Y.; Liang, T.R.; Peng, S.Y. An in-vivo study into the effects of schisandrin b in the liver, spleen, kidney, and brain of acute thioacetamide-intoxicated mice. Iran. J. Pharm. Res. 2021, 20, 300–3014. [Google Scholar]
- El-Agamy, D.S. Pirfenidone ameliorates concanavalin A-induced hepatitis in mice via modulation of reactive oxygen species/nuclear factor kappa B signalling pathways. J. Pharm. Pharmacol. 2016, 68, 1559. [Google Scholar] [CrossRef] [PubMed]
- Toprak, T.; Sekerci, C.A.; Aydın, H.R.; Ramazanoglu, M.A.; Arslan, F.D.; Basok, B.I.; Kucuk, H.; Kocakgol, H.; Aksoy, H.Z.; Asci, S.S.; et al. Protective effect of chlorogenic acid on renal ischemia/reperfusion injury in rats. Arch. Ital. Urol. Androl. 2020, 92, 153. [Google Scholar] [CrossRef] [PubMed]
- El-Agamy, D.S.; Ibrahim, S.R.M.; Ahmed, N.; Khoshhal, S.; Abo-Haded, H.M.; Elkablawy, M.A.; Aljuhani, N.; Mohamed, G.A. Aspernolide F, as a new cardioprotective butyrolactone against doxorubicin–induced cardiotoxicity. Int. Immunopharmacol. 2019, 72, 429–436. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-S.; Kim, S.-H.; Jeong, S.-H.; Jeong, U.-Y.; Jung, J.-E.; Lee, S.-K.; Lee, S.-H. Antioxidant and anti-inflammatory eEffects of ethanol extract from whole onion (Allium cepa L.) with leaves. Agriculture 2022, 12, 963. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.Q.; Yan, H.C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Naeini, F.; Asghari Azar, V.; Hasanzadeh, M.; Ostadrahimi, A.; Niazkar, H.R.; Mobasseri, M.; Tutunchi, H. A Comprehensive systematic review of the therapeutic effects and mechanisms of action of quercetin in sepsis. Phytomedicine 2021, 86, 153567. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Cho, D.Y.; Haque, M.E.; Kim, I.S.; Choi, D.K. The methanol extract of Allium cepa L. protects inflammatory markers in LPS-induced BV-2 microglial cells and upregulates the antiapoptotic gene and antioxidant enzymes in N27-A cells. Antioxidants 2019, 8, 348. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Yang, Y.; Gou, Y.; Wang, Z.; Yang, D.; Li, C. Protective effects of silymarin against D-Gal/LPS-induced organ damage and inflammation in mice. Drug Des. Dev. Ther. 2021, 15, 1903. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, M.; Li, M.; Kan, Y.; Li, B.; Xu, R.; Wu, Y.; Wang, S.; Zheng, X.; Feng, W. Protopine protects mice against LPS-induced acute kidney injury by inhibiting apoptosis and inflammation via the TLR4 signaling pathway. Molecules 2019, 25, 15. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of acute kidney injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhao, Y.; Su, J.; Liu, Z.; Fang, S.; Li, L.; Deng, J.; Fan, G. Toddalolactone protects lipopolysaccharide-induced sepsis and attenuates lipopolysaccharide-induced inflammatory response by modulating HMGB1-NF-κB translocation. Front. Pharmacol. 2020, 11, 109. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Su, J.; Liu, Z.; Fang, S.; Li, L.; Deng, J.; Fan, G. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557. [Google Scholar]
