Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Dietary Treatments
2.2. Growth Performance and Diarrhea
2.3. Sample Collections
2.4. Digestive Enzyme Activity
2.5. Morphological Analysis of Small Intestine
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Relative Quantitative Real-Time PCR(qRT-PCR)
2.8. Western Blot Analysis of Intestinal Mucosa Tight Junction Proteins
2.9. Analysis of Microbiota in Cecum Digesta
2.10. Measurement of Organic Acid in Cecum Digesta
2.11. Statistical Analysis
3. Results
3.1. BL-S6 Supplementation of Weaning Piglets’ Diets Affects Growth Performance and Diarrhea
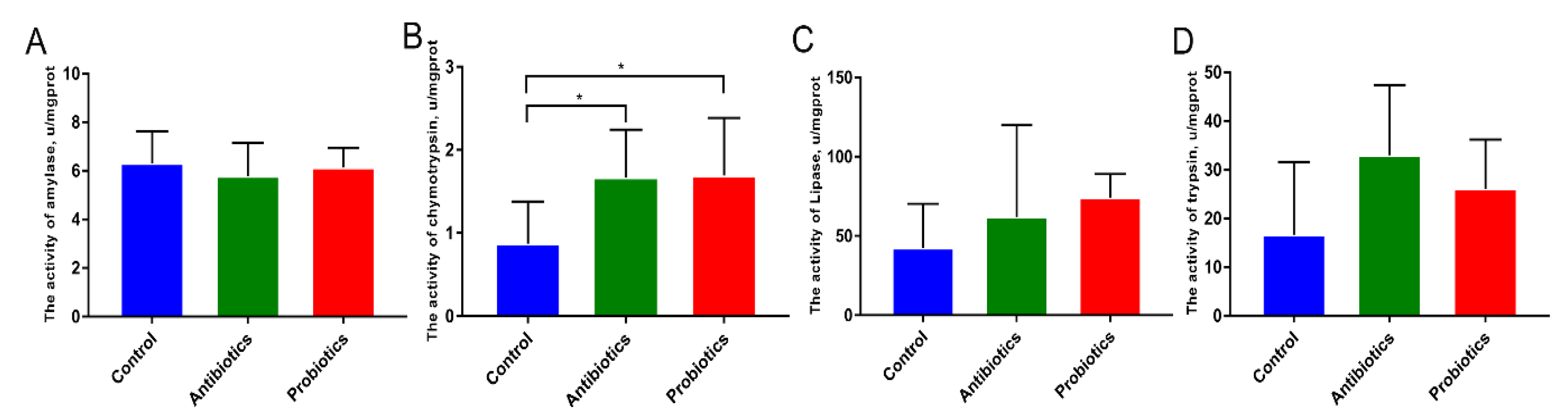
3.2. Effect of Dietary BL-S6 Supplementation on Digestive Enzyme Activity
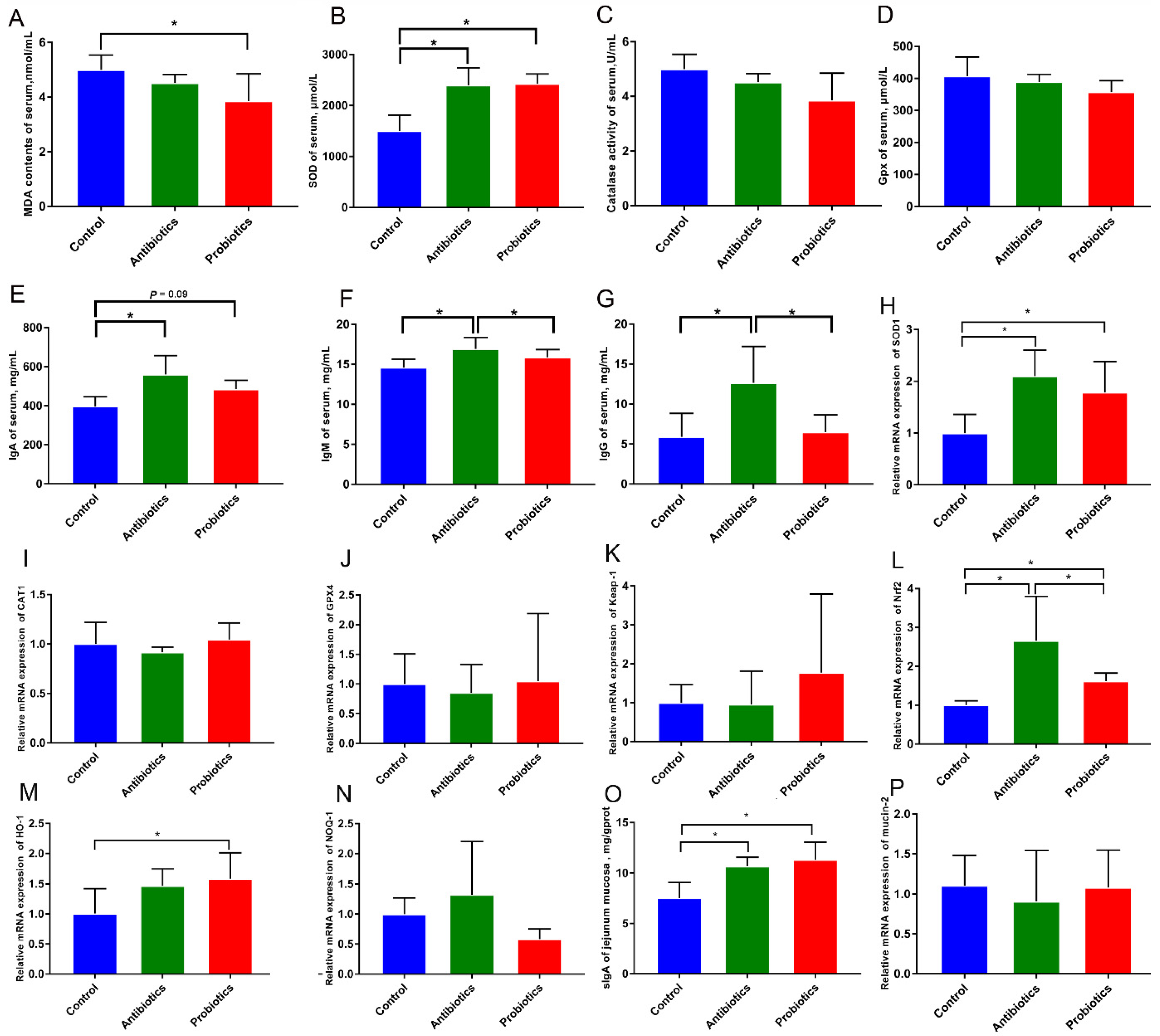
3.3. Dietary BL-S6 Supplementation Improves the Immune Antioxidant Status of Serum and Jejunum Mucosa
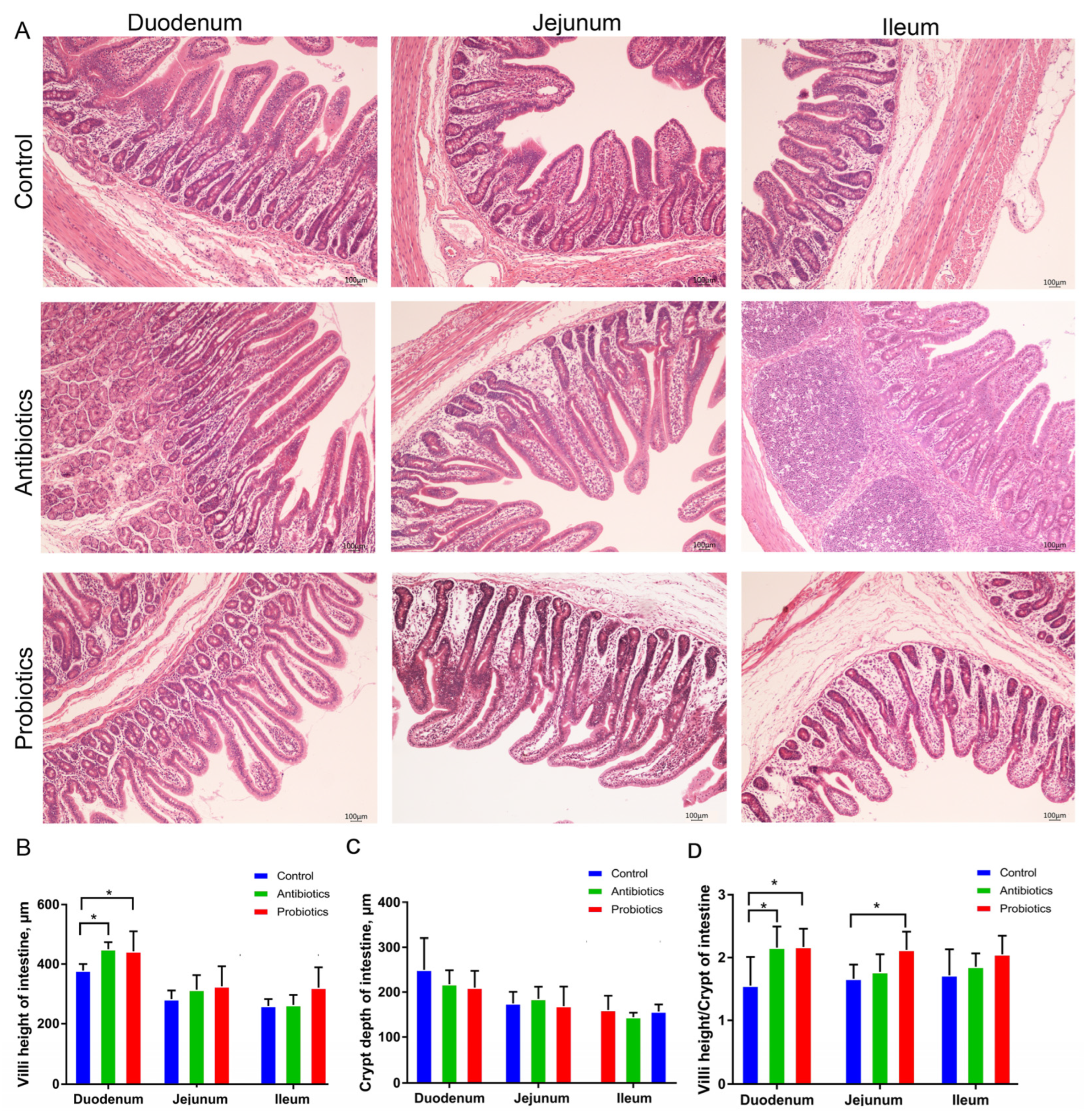
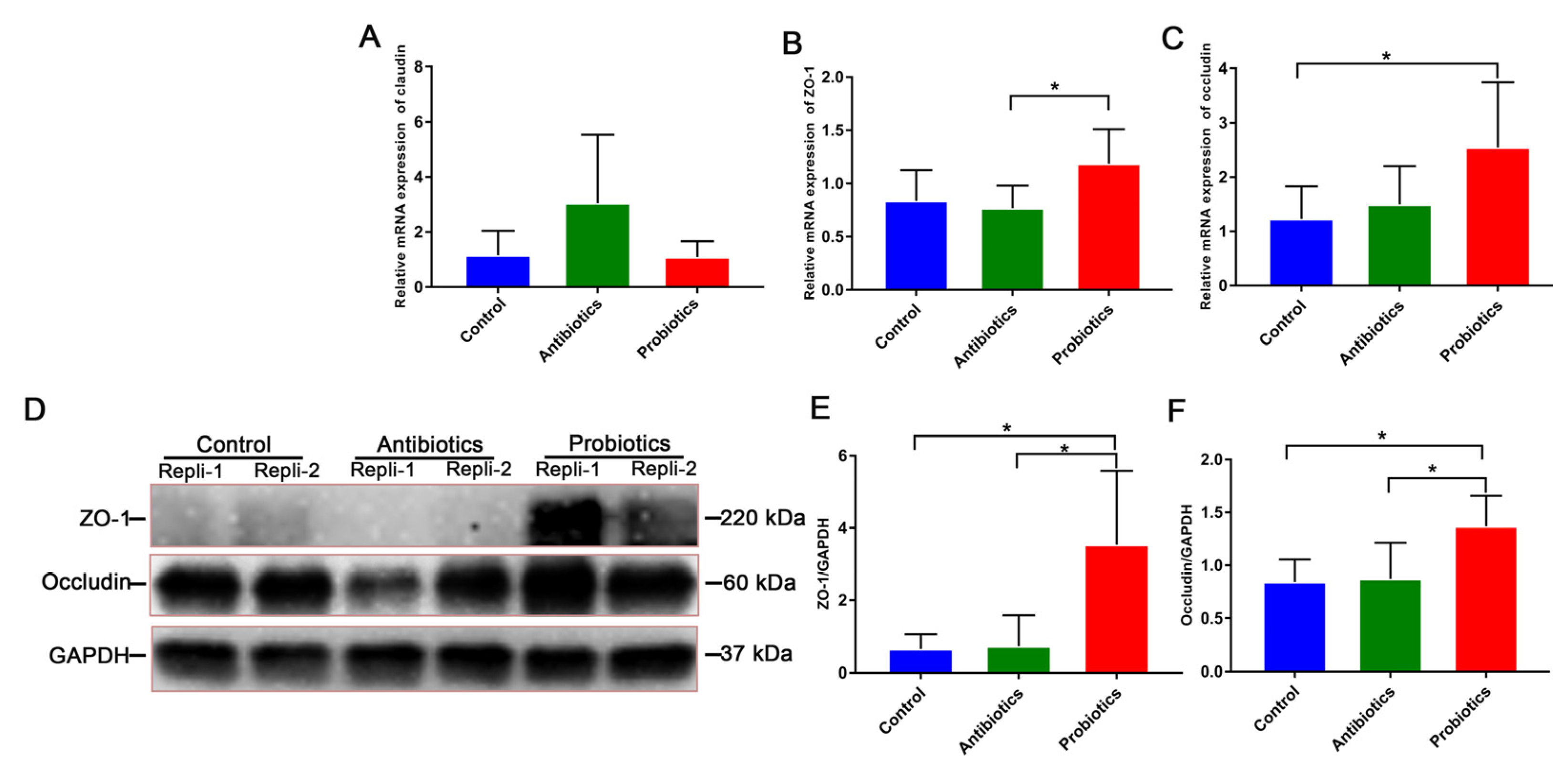
3.4. A Diet Containing BL-S6 Improves Weaned Pigs’ Intestinal Morphology and Epithelial Barrier Function
3.5. Microbiota Diversity in the Cecum Digesta in Response to BL-S6
3.6. The Effects of BL-S6 on the Bacterial Abundance in the Cecum Digesta
3.7. Effects of BL-S6 on the Contents of Organic Acid in Cecum Digesta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boudry, G.; Péron, V.; Le Huërou-Luron, I.; Lallès, J.P.; Sève, B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J. Nutr. 2004, 134, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.P.; Evans, S.L.; Price, L.B.; Silbergeld, E.K. Fate of antimicrobial-resistant enterococci and staphylococci and resistance determinants in stored poultry litter. Environ. Res. 2009, 109, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, Z.; Xu, T.; Qian, M.; Jiang, X.; Zhan, X.; Han, X. Chlortetracycline alters microbiota of gut or faeces in pigs and leads to accumulation and migration of antibiotic resistance genes. Sci. Total Environ. 2021, 796, 148976. