Potential Geographic Range of the Endangered Reed Parrotbill Paradoxornis heudei under Climate Change
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Occurrence Records
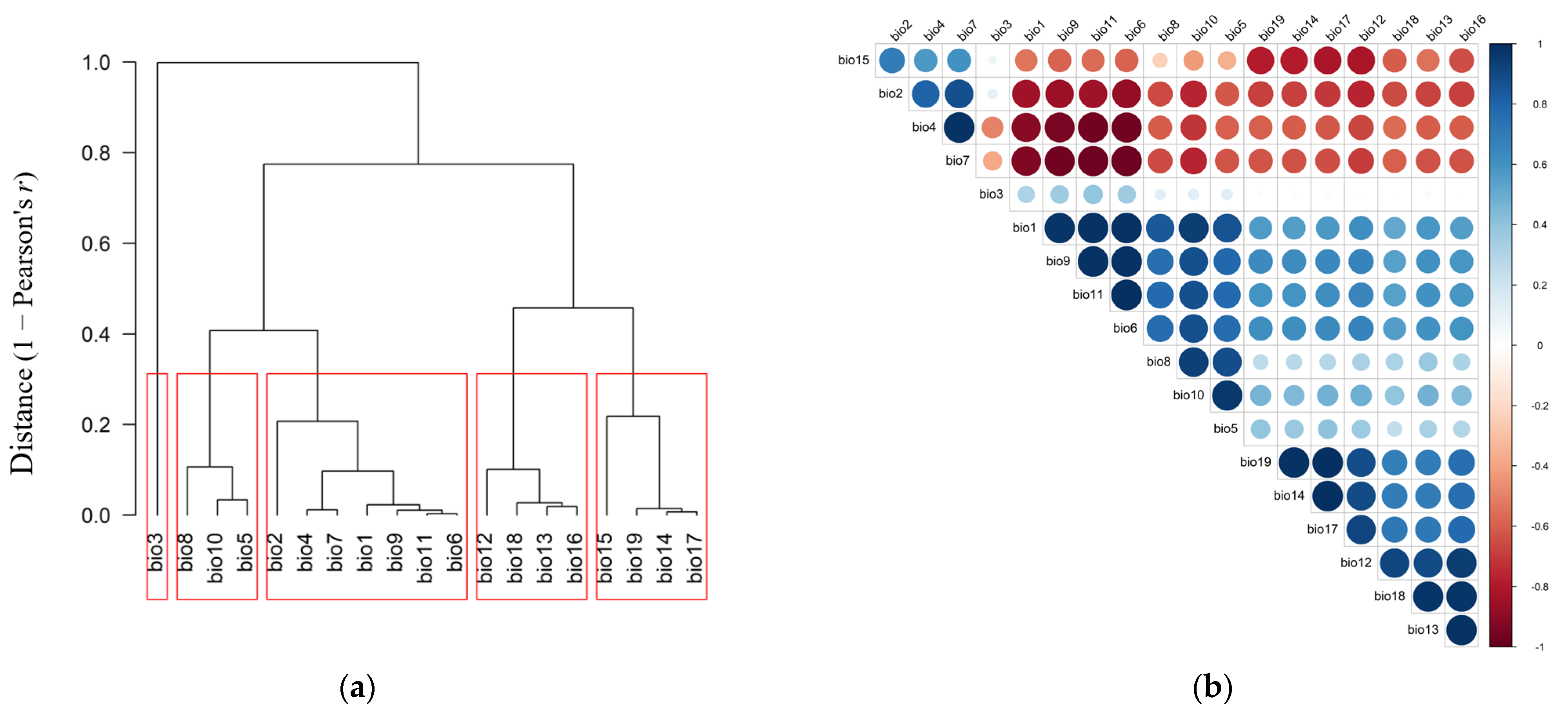
2.2. Environmental Predictors
2.3. Modeling Procedure
3. Results
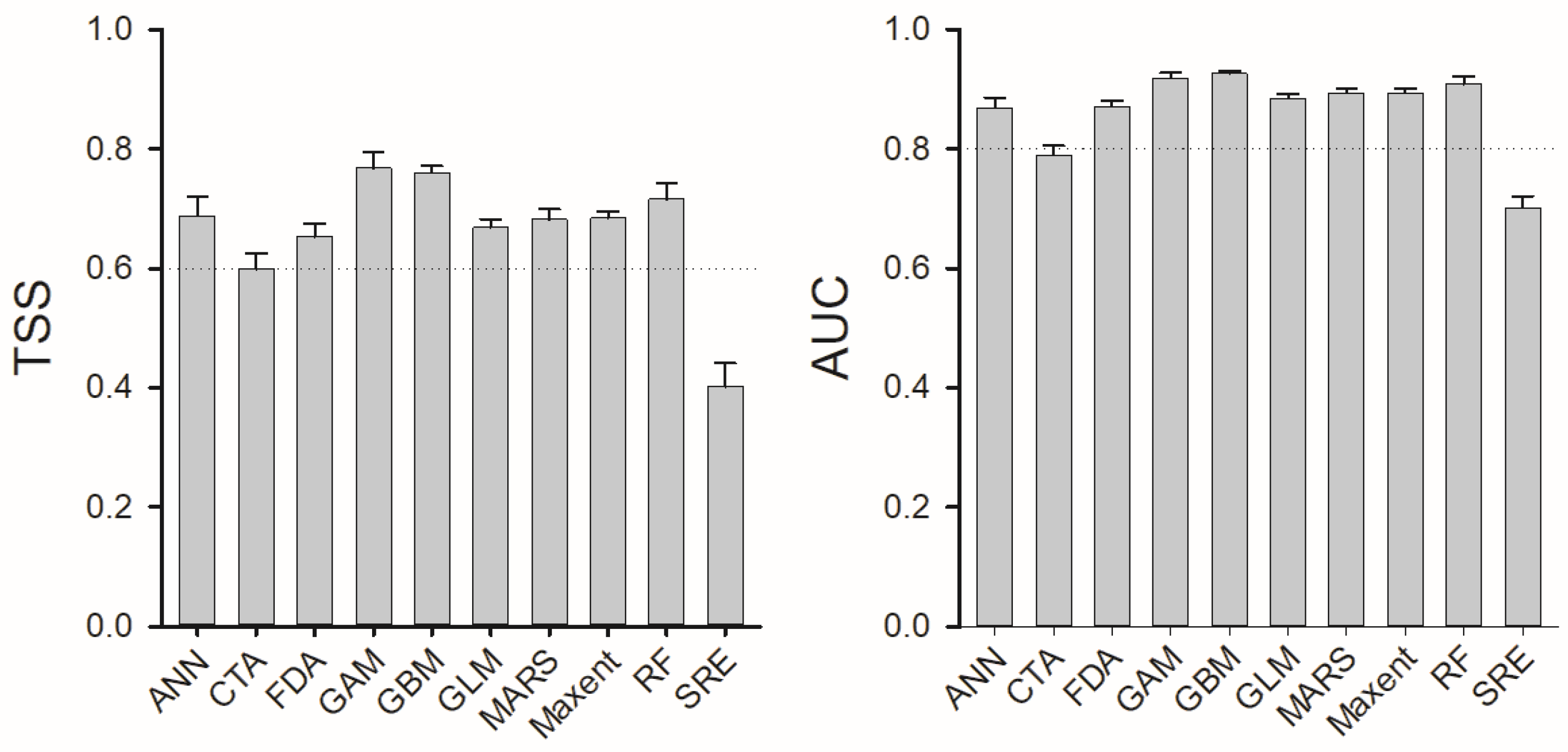
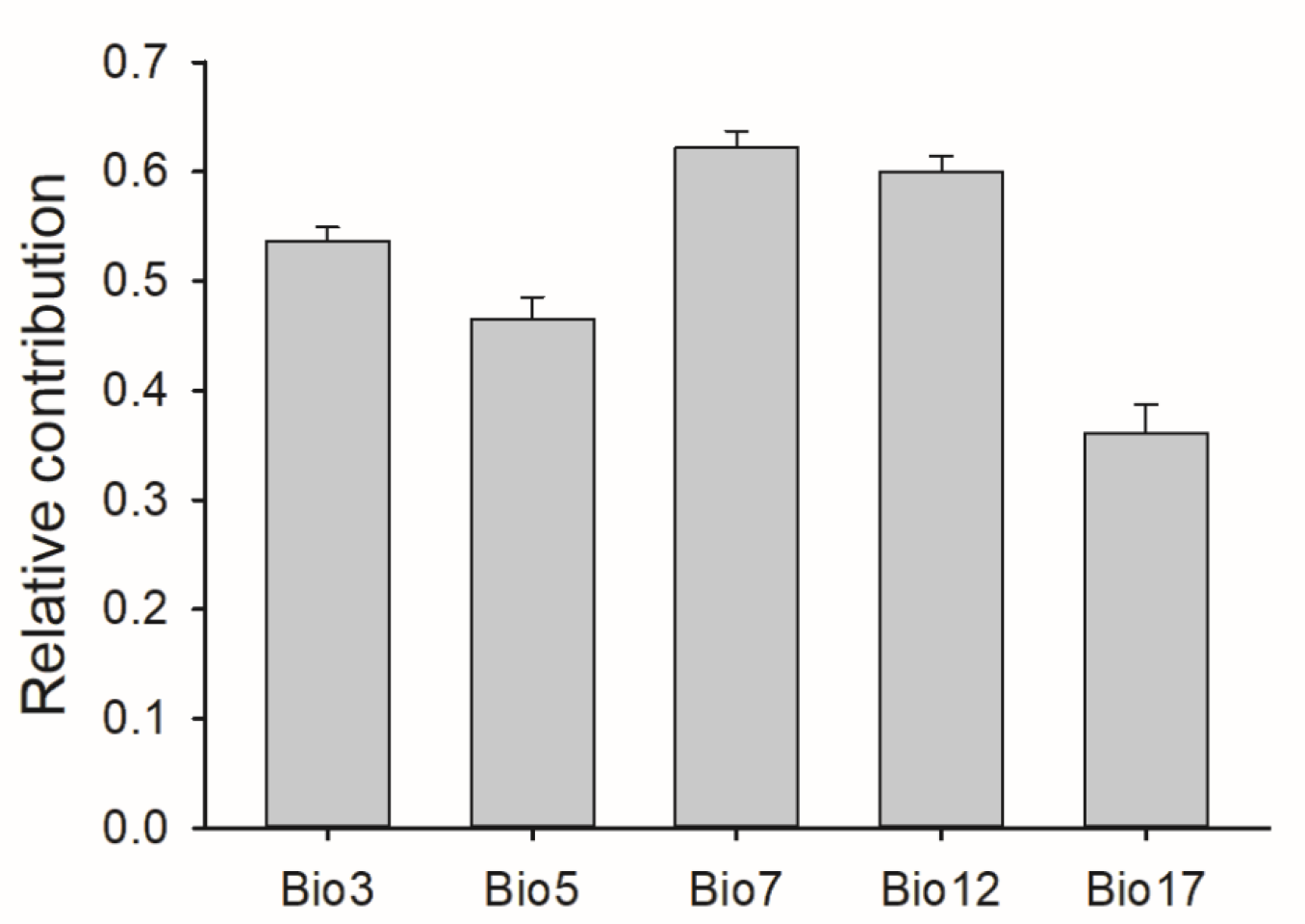
3.1. Predictor Variable Contributions and Model Performance
3.2. Current Potential Distribution
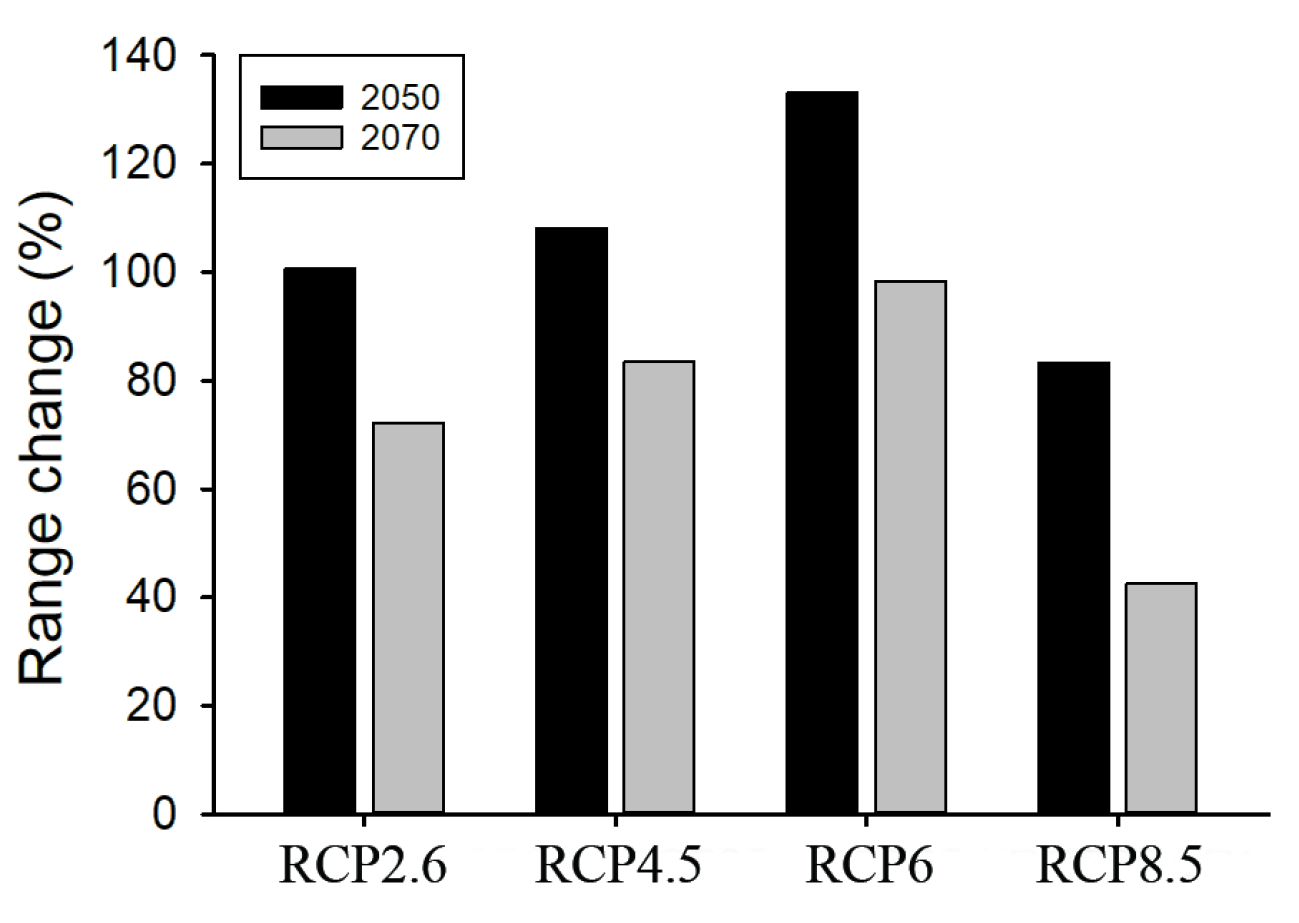
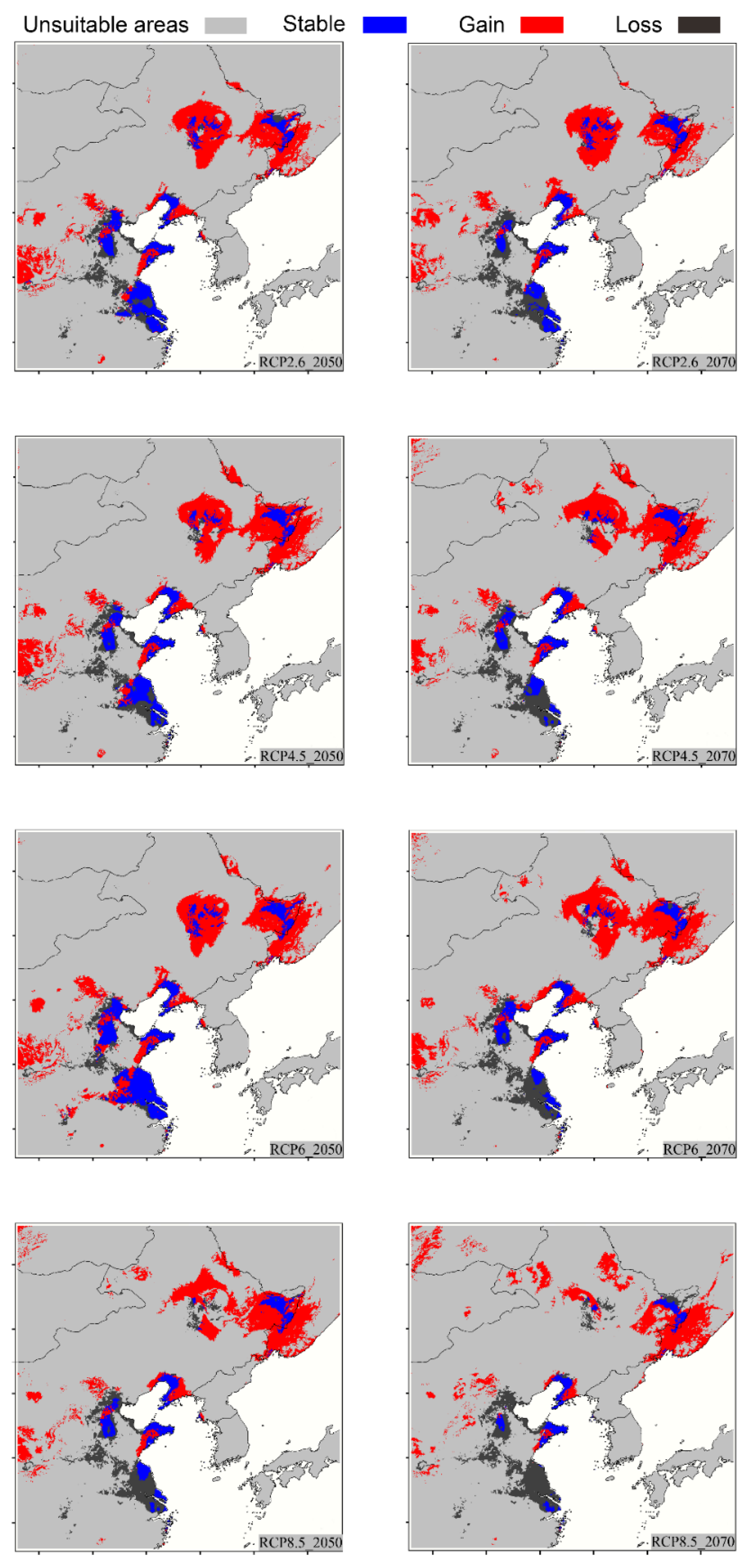
3.3. Projected Potential Distribution in the Future Considering Climate Change
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yousefi, M.; Jouladeh-Roudbar, A.; Kafash, A. Using endemic freshwater fishes as proxies of their ecosystems to identify high priority rivers for conservation under climate change. Ecol. Indic. 2020, 112, 106137. [Google Scholar] [CrossRef]
- Grünig, M.; Mazzi, D.; Calanca, P.; Karger, D.N.; Pellissier, L. Crop and forest pest metawebs shift towards increased linkage and suitability overlap under climate change. Commun. Biol. