A Cretaceous Chafer Beetle (Coleoptera: Scarabaeidae) with Exaggerated Hind Legs—Insight from Comparative Functional Morphology into a Possible Spring Movement
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Nomenclatural Acts
2.3. Images
2.4. Phylogenetic Position
2.5. FEA Simulation for the Bumping Protection Function
2.5.1. Testing Hypotheses
2.5.2. The 3D Model Reconstruction of Legs
2.5.3. FEA Simulation
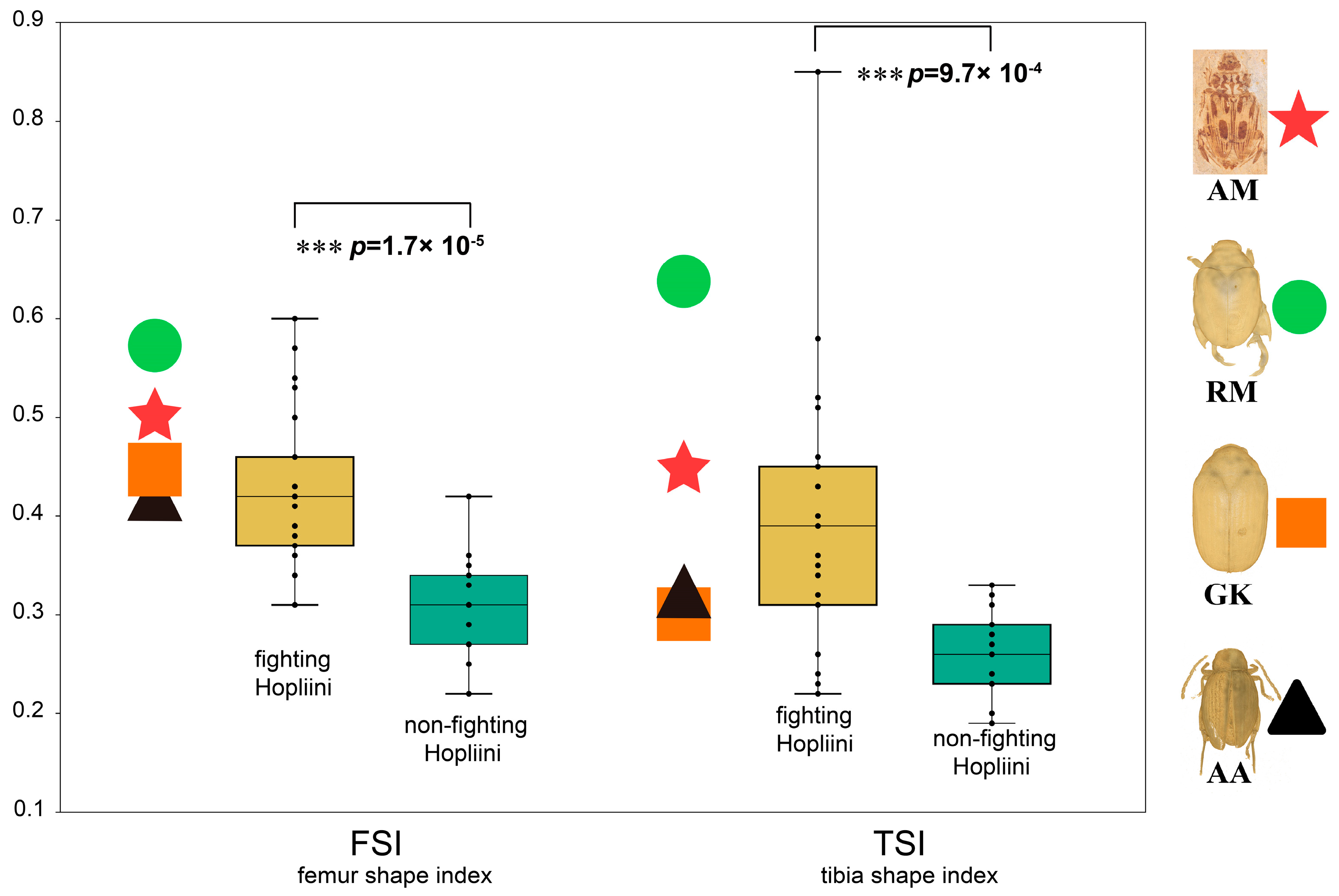
2.6. Morphometric Analyses for the Fighting Function of the Hind Legs
3. Results
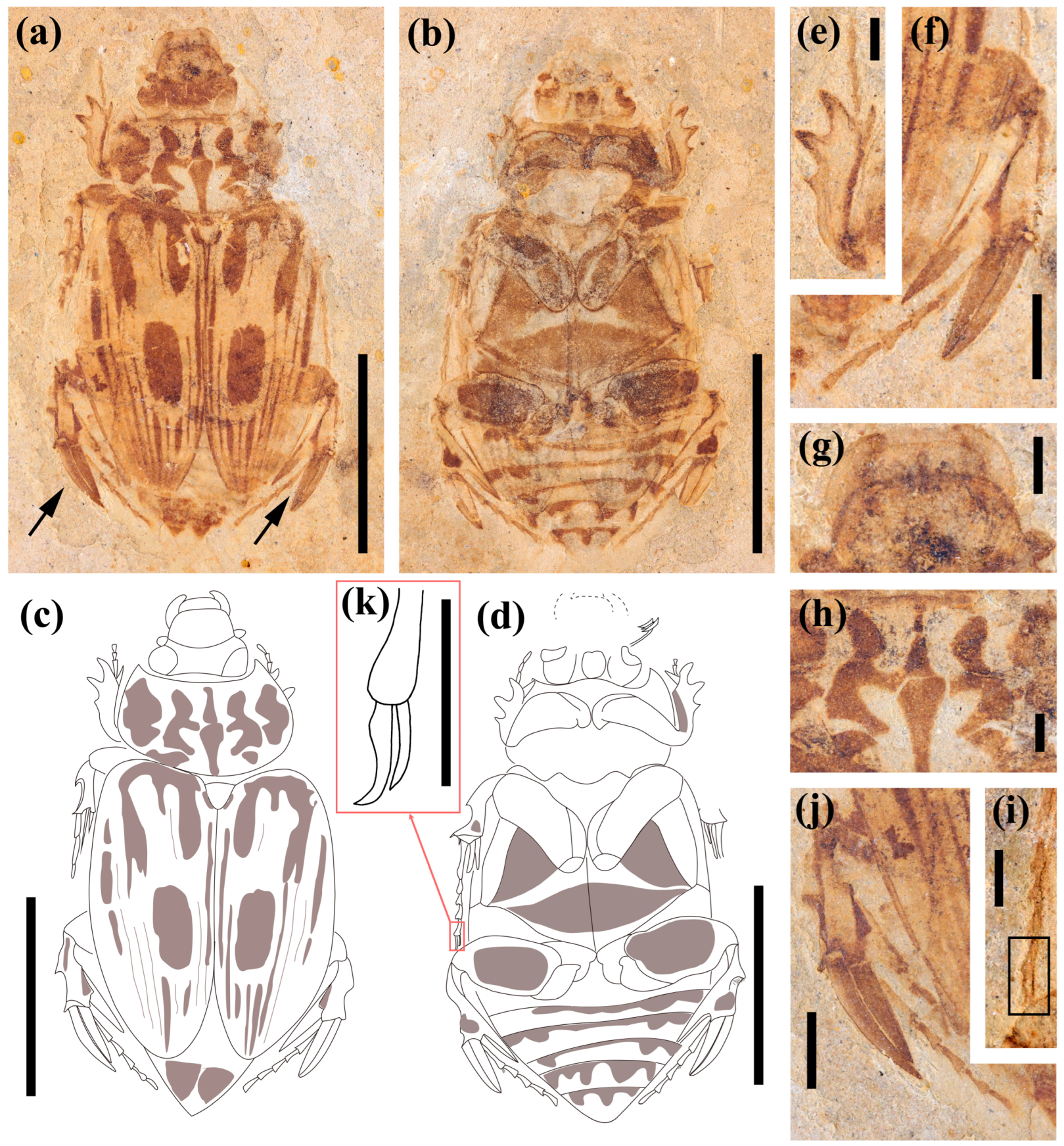
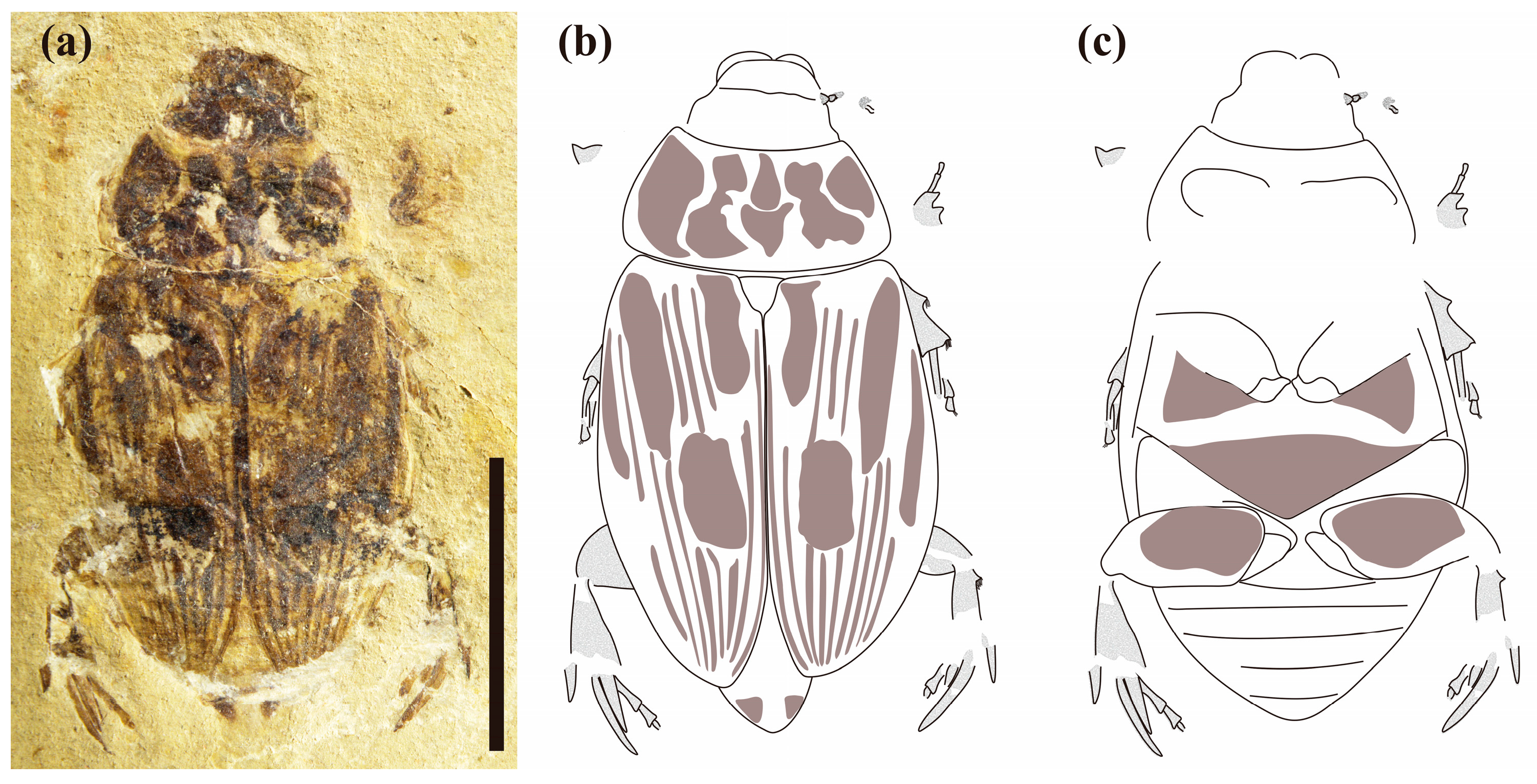
3.1. Systematic Paleontology
- Coleoptera Linnaeus, 1758
- Family: Scarabaeidae Latreille, 1802
- Subfamily: incertae sedis
3.2. Comparative Morphology and Phylogenetic Analyses
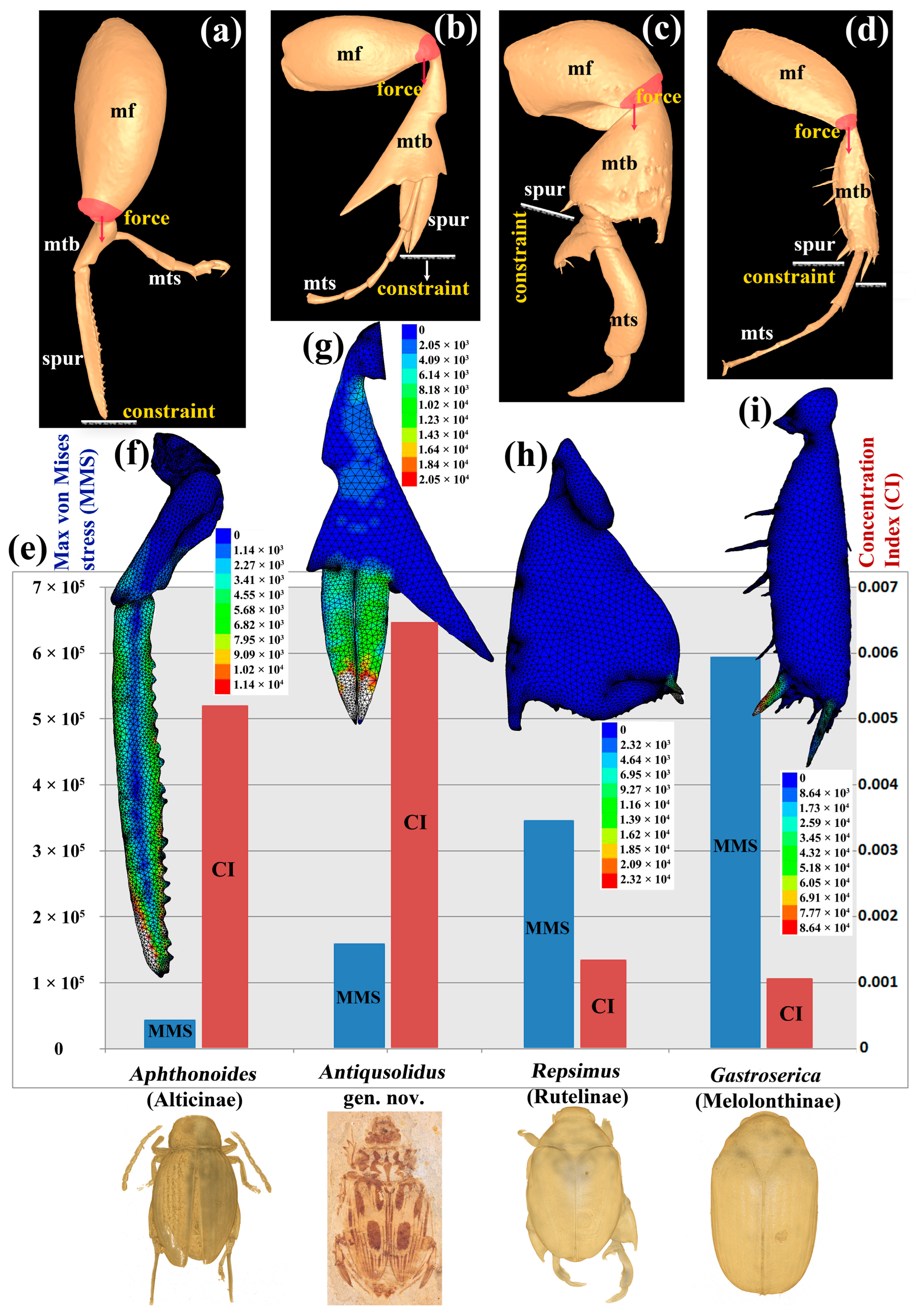
3.3. The Function of the Exaggerated Hind Legs
4. Discussion
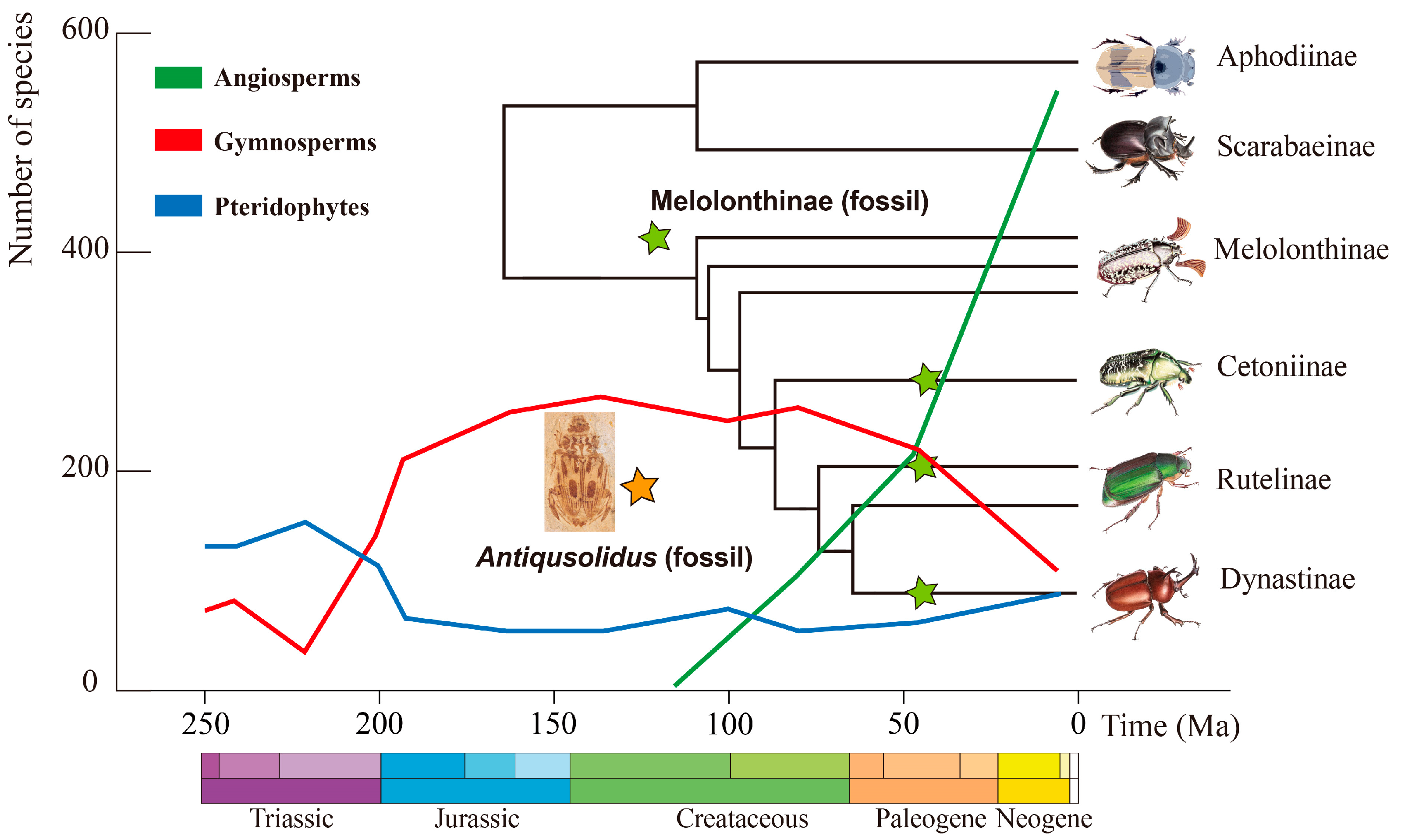
4.1. Phylogenetic Position of Antiqusolidus gen. n.
4.1.1. The Earliest Record of Phytophagous Scarab Beetles
4.1.2. Intraspecific Morphological Variability
4.2. Exaggerated Hind Legs and Their Possible Functions
4.2.1. Exaggerated Spurs on Hind Legs with Possible “Springing” Function
4.2.2. Elongated Process with Possible “Fighting” Function
4.3. Possible Biology Inferred from Marking Pattern
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavine, L.; Gotoh, H.; Brent, C.S.; Dworkin, I.; Emlen, D.J. Exaggerated Trait Growth in Insects. Annu. Rev. Entomol. 2015, 60, 453–472. [Google Scholar] [CrossRef]
- Dietrich, C.H.; McKamey, S.; Deitz, L. Morphology-based phylogeny of the treehopper family Membracidae (Hemiptera: Cicadomorpha: Membracoidea). Syst. Entomol. 2001, 26, 213–239. [Google Scholar] [CrossRef]
- Emlen, D.J.; Lavine, L.C.; Ewen-Campen, B. On the origin and evolutionary diversification of beetle horns. Proc. Natl. Acad. Sci. USA 2007, 104, 8661–8668. [Google Scholar] [CrossRef]
- Emlen, D.J.; Philips, T.K. Phylogenetic evidence for an association between tunneling behavior and the evolution of horns in dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopt. Bull. 2006, 60, 47–56. [Google Scholar] [CrossRef]