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. Probiotic Strains as a Potential Tool for Limiting the Use of Antibiotics, and Improving the Growth and Health of Pigs and Chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Z.; Azad, M.A.K.; Zhang, W.; Blachier, F.; Wang, Z.; Kong, X. Dietary supplementation with Bacillus mixture modifies the intestinal ecosystem of weaned piglets in an overall beneficial way. J. Appl. Microbiol. 2021, 130, 233–246. [Google Scholar] [CrossRef]
- Fu, J.; Wang, T.; Xiao, X.; Cheng, Y.; Wang, F.; Jin, M.; Wang, Y.; Zong, X. Clostridium Butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets. Cells 2021, 10, 527. [Google Scholar] [CrossRef]
- Lin, K.H.; Yu, Y.H. Evaluation of Bacillus licheniformis-Fermented Feed Additive as an Antibiotic Substitute: Effect on the Growth Performance, Diarrhea Incidence, and Cecal Microbiota in Weaning Piglets. Animals 2020, 10, 1649. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Q.; Guo, Q.; Zhang, J.; Sun, Y.; Wei, H.; Shen, L. Isolation and Characterization of a Deoxynivalenol-Degrading Bacterium Bacillus licheniformis YB9 with the Capability of Modulating Intestinal Microbial Flora of Mice. Toxins 2020, 12, 184. [Google Scholar] [CrossRef]
- Hsu, T.C.; Yi, P.J.; Lee, T.Y.; Liu, J.R. Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. PLoS ONE 2018, 13, e0194866. [Google Scholar] [CrossRef]
- Yu, X.; Cui, Z.; Qin, S.; Zhang, R.; Wu, Y.; Liu, J.; Yang, C. Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets. Animals 2022, 12, 1609. [Google Scholar] [CrossRef]
- Lan, R.; Kim, I.H. Effects of Bacillus licheniformis and Bacillus subtilis complex on growth performance and faecal noxious gas emissions in growing-finishing pigs. J. Sci. Food Agric. 2019, 99, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Li, Y.L.; Yu, D.Y.; Rajput, I.R.; Li, W.F. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 2013, 92, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.R.; Agazzi, A.; Awati, A.; Vitari, F.; Bento, H.; Ferrari, A.; Alborali, G.L.; Crestani, M.; Domeneghini, C.; Bontempo, V. Influence of a blend of essential oils and an enzyme combination on growth performance, microbial counts, ileum microscopic anatomy and the expression of inflammatory mediators in weaned piglets following an Escherichia coli infection. Anim. Feed. Sci. Technol. 2015, 209, 219–229. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Y.; Shen, Y.; Li, Q.; Lan, J.; Wu, Y.; Zhang, R.; Cao, G.; Yang, C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021, 100, 101358. [Google Scholar] [CrossRef]
- Liu, X.; Xia, B.; He, T.; Li, D.; Su, J.H.; Guo, L.; Wang, J.F.; Zhu, Y.H. Oral Administration of a Select Mixture of Lactobacillus and Bacillus Alleviates Inflammation and Maintains Mucosal Barrier Integrity in the Ileum of Pigs Challenged with Salmonella Infantis. Microorganisms 2019, 7, 135. [Google Scholar] [CrossRef]
- Wang, K.; Cao, G.; Zhang, H.; Li, Q.; Yang, C. Effects of Clostridium butyricum and Enterococcus faecalis on growth performance, immune function, intestinal morphology, volatile fatty acids, and intestinal flora in a piglet model. Food Funct. 2019, 10, 7844–7854. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Pumford, N.R.; Tang, Z.X.; Lassiter, K.; Ojano-Dirain, C.; Wing, T.; Cooper, M.; Bottje, W. Compromised liver mitochondrial function and complex activity in low feed efficient broilers are associated with higher oxidative stress and differential protein expression. Poult. Sci. 2005, 84, 933–941. [Google Scholar] [CrossRef]
- Klasing, K.C. Nutritional aspects of leukocytic cytokines. J. Nutr. 1988, 118, 1436–1446. [Google Scholar] [CrossRef]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Fu, R.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Yu, B. Effect of dietary supplementation of Bacillus coagulans or yeast hydrolysates on growth performance, antioxidant activity, cytokines and intestinal microflora of growing-finishing pigs. Anim. Nutr. 2019, 5, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; He, Y.; Xiong, X.; Ehrlich, A.; Li, X.; Raybould, H.; Atwill, E.R.; Maga, E.A.; Jørgensen, J.; Liu, Y. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J. Anim. Sci. Biotechnol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Ma, J.; Long, S.; Wang, J.; Gao, J.; Piao, X. Microencapsulated essential oils combined with organic acids improves immune antioxidant capacity and intestinal barrier function as well as modulates the hindgut microbial community in piglets. J. Anim. Sci. Biotechnol. 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shi, J.; Shi, X.; Dong, Y.; Wu, X.; Li, Z.; Fang, Z.; Lin, Y.; Che, L.; Li, J.; et al. Effects of dietary supplementation with lysozyme during late gestation and lactation stage on the performance of sows and their offspring. J. Anim. Sci. 2018, 96, 4768–4779. [Google Scholar] [CrossRef]
- Rajput, I.R.; Li, W.F.; Li, Y.L.; Jian, L.; Wang, M.Q. Application of probiotic (Bacillus subtilis) to enhance immunity, antioxidation, digestive enzymes activity and hematological profile of Shaoxing duck. Pak. Vet. J. 2013, 33, 69–72. [Google Scholar]
- Xu, J.M.A.; Xu, C.P.D.; Chen, X.P.D.; Cai, X.P.D.; Yang, S.M.A.; Sheng, Y.M.A.; Wang, T.P.D. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 2014, 30, 584–589. [Google Scholar] [CrossRef]
- Yousefi, M.; Ahmadifar, M.; Mohammadzadeh, S.; Kalhor, N.; Esfahani, D.E.; Bagheri, A.; Mashhadizadeh, N.; Moghadam, M.S.; Ahmadifar, E. Individual and combined effects of the dietary Spirulina platensis and Bacillus licheniformis supplementation on growth performance, antioxidant capacity, innate immunity, relative gene expression and resistance of goldfish, Carassius auratus to Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 127, 1070–1078. [Google Scholar] [CrossRef]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.; Blikslager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef]