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Newbold, T. Future effects of climate and land-use change on terrestrial vertebrate community diversity under different scenarios. Proc. R. Soc. B 2018, 285, 20180792. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Scridel, D.; Brambilla, M.; Martin, K.; Lehikoinen, A.; Iemma, A.; Matteo, A.; Jähnig, S.; Caprio, E.; Bogliani, G.; Pedrini, P. A review and meta-analysis of the effects of climate change on Holarctic mountain and upland bird populations. Ibis 2018, 160, 489–515. [Google Scholar] [CrossRef]
- Buckley, L.B.; Khaliq, I.; Swanson, D.L.; Hof, C. Does metabolism constrain bird and mammal ranges and predict shifts in response to climate change? Ecol. Evol. 2018, 8, 12375–12385. [Google Scholar] [CrossRef]
- Subba, B.; Sen, S.; Ravikanth, G.; Nobis, M.P. Direct modelling of limited migration improves projected distributions of Himalayan amphibians under climate change. Biol. Conserv. 2018, 227, 352–360. [Google Scholar] [CrossRef]
- Kafash, A.; Ashrafi, S.; Ohler, A.; Yousefi, M.; Malakoutikhah, S.; Koehler, G.; Schmidt, B.R. Climate change produces winners and losers: Differential responses of amphibians in mountain forests of the Near East. Glob. Ecol. Conserv. 2018, 16, e00471. [Google Scholar] [CrossRef]