- Gill, B.; Howden, H. A review of the North American genus Aphonus LeConte (Coleoptera: Scarabaeidae: Dynastinae). Coleopt. Bull. 1985, 39, 119–129. [Google Scholar]
- Scudder, G. Monosteira unicostata (Mulsant & Rey) (Hemiptera: Tingidae) established in North America, with a key to the genera of Tingidae in Canada. Entomol. Am. 2013, 118, 295–297. [Google Scholar]
- Kawano, K. Horn and wing allometry and male dimorphism in giant rhinoceros beetles (Coleoptera: Scarabaeidae) of tropical Asia and America. Ann. Entomol. Soc. Am. 1995, 88, 92–99. [Google Scholar] [CrossRef]
- Emlen, D.J.; Nijhout, H.F. The development and evolution of exaggerated morphologies in insects. Annu. Rev. Entomol. 2000, 45, 661–708. [Google Scholar] [CrossRef]
- Wang, Q.; Shih, C.K.; Ren, D. The earliest case of extreme sexual display with exaggerated male organs by two middle Jurassic mecopterans. PLoS ONE 2013, 8, e71378. [Google Scholar] [CrossRef]
- Perrichot, V.; Wan, B.; Engel, M.S. Extreme morphogenesis and ecological specialization among cretaceous basal ants. Curr. Biol. 2016, 26, 1468–1472. [Google Scholar] [CrossRef]
- Konstantinov, A.S. Possible living fossil in Bolivia: A new genus of flea beetles with modified hind legs (Coleoptera, Chrysomelidae, Galerucinae, Alticini). ZooKeys 2016, 592, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Nel, A.; Jarzembowski, E.A.; Chang, S.C.; Zhang, H.; Xia, F.; Liu, H.; Wang, B. Extreme adaptations for probable visual courtship behaviour in a Cretaceous dancing damselfly. Sci. Rep. 2017, 7, 44932. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F. The Insects: Structure and Function, 5th ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Bußhardt, P.; Kunze, D.; Gorb, S.N. Interlocking-based attachment during locomotion in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). Sci. Rep. 2014, 4, 6998. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sha, J.; Zhou, Z.; Fürsich, F.T. The Jehol Biota: Definition and distribution of exceptionally preserved relicts of a continental Early Cretaceous ecosystem. Cretac. Res. 2013, 44, 30–38. [Google Scholar] [CrossRef]