- Aperce, C.C.; Burkey, T.E.; KuKanich, B.; Crozier-Dodson, B.A.; Dritz, S.S.; Minton, J.E. Interaction of Bacillus species and Salmonella enterica serovar Typhimurium in immune or inflammatory signaling from swine intestinal epithelial cells. J. Anim. Sci. 2010, 88, 1649–1656. [Google Scholar] [CrossRef]
- Wang, M.; Yang, C.; Wang, Q.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Yang, H.; Yin, Y. The relationship between villous height and growth performance, small intestinal mucosal enzymes activities and nutrient transporters expression in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 606–615. [Google Scholar] [CrossRef]
- Huang, P.; Cui, X.; Wang, Z.; Xiao, C.; Ji, Q.; Wei, Q.; Huang, Y.; Bao, G.; Liu, Y. Effects of clostridium butyricum and a bacteriophage cocktail on growth performance, serum biochemistry, digestive enzyme activities, intestinal morphology, immune responses, and the intestinal microbiota in rabbits. Antibiotics 2021, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kan, L.; Guo, F.; Liu, Y.; Pham, V.H.; Guo, Y.; Wang, Z. Probiotics Bacillus licheniformis Improves Intestinal Health of Subclinical Necrotic Enteritis-Challenged Broilers. Front. Microbiol. 2021, 12, 623739. [Google Scholar] [CrossRef]
- Wang, Y.; Du, W.; Lei, K.; Wang, B.; Wang, Y.; Zhou, Y.; Li, W. Effects of Dietary Bacillus licheniformis on Gut Physical Barrier, Immunity, and Reproductive Hormones of Laying Hens. Probiotics Antimicrob. Proteins 2017, 9, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Y.H.; Zhou, D.; Wu, Q.; Song, D.; Dicksved, J.; Wang, J.F. Oral Administration of a Select Mixture of Bacillus Probiotics Affects the Gut Microbiota and Goblet Cell Function following Escherichia coli Challenge in Newly Weaned Pigs of Genotype MUC4 That Are Supposed to Be Enterotoxigenic E. coli F4ab/ac Receptor Negative. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Otte, J.M.; Podolsky, D.K. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G613–G626. [Google Scholar] [CrossRef]