- Adler, C.; Athanassiou, C.; Carvalho, M.O.; Emekci, M.; Gvozdenac, S.; Hamel, D.; Riudavets, J.; Stejskal, V.; Trdan, S.; Trematerra, P. Changes in the distribution and pest risk of stored product insects in Europe due to global warming: Need for pan-European pest monitoring and improved food-safety. J. Stored Prod. Res. 2022, 97, 101977. [Google Scholar] [CrossRef]
- Moreno-Letelier, A.; Mastretta-Yanes, A.; Barraclough, T.G. Late M iocene lineage divergence and ecological differentiation of rare endemic Juniperus blancoi: Clues for the diversification of North American conifers. New Phytol. 2014, 203, 335–347. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Cursach, J.; Far, A.J.; Ruiz, M. Geospatial analysis to assess distribution patterns and predictive models for endangered plant species to support management decisions: A case study in the Balearic Islands. Biodivers. Conserv. 2020, 29, 3393–3410. [Google Scholar] [CrossRef]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef]
- Eyres, A.; Eronen, J.T.; Hagen, O.; Böhning-Gaese, K.; Fritz, S.A. Climatic effects on niche evolution in a passerine bird clade depend on paleoclimate reconstruction method. Evolution 2021, 75, 1046–1060. [Google Scholar] [CrossRef]