- Ahrens, D.; Schwarzer, J.; Vogler, A.P. The evolution of scarab beetles tracks the sequential rise of angiosperms and mammals. Proc. R. Soc. B-Biol. Sci. 2014, 281, 20141470. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Shih, C.; Rasnitsyn, A.P.; Xu, X.; Wang, S.; Ren, D. New transitional fleas from China highlighting diversity of early Cretaceous ectoparasitic Insects. Curr. Biol. 2013, 23, 1261–1266. [Google Scholar] [CrossRef]
- Ren, D.; Shih, C.; Gao, T.; Wang, Y.; Yao, Y. (Eds.) Rhythms of Insect Evolution-Evidence from the Jurassic and Cretaceous in Northern China; Wiley Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Yao, Y.; Cai, W.; Xu, X.; Shih, C.; Engel, M.S.; Zheng, X.; Zhao, Y.; Ren, D. Blood-feeding true bugs in the early Cretaceous. Curr. Biol. 2014, 24, 1786–1792. [Google Scholar] [CrossRef]
- Zhao, H.; Bai, M.; Shih, C.; Ren, D. Two new glaphyrids (Coleoptera, Scarabaeoidea) from the Jehol Biota, China. Cretac. Res. 2016, 59, 1–9. [Google Scholar] [CrossRef]
- Xiao, L.; Labandeira, C.C.; Dilcher, D.L.; Ren, D. Arthropod and fungal herbivory at the dawn of angiosperm diversification: The Rose Creek plant assemblage of Nebraska, U.S.A. Cretac. Res. 2022, 131, 105088. [Google Scholar] [CrossRef]
- Howden, H.F. Larval and adult characters of Frickius germain, its relationship to the Geotrupini, and a phylogeny of some major taxa in the Scarabaeoidea (Insecta, Coleoptera). Can. J. Zool. 1982, 60, 2713–2724. [Google Scholar] [CrossRef]
- Browne, J.; Scholtz, C.H. Evolution of the scarab hindwing articulation and wing base: A contribution toward the phylogeny of the Scarabaeidae (Scarabaeoidea: Coleoptera). Syst. Entomol. 1998, 23, 307–326. [Google Scholar] [CrossRef]
- Li, S.; Lu, Y.; Wang, B.; Li, J.; Yang, X.; Bai, M. †Electrorubesopsinae, a new subfamily from Cretaceous Burmese amber, as the possible sister group of Dynamopodinae (Coleoptera: Scarabaeidae). J. Syst. Palaeontol. 2019, 17, 349–357. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Takahashi, Y. First and oldest Leptochirini rove beetles illuminate diverse cephalic structures in the Cretaceous (Coleoptera: Staphylinidae: Osoriinae). Syst. Entomol. 2019, 44, 588–611. [Google Scholar] [CrossRef]
- Von Mises, R. Mechanik der festen Körper im plastisch deformablen Zustand. Nachr. Von Der Königlichen Ges. Der Wiss. Zu Göettingen (Math. Phys. Kl.) 1913, 1, 582–592. [Google Scholar]