- Hu, J.; Chen, L.; Zheng, W.; Shi, M.; Liu, L.; Xie, C.; Wang, X.; Niu, Y.; Hou, Q.; Xu, X.; et al. Lactobacillus frumenti Facilitates Intestinal Epithelial Barrier Function Maintenance in Early-Weaned Piglets. Front. Microbiol. 2018, 9, 897. [Google Scholar] [CrossRef]
- Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011, 32, 256–264. [Google Scholar] [CrossRef]
- Cao, H.; Huang, X.; Gu, Y.; Zheng, X.; Xu, L.; Gai, C. Protective effects of Bacillus licheniformis against Citrobacter freundii infection in Chinese mitten crab Eriocheir sinensis. J. Invertebr. Pathol. 2022, 193, 107805. [Google Scholar] [CrossRef]
- Zhao, D.; Song, S.; Wang, Q.; Zhang, X.; Hu, S.; Chen, L. Discovery of immune-related genes in Chinese mitten crab (Eriocheir sinensis) by expressed sequence tag analysis of haemocytes. Aquaculture 2009, 287, 297–303. [Google Scholar] [CrossRef]
- Qin, D.; Bai, Y.; Li, Y.; Huang, Y.; Li, L.; Wang, G.; Qu, Y.; Wang, J.; Yu, L.Y.; Hou, X. Changes in Gut Microbiota by the Lactobacillus casei Anchoring the K88 Fimbrial Protein Prevented Newborn Piglets from Clinical Diarrhea. Front. Cell Infect. Microbiol. 2022, 12, 842007. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zeng, D.; Wang, H.; Sun, N.; Zhao, Y.; Dan, Y.; Pan, K.; Jing, B.; Ni, X. Probiotic Lactobacillus johnsonii BS15 Promotes Growth Performance, Intestinal Immunity, and Gut Microbiota in Piglets. Probiotics Antimicrob. Proteins 2020, 12, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Wang, L.; Xiong, Y.; Wen, X.; Wang, Z.; Yang, X.; Gao, K.; Jiang, Z. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 2018, 96, 2342–2351. [Google Scholar] [CrossRef]
- Shandilya, U.K.; Sharma, A.; Kapila, R.; Kansal, V.K. Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum modulates immunoglobulin levels and cytokines expression in whey proteins sensitised mice. J. Sci. Food Agric. 2016, 96, 3180–3187. [Google Scholar] [CrossRef]