- An, A.; Zhang, Y.; Cao, L.; Jia, Q.; Wang, X. A potential distribution map of wintering Swan Goose (Anser cygnoides) in the middle and lower Yangtze River floodplain, China. Avian Res. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Austin, M.P. Spatial prediction of species distribution: An interface between ecological theory and statistical modelling. Ecol. Model. 2002, 157, 101–118. [Google Scholar] [CrossRef]
- Palacio, F.X.; Girini, J.M. Biotic interactions in species distribution models enhance model performance and shed light on natural history of rare birds: A case study using the straight-billed reedhaunter Limnoctites rectirostris. J. Avian Biol. 2018, 49, e01743. [Google Scholar] [CrossRef]
- Zhang, Z.; Mammola, S.; Liang, Z.; Capinha, C.; Wei, Q.; Wu, Y.; Zhou, J.; Wang, C. Future climate change will severely reduce habitat suitability of the Critically Endangered Chinese giant salamander. Freshw. Biol. 2020, 65, 971–980. [Google Scholar] [CrossRef]
- Thapa, A.K.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.; Butchart, S.H.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R. Assessing species vulnerability to climate change. Nat. Clim. Chang. 2015, 5, 215–224. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C., Jr.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Araújo, M.B.; Peterson, A.T. Uses and misuses of bioclimatic envelope modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017; pp. 62–63. [Google Scholar]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F. Biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 3.3-7.1. 2019. Available online: https://CRAN.R-project.org/package=biomod2 (accessed on 13 November 2022).
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Zheng, G. A Checklist on the Classification and Distribution of the Birds of China, 3rd ed.; Science Press: Beijing, China, 2017. [Google Scholar]
- BirdLife International. Paradoxornis heudei. The IUCN Red List of Threatened Species 2017 e.T22716844A111640218. Available online: https://www.iucnredlist.org/species (accessed on 13 November 2022).
- Chen, P.; Chen, T.; Liu, B.; Zhang, M.; Lu, C.; Chen, Y. Snakes are the principal nest predators of the threatened reed parrotbill in a coastal wetland of eastern China. Glob. Ecol. Conserv. 2020, 23, e01055. [Google Scholar] [CrossRef]