- Sun, J.Y.; Tong, J.; Ma, Y.H. Nanomechanical behaviours of cuticle of three kinds of beetle. J. Bionic. Eng. 2008, 5, 152–157. [Google Scholar] [CrossRef]
- Nikolov, S.; Petrov, M.; Lymperakis, L.; Friák, M.; Sachs, C.; Fabritius, H.-O.; Raabe, D.; Neugebauer, J. Revealing the design principles of high-performance biological composites using ab initio and multiscale simulations: The example of lobster cuticle. Adv. Mater. 2010, 22, 519–526. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Z.; Guo, C. Morphology and mechanical properties of cybister elytra. Chin. Sci. Bull. 2010, 55, 771–776. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Yu, Q.; Dai, Z. Measurements on mechanical parameters and studies on microstructure of elytra in beetles. Acta. Mater. Comp. Sin. 2007, 24, 92–98. [Google Scholar]
- Gil, L.L.; Marcé-Nogué, J.; Sánchez, M. Insights on the controversy about material data for the comparison of biomechanical performance of vertebrates. Palaeontol. Electron. 2015, 18, 1–24. [Google Scholar]
- Goyens, J.; Soons, J.; Aerts, P.; Dirckx, J. Finite-element modelling reveals force modulation of jaw adductors in stag beetles. J. R. Soc. Interface 2014, 11, 20140908. [Google Scholar] [CrossRef] [PubMed]
- Colville, J.F.; Picker, M.D.; Cowling, R.M. Feeding ecology and sexual dimorphism in a speciose flower beetle clade (Hopliini: Scarabaeidae). PeerJ 2018, 6, e4632. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, D.; Scott, M.; Vogler, A.P. A phylogeny of monkey beetles based on mitochondrial and ribosomal DNA (Coleoptera: Scarabaeidae: Hopliini). Mol. Phylogenet. Evol. 2011, 60, 408–415. [Google Scholar] [CrossRef]
- Krell, F.T. The fossil record of Mesozoic and Tertiary Scarabaeoidea (Coleoptera: Polyphaga). Invertebr. Taxon. 2000, 14, 871–905. [Google Scholar] [CrossRef]
- Scholtz, C.H.; Grebennikov, V.V. 12 Scarabaeiformia Crowson, 1960. In Handbook of Zoology; De Gruyter: Berlin, Germany, 2016; Volume IV, Part 38; pp. 345–425. [Google Scholar]
- Huchet, J.B. Insecta Coleoptera Chironidae. Faune De Madag. 2003, 90, 1–91. [Google Scholar]
- Stebnicka, Z.T.; Dellacasa, M.; Skelley, P.E. Review of New World Aegialiini (Coleoptera: Scarabaeidae: Aphodiinae), with descriptions of two new genera from South America. Insecta Mundi 2004, 17, 73–83. [Google Scholar]