- Verma, S.K.; Molitoris, B.A. Renal endothelial injury and microvascular dysfunction in acute kidney injury. Semin. Nephrol. 2015, 35, 96. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.O.; Nadeem, A.; Ahmad, S.F.; Alanazi, M.M.; Aldossari, A.A.; Alasmari, F. Amelioration of sepsis-induced acute kidney injury through inhibition of inflammatory cytokines and oxidative stress in dendritic cells and neutrophils respectively in mice: Role of spleen tyrosine kinase signaling. Biochimie 2019, 158, 102. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, F.; Yi, S.; Li, S.; Zhu, A.; Tang, Y.; Li, A. Protective role of crocin against sepsis-induced injury in the liver, kidney and lungs via inhibition of p38 MAPK/NF-κB and Bax/Bcl-2 signalling pathways. Pharm. Biol. 2022, 60, 543–552. [Google Scholar] [CrossRef]
- Huang, B.; He, D.; Chen, G.; Ran, X.; Guo, W.; Kan, X.; Wang, W.; Liu, D.; Fu, S.; Liu, J. α-Cyperone inhibits LPS-induced inflammation in BV-2 cells through activation of Akt/Nrf2/HO-1 and suppression of the NF-jB pathway. Food Funct. 2018, 9, 2735. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wang, X.; He, Z.; Liu, F.; Zhi, W.; Zhang, H.; Niu, X. Esculin attenuates endotoxin shock induced by lipopolysaccharide in mouse and NO production in vitro through inhibition of NF-κB activation. Eur. J. Pharmacol. 2016, 791, 726–734. [Google Scholar] [CrossRef]
- Wang, Z.; Ka, S.O.; Han, Y.T.; Bae, E.J. Dihydropyranoaurone compound damaurone D inhibits LPS-induced inflammation and liver injury by inhibiting NF-κB and MAPK signaling independent of AMPK. Arch. Pharm. Res. 2018, 41, 314–323. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, W.; Zhang, Z.; Zhu, J.; Li, W.; Yi, Y.; Yang, X.; Ma, W.; Liang, H. The water extract of “Jiao Mei Gu” attenuates the lipopolysaccharide-induced inflammatory response via inhibiting NF-κB activity in mice. J. Ethnopharmacol. 2020, 259, 112882. [Google Scholar] [CrossRef]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef]
- Huang, G.; Bao, J.; Shao, X.; Zhou, W.; Wu, B.; Ni, Z.; Wang, L. Inhibiting pannexin-1 alleviates sepsis-induced acute kidney injury via decreasing NLRP3 inflammasome activation and cell apoptosis. Life Sci. 2020, 254, 117791. [Google Scholar] [CrossRef]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine attenuates LPS-induced liver injury and improves survival of mice through suppressing oxidative stress, inflammation, and apoptosis. Mediators Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef]
- Hong, M.K.; Hu, L.L.; Zhang, Y.X.; Xu, Y.L.; Liu, X.Y.; He, P.K.; Jia, Y.H. 6-Gingerol ameliorates sepsis-induced liver injury through the Nrf2 pathway. Int. Immunopharmacol. 2020, 80, 106196. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, W.; Zhang, N.; Wang, Y.; Tian, Y.; Sun, H.; Li, X.; Wang, Y.; Liu, J. Lactobacillus plantarum Lp2 improved LPS-induced liver injury through the TLR-4/MAPK/NFκB and Nrf2-HO-1/CYP2E1 pathways in mice. Food Nutr. Res. 2022, 66, 5459. [Google Scholar] [CrossRef]
- Qiongyue, Z.; Xin, Y.; Meng, P.; Sulin, M.; Yanlin, W.; Xinyi, L.; Xuemin, S. Post-treatment With Irisin attenuates acute kidney injury in sepsis mice through anti-ferroptosis via the SIRT1/Nrf2 pathway. Front. Pharmacol. 2022, 13, 857067. [Google Scholar] [CrossRef]
- Qiu, W.; An, S.; Wang, T.; Li, J.; Yu, B.; Zeng, Z.; Chen, Z.; Lin, B.; Lin, X.; Gao, Y. Melatonin suppresses ferroptosis via activation of the Nrf2/HO-1 signaling pathway in the mouse model of sepsis-induced acute kidney injury. Int. Immunopharmacol. 2022, 112, 109162. [Google Scholar] [CrossRef]


| Gene (Mouse) | PCR Product (bp) | Sequence (5′-3′) |
|---|---|---|
| TNF-α | 99 | F: TGAACTTCGGGGTGATCGGT |