- Xiong, L.-H.; Lu, J.-J. Habitat specialization in the Reed Parrotbill Paradoxornis heudei–evidence from its distribution and habitat use. Forktail 2013, 29, 64–70. [Google Scholar]
- Boulord, A.; Mei, Z.; Tian-Hou, W.; Xiao-Ming, W.; Jiguet, F. Reproductive success of the threatened Reed Parrotbill Paradoxornis heudei in non-harvested and harvested reedbeds in the Yangtze River estuary, China. Bird Conserv. Int. 2012, 22, 339–347. [Google Scholar] [CrossRef]
- Chang, Q.; Shen, F.; Xie, W.; Zhang, B.; Zhou, K. Isolation and characterization of microsatellite markers from reed parrotbill, Paradoxornis heudei (Aves: Timaliidae). Conserv. Genet. 2009, 10, 237–239. [Google Scholar] [CrossRef]
- Chen, P.; Sun, C.; Liu, B.; Lu, C.; Chen, Y. Complete mitochondrial genome of the reed parrotbill Paradoxornis heudei (Aves: Passeriformes: Muscicapidae). Mitochondrial DNA Part B 2020, 5, 1467–1468. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.; Liu, J.; Yang, C.; Liang, W.; Møller, A.P. Adaptation or ecological trap? Altered nest-site selection by Reed Parrotbills after an extreme flood. Avian Res. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.ybx2fq (accessed on 5 October 2022).