- Elshewy, D.A. Taxonomic revision on subfamily Eremazinae, (Coleoptera: Scarabaeidae) in Egypt. Egypt. Acad. J. Biol. Sci. A Entomol. 2018, 11, 13–19. [Google Scholar] [CrossRef]
- Neita-Moreno, J.C.; Agrain, F.A.; Eberle, J.; Ahrens, D.; Pereyra, V. On the phylogenetic position and systematics of extant and fossil Aclopinae (Coleoptera: Scarabaeidae). Syst. Entomol. 2019, 44, 709–727. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Hastings, A.M.; Dallwitz, M.J.; Paine, T.A.; Zurcher, E.J. Beetles of the World; Version 1.0. Licensed Data Set; CSIRO Entomology: Canberra, Australia, 1999. [Google Scholar]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef]
- Gunter, N.L.; Weir, T.A.; Slipinksi, A.; Bocak, L.; Cameron, S.L. If dung beetles (Scarabaeidae: Scarabaeinae) arose in association with dinosaurs, did they also suffer a mass co-extinction at the K-Pg boundary? PLoS ONE 2016, 11, e0153570. [Google Scholar] [CrossRef]
- Krell, F.T. Catalogue of fossil Scarabaeoidea (Coleoptera: Polyphaga) of the Mesozoic and Tertiary-Version 2007. Denver Mus. Nat. Sci. Tech. Rep. 2007, 8, 1–79. [Google Scholar]
- Ritcher, P.O. Biology of Scarabaeidae. Annu. Rev. Entomol. 1958, 3, 311–334. [Google Scholar] [CrossRef]
- Krell, F.T. Fossil record and evolution of Scarabaeoidea (Coleoptera: Polyphaga). Coleopt. Soc. Monogr. Patricia Vaurie Ser. 2006, 5, 120–143. [Google Scholar]
- Woolley, C. The first scarabaeid beetle (Coleoptera, Scarabaeidae, Melolonthinae) described from the Mesozoic (Late-Cretaceous) of Africa. Afr. Invertebr. 2016, 57, 53–66. [Google Scholar] [CrossRef]
- Nikolajev, G.V. Mezozoiskii Etap Evolyutsii Plastinchatousykh (Insecta: Coleoptera: Scarabaeoidea); Kazak Universiteti: Almaty, Kazakhstan, 2007. [Google Scholar]
- Stephen, F.M.; Dahlsten, D.L. Arrival sequence of arthropod complex following attack by Dendroctonus brevicomis (Coleoptera: Scolytidae) in ponderosa pine. Can. Entomol. 1976, 108, 283–304. [Google Scholar] [CrossRef]
- Cai, C.; Escalona, H.E.; Li, L.; Yin, Z.; Huang, D.; Engel, M.S. Beetle Pollination of Cycads in the Mesozoic. Curr. Biol. 2018, 28, 2806–2812. [Google Scholar] [CrossRef]
- Curoe, D.; Moron, M.A. A new species of Promacropoides Sigwalt (Coleoptera: Scarabaeidae: Rutelinae) from Panama. Zootaxa 2003, 312, 1–8. [Google Scholar] [CrossRef]