| R: GGTGGTTTGTGAGTGTGAGGG | ||
| IL-6 | 79 | F: AGTCCTTCCTACCCCAATTTCC |
| R: GGTCTTGGTCCTTAGCCACT | ||
| IL-1β | 81 | F: GCAACTGTTCCTGAACTCAACT |
| R: GGGTCCGTCAACTTCAAAGA | ||
| iNOS | 75 | F: TGGTGAAGGGACTGAGCTGT |
| R: GCTACTCCGTGGAGTGAACA | ||
| Bcl2 | 123 | F: CCTGTGGATGACTGAGTACCTG |
| R: AGCCAGGAGAAATCAAACAGAGG | ||
| Caspase-3 | 74 | F: ATGGAGAACAACAAAACCTCAGT |
| R: TTGCTCCCATGTATGGTCTTTAC | ||
| Bax | 140 | F: TGAAGACAGGGGCCTTTTTG |
| R: AATTCGCCGGAGACACTCG | ||
| Nrf2 | 170 | F: AAGAATAAAGTCGCCGCCCA |
| R: AGATACAAGGTGCTGAGCCG | ||
| HO-1 | 122 | F: GAAATCATCCCTTGCACGCC |
| R: CCTGAGAGGTCACCCAGGTA | ||
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | 123 | F: AGGTCGGTGTGAACGGATTTG |
| R: TGTAGACCATGTAGTTGAGGTCA |
| Parameters | Groups | ||||
|---|---|---|---|---|---|
| Control | CBs | LPS | CBs (40 mg/kg) + LPS | CBs (60 mg/kg) + LPS | |
| MDA (nmol/g tissue) | |||||
| Liver | 29.5 ± 3.0 | 22.8 ± 2.1 | 73.1 ± 4.9 *** | 52.6 ± 4.3 **## | 35.3 ± 4.0 ### |
| Kidney | 23.6 ± 2.8 | 20.9 ± 2.4 | 65.4 ± 5.4 *** | 46.6 ± 3.7 **## | 28.8 ± 2.9 ### |
| 4-HNE (µmol/mL) | |||||
| Liver | 0.36 ± 0.05 | 0.31 ± 0.04 | 1.02 ± 0.1 *** | 0.7 ± 0.06 *# | 0.57 ± 0.07 ### |
| Kidney | 0.42 ± 0.05 | 0.38 ± 0.05 | 1.24 ± 0.08 *** | 0.82 ± 0.05 ***### | 0.6 ± 0.04 ### |
| TAC (nmol/g tissue) | |||||
| Liver | 0.75 ± 0.06 | 0.81 ± 0.04 | 0.36 ± 0.03 *** | 0.57 ± 0.03 *# | 0.71 ± 0.04 ### |
| Kidney | 0.58 ± 0.08 | 0.67 ± 0.02 | 0.25 ± 0.01 *** | 0.48 ± 0.03 ## | 0.54 ± 0.02 ## |
| SOD (U/g tissue) | |||||
| Liver | 22.6 ± 2.2 | 26.9 ± 1.2 | 7.9 ± 0.3 *** | 16.4 ± 1.9 **## | 18.6 ± 4.6 ### |
| Kidney | 20.2 ± 1.9 | 25.1 ± 2.7 | 8.7 ± 0.8 *** | 13.9 ± 1.5 *## | 15.1 ± 2.1 ### |
| GSH (µmol/g tissue) | |||||
| Liver | 14.8 ± 1.9 | 17.9 ± 1.5 | 4.7 ± 0.4 *** | 8.3 ± 0.8 **## | 12.3 ± 1.4 ### |
| Kidney | 16.3 ± 1.3 | 14.5 ± 1.3 | 6.9 ± 0.2 *** | 10.1 ± 1.5 **## | 13.1 ± 1.1 ### |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizq, A.T.; Sirwi, A.; El-Agamy, D.S.; Abdallah, H.M.; Ibrahim, S.R.M.; Mohamed, G.A. Cepabiflas B and C as Novel Anti-Inflammatory and Anti-Apoptotic Agents against Endotoxin-Induced Acute Kidney and Hepatic Injury in Mice: Impact on Bax/Bcl2 and Nrf2/NF-κB Signalling Pathways. Biology 2023, 12, 938. https://doi.org/10.3390/biology12070938
Rizq AT, Sirwi A, El-Agamy DS, Abdallah HM, Ibrahim SRM, Mohamed GA. Cepabiflas B and C as Novel Anti-Inflammatory and Anti-Apoptotic Agents against Endotoxin-Induced Acute Kidney and Hepatic Injury in Mice: Impact on Bax/Bcl2 and Nrf2/NF-κB Signalling Pathways. Biology. 2023; 12(7):938. https://doi.org/10.3390/biology12070938
Chicago/Turabian StyleRizq, Akaber T., Alaa Sirwi, Dina S. El-Agamy, Hossam M. Abdallah, Sabrin R. M. Ibrahim, and Gamal A. Mohamed. 2023. "Cepabiflas B and C as Novel Anti-Inflammatory and Anti-Apoptotic Agents against Endotoxin-Induced Acute Kidney and Hepatic Injury in Mice: Impact on Bax/Bcl2 and Nrf2/NF-κB Signalling Pathways" Biology 12, no. 7: 938. https://doi.org/10.3390/biology12070938
APA StyleRizq, A. T., Sirwi, A., El-Agamy, D. S., Abdallah, H. M., Ibrahim, S. R. M., & Mohamed, G. A. (2023). Cepabiflas B and C as Novel Anti-Inflammatory and Anti-Apoptotic Agents against Endotoxin-Induced Acute Kidney and Hepatic Injury in Mice: Impact on Bax/Bcl2 and Nrf2/NF-κB Signalling Pathways. Biology, 12(7), 938. https://doi.org/10.3390/biology12070938