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Santillan, V.; Quitián, M.; Tinoco, B.A.; Zárate, E.; Schleuning, M.; Böhning-Gaese, K.; Neuschulz, E.L. Spatio-temporal variation in bird assemblages is associated with fluctuations in temperature and precipitation along a tropical elevational gradient. PLoS ONE 2018, 13, e0196179. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A. A standard protocol for reporting species distribution models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Hurtt, G.C.; Chini, L.P.; Frolking, S.; Betts, R.; Feddema, J.; Fischer, G.; Fisk, J.; Hibbard, K.; Houghton, R.; Janetos, A. Harmonization of land-use scenarios for the period 1500–2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands. Clim. Chang. 2011, 109, 117–161. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Menke, S.; Holway, D.; Fisher, R.; Jetz, W. Characterizing and predicting species distributions across environments and scales: Argentine ant occurrences in the eye of the beholder. Glob. Ecol. Biogeogr. 2009, 18, 50–63. [Google Scholar] [CrossRef]
- Rushing, C.S.; Royle, J.A.; Ziolkowski, D.J., Jr.; Pardieck, K.L. Migratory behavior and winter geography drive differential range shifts of eastern birds in response to recent climate change. Proc. Natl. Acad. Sci. USA 2020, 117, 12897–12903. [Google Scholar] [CrossRef] [PubMed]
- Barbet-Massin, M.; Jetz, W. A 40-year, continent-wide, multispecies assessment of relevant climate predictors for species distribution modelling. Divers. Distrib. 2014, 20, 1285–1295. [Google Scholar] [CrossRef]
- Xie, W.; Song, K.; Klaus, S.; Swenson, J.E.; Sun, Y.-H. The past, present, and future of the Siberian Grouse (Falcipennis falcipennis) under glacial oscillations and global warming. Avian Res. 2022, 13, 100009. [Google Scholar] [CrossRef]
- Zuckerberg, B.; Woods, A.M.; Porter, W.F. Poleward shifts in breeding bird distributions in New York State. Glob. Chang. Biol. 2009, 15, 1866–1883. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Song, G.; Yu, L.; Gao, B.; Zhang, R.; Qu, Y.; Lambert, D.M.; Li, S.; Zhou, T.; Lei, F. Gene flow maintains genetic diversity and colonization potential in recently range-expanded populations of an oriental bird, the light-vented bulbul (Pycnonotus sinensis, Aves: Pycnonotidae). Divers. Distrib. 2013, 19, 1248–1262. [Google Scholar] [CrossRef]
- Ju, L.; Wang, H.; Jiang, D. Simulation of the Last Glacial Maximum climate over East Asia with a regional climate model nested in a general circulation model. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 248, 376–390. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Evans, T.A.; Williams, S.E.; Edwards, D.P. Microhabitats in the tropics buffer temperature in a globally coherent manner. Biol. Lett. 2014, 10, 20140819. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Miao, K.; Guo, K.; Qian, W.; Sun, W.; Wang, H.; Chang, Q.; Hu, C. Potential Geographic Range of the Endangered Reed Parrotbill Paradoxornis heudei under Climate Change. Biology 2023, 12, 560. https://doi.org/10.3390/biology12040560
Chen W, Miao K, Guo K, Qian W, Sun W, Wang H, Chang Q, Hu C. Potential Geographic Range of the Endangered Reed Parrotbill Paradoxornis heudei under Climate Change. Biology. 2023; 12(4):560. https://doi.org/10.3390/biology12040560
Chicago/Turabian StyleChen, Wan, Keer Miao, Kun Guo, Weiya Qian, Wan Sun, Hao Wang, Qing Chang, and Chaochao Hu. 2023. "Potential Geographic Range of the Endangered Reed Parrotbill Paradoxornis heudei under Climate Change" Biology 12, no. 4: 560. https://doi.org/10.3390/biology12040560
APA StyleChen, W., Miao, K., Guo, K., Qian, W., Sun, W., Wang, H., Chang, Q., & Hu, C. (2023). Potential Geographic Range of the Endangered Reed Parrotbill Paradoxornis heudei under Climate Change. Biology, 12(4), 560. https://doi.org/10.3390/biology12040560