- Delgado, L.; Blackaller-Bages, J. A new Mexican species of Homoiosternus (Coleoptera: Melolonthidae: Rutelinae). J. N. Y. Entomol. Soc. 1997, 105, 170–179. [Google Scholar]
- Moron, M.A.; Howden, H.F. A second species of Plesiosternus Moron with notes on other Heterosternina (Coleoptera: Scarabaeidae: Rutelinae). Coleopt. Bull. 1992, 46, 15–19. [Google Scholar]
- Moron, R.M.A. A revision of the subtribe Heterosternina (Coleoptera, Melolonthidae, Rutelinae). Folia Entomol. Mex. 1983, 55, 31–101. [Google Scholar]
- Katovich, K. A generic-level phylogenetic review of the Macrodactylini (Coleoptera: Scarabaeidae: Melolonthinae). Insecta Mundi 2008, 23, 1–78. [Google Scholar]
- Katsuki, M.; Yokoi, T.; Funakoshi, K. Enlarged hind legs and sexual behavior with male-male interaction in Sagra femorata (Coleoptera: Chrysomelidae). Entomol. News 2014, 124, 211–220. [Google Scholar] [CrossRef]
- Eberhard, W.G. Sexual behavior of Acanthocephala declivis guatemalana (Hemiptera: Coreidae) and the allometric scaling of their modified hind legs. Ann. Entomol. Soc. Am. 1998, 91, 863–871. [Google Scholar] [CrossRef]
- Miyatake, T. Functional morphology of the hind legs as weapons for male contests in Leptoglossus australis (Heteroptera: Coreidae). J. Insect Behav. 1997, 10, 727–735. [Google Scholar] [CrossRef]
- Shamim, M. The genus Streblocera Westwood (Hymenoptera: Braconidae: Euphorinae) from India, with descriptions of 9 new species. Turk. J. Zool. 2013, 37, 385–405. [Google Scholar] [CrossRef]
- Ruan, Y.; Konstantinov, A.S.; Shi, G.; Tao, Y.; Li, Y.; Johnson, A.J.; Luo, X.; Zhang, X.; Zhang, M.; Wu, J.; et al. The jumping mechanism of flea beetles (Coleoptera, Chrysomelidae, Alticini), its application to bionics and preliminary design for a robotic jumping leg. ZooKeys 2020, 915, 87–105. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, C.; He, J.; Yue, Y.; Wang, J.; Xiao, D. Observation and analysis of diving beetle movements while swimming. Sci. Rep. 2021, 11, 16581. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.M.; Hu, Y.; Moczek, A.P. The origins of novelty from within the confines of homology: The developmental evolution of the digging tibia of dung beetles. Proc. Royal Soc. B 2019, 286, 20182427. [Google Scholar] [CrossRef]
- Crowson, R. The Biology of the Coleoptera; Academic Press: London, UK, 1981. [Google Scholar]
- Dumont, E.R.; Grosse, I.R.; Slater, G.J. Requirements for comparing the performance of finite element models of biological structures. J. Theor. Biol. 2009, 256, 96–103. [Google Scholar] [CrossRef]
- Emlen, D.J. The Evolution of Animal Weapons. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 387–413. [Google Scholar] [CrossRef]
- Bai, M.; Beutel, R.G.; Zhang, W.; Wang, S.; Hörnig, M.K.; Gröhn, C.; Yan, E.; Yang, X.; Wipfler, B. A new Cretaceous insect with a unique cephalo-thoracic scissor device. Curr. Biol. 2018, 28, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Shih, C.; Labandeira, C.C.; Santiago-Blay, J.A.; Yao, Y.; Ren, D. Convergent evolution of ramified antennae in insect lineages from the Early Cretaceous of Northeastern China. Proc. R. Soc. B-Biol. Sci. 2016, 283, 20161448. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shih, C.; Wang, C.; Pang, H.; Ren, D. Forever love: The hitherto earliest record of copulating insects from the middle Jurassic of China. PLoS ONE 2013, 8, e78188. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, A.; Peñalver, E.; Delclòs, X.; Engel, M.S. Engel Mating and aggregative behaviors among basal hexapods in the Early Cretaceous. PLoS ONE 2018, 13, e0191669. [Google Scholar] [CrossRef]
- Li, L.; Ren, D.; Wang, Z. New prophalangopsids from Late Mesozoic of China (Orthoptera, Prophalangopsidae, Aboilinae). Acta Zool. Sin. 2007, 32, 412–422. [Google Scholar]
- Ren, D.; Yin, J. New ‘osmylid-like’ fossil Neuroptera from the Middle Jurassic of Inner Mongolia, China. J. N. Y. Entomol. Soc. 2003, 111, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, D.; Shih, C.K. New discovery of Palaeontinid fossils from the Middle Jurassic in Daohugou, Inner Mongolia (Homoptera, Palaeontinidae). Sci. China Ser. D-Earth Sci. 2007, 50, 481–486. [Google Scholar] [CrossRef]
- Marianne, K. Diversity and phenotypes of diurnal and nocturnal coleopterans in the Monteverde cloud forest zone. In Tropical Ecology Collection; Monteverde Institute: Monteverde, Costa Rica, 2016; p. 178. Available online: https://digitalcommons.usf.edu/tropical_ecology/178 (accessed on 20 December 2022).
- Bocek, M.; Kusy, D.; Motyka, M.; Bocak, L. Persistence of multiple patterns and intraspecific polymorphism in multi-species Müllerian communities of net-winged beetles. Front. Zool. 2019, 16, 38. [Google Scholar] [CrossRef]
- Motyka, M.; Bocek, M.; Kusy, D.; Bocak, L. Interactions in multi-pattern Müllerian communities support origins of new patterns, false structures, imperfect resemblance and mimetic sexual dimorphism. Sci. Rep. 2020, 10, 11193. [Google Scholar] [CrossRef]
- Arrow, G.J. The fauna of British India, including Ceylon and Burma. In Coleoptera: Lamellicornia Part II (Rutelinae, Desmonycinae and Euchirinae; Taylor & Francis: Abingdon-on-Thames, UK, 1917. [Google Scholar]
- Parker, A.R.; McKenzie, D.R.; Large, M.C.J. Multilayer reflectors in animals using green and gold beetles as contrasting examples. J. Exp. Biol. 1998, 201, 1307–1313. [Google Scholar] [CrossRef]
- Jameson, M.L. Phylogenetic analysis of the subtribe rutelina and revision of the Rutela generic groups (Coleoptera: Scarabaeidae: Rutelinae: Rutelini). Bull. Univ. Nebr. State Mus. 1997, 14, 1–184. [Google Scholar]
- Šípek, P.; Fabrizi, S.; Eberle, J.; Ahrens, D. A molecular phylogeny of rose chafers (Coleoptera: Scarabaeidae: Cetoniinae) reveals a complex and concerted morphological evolution related to their flight mode. Mol. Phylogenet. Evol. 2016, 101, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Calisto, V.; Morelli, E. Description of the immature stages of Rutela lineola (Linnaeus, 1767) (Coleoptera: Melolonthidae: Rutelinae). Acta Zool. Mex. 2011, 27, 67–76. [Google Scholar] [CrossRef]
| Characters | Melolonthinae | Dynastinae | Rutelinae | Antiqusolidus gen.n. |
|---|---|---|---|---|
| Mandible | Invisible from the dorsal view | Visible from the dorsal view | Invisible from the dorsal view, or sometimes slightly visible | Visible from the dorsal view |
| Labrum | At least partly visible, or concealed beneath clypeus or apparently absent | Concealed beneath clypeus or apparently absent | At least partly visible, or concealed beneath clypeus or apparently absent | Partly visible |
| Color | Usually reddish brown or black, sometimes with metallic blue or green luster or distinctly marked with patches of scales | Usually testaceous, brown or black | Dull browns and yellows (nocturnal species) to brightly patterned and brilliantly metallic, even in silver and gold | Color marking pattern |
| Claws | Simple, cleft, toothed, serrate or pectinate usually paired, equal in thickness and length | All subequal in size | Unequal in length or size, and frequently weakly split at apex; one claw of each pair reduced | Seems unequal in length or size |
| Eyes | Not or only slightly protuberant, or strongly protuberant | Not or only slightly protuberant | Not or only slightly protuberant, or strongly protuberant | Strongly protuberant |
| Frontoclypeal suture | Absent or incomplete, or indistinctly impressed, or distinctly impressed | Absent or incomplete | Absent or incomplete, or indistinctly impressed, or distinctly impressed | Distinctly impressed |
| Ratio of elytral length to pronotum length | 1.55–4.55 | 0.45–2.52 | 1.6–4.1 | 3 |
| Mesoventral process | Absent or not extending to middle of mesocoxal cavity, or extending at least to middle of mesocoxal cavity | Extending at least to middle of mesocoxal cavity | Absent or not extending to middle of mesocoxal cavity, or extending at least to middle of mesocoxal cavity | Absent |
| Abdominal process | Broadly rounded or angulate, or absent | Acute or narrowly rounded, or broadly rounded or angulate | Acute or narrowly rounded, or broadly rounded or angulate, or absent | Acute or narrowly rounded |
| Tarsi | Normally thin and undeveloped | Normally stubby and developed | Normally stubby and developed | Thin and undeveloped |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Ahrens, D.; Shih, C.; Shaw, J.J.; Yang, X.; Ren, D.; Bai, M. A Cretaceous Chafer Beetle (Coleoptera: Scarabaeidae) with Exaggerated Hind Legs—Insight from Comparative Functional Morphology into a Possible Spring Movement. Biology 2023, 12, 237. https://doi.org/10.3390/biology12020237
Lu Y, Ahrens D, Shih C, Shaw JJ, Yang X, Ren D, Bai M. A Cretaceous Chafer Beetle (Coleoptera: Scarabaeidae) with Exaggerated Hind Legs—Insight from Comparative Functional Morphology into a Possible Spring Movement. Biology. 2023; 12(2):237. https://doi.org/10.3390/biology12020237
Chicago/Turabian StyleLu, Yuanyuan, Dirk Ahrens, Chungkun Shih, Josh Jenkins Shaw, Xingke Yang, Dong Ren, and Ming Bai. 2023. "A Cretaceous Chafer Beetle (Coleoptera: Scarabaeidae) with Exaggerated Hind Legs—Insight from Comparative Functional Morphology into a Possible Spring Movement" Biology 12, no. 2: 237. https://doi.org/10.3390/biology12020237
APA StyleLu, Y., Ahrens, D., Shih, C., Shaw, J. J., Yang, X., Ren, D., & Bai, M. (2023). A Cretaceous Chafer Beetle (Coleoptera: Scarabaeidae) with Exaggerated Hind Legs—Insight from Comparative Functional Morphology into a Possible Spring Movement. Biology, 12(2), 237. https://doi.org/10.3390/biology12020237