- Garcia, G.R.; Dogi, C.A.; Poloni, V.L.; Fochesato, A.S.; De Moreno de Leblanc, A.; Cossalter, A.M.; Payros, D.; Oswald, I.P.; Cavaglieri, L.R. Beneficial effects of Saccharomyces cerevisiae RC016 in weaned piglets: In vivo and ex vivo analysis. Benef. Microbes 2019, 10, 33–42. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, L.; Zhang, E.; Yuan, C.; Yang, Q. Oral administration of Bacillus subtilis promotes homing of CD3(+) T cells and IgA-secreting cells to the respiratory tract in piglets. Res. Vet. Sci. 2021, 136, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kalita, A.; Talukdar, M.; Sarma, K.; Kalita, P.C.; Barman, N.N.; Roychoudhury, P.; Kalita, G.; Choudhary, O.P.; Doley, P.J.; Debroy, S.; et al. Lymphocyte subsets in the small intestine of piglets fed with probiotic and zinc: A qualitative and quantitative micro-anatomical study. Folia Morphol. 2022, 81, 82–90. [Google Scholar] [CrossRef]
- Xia, B.; Yu, J.; He, T.; Liu, X.; Su, J.; Wang, M.; Wang, J.; Zhu, Y. Lactobacillus johnsonii L531 ameliorates enteritis via elimination of damaged mitochondria and suppression of SQSTM1-dependent mitophagy in a Salmonella infantis model of piglet diarrhea. FASEB J. 2020, 34, 2821–2839. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, P.; Luise, D.; Correa, F.; Bosi, P. Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health. Microorganisms 2021, 9, 313. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, J.; Yang, S.; Xiao, Q.; Wang, X.; Zhou, Z.; Xiao, Y.; Shi, D. Different Responses of Microbiota across Intestinal Tract to Enterococcus faecium HDRsEf1 and Their Correlation with Inflammation in Weaned Piglets. Microorganisms 2021, 9, 1767. [Google Scholar] [CrossRef]
- Shaaban, S.; Hamad, G.M.; Genena, S.; Meheissen, M.A.; Moussa, S. Evaluation of the antibacterial activity of Lactobacilli probiotics supernatants against Enterococcus faecalis (in-vitro study). BMC Oral Health 2022, 22, 407. [Google Scholar] [CrossRef] [PubMed]
- Valeriano, V.D.; Balolong, M.P.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, N.; Yang, Y.; Lei, Y.; Lyu, J.; Dai, Z.; Kim, I.H.; Li, J.; Wu, Z.; Li, D. Flavor supplementation during late gestation and lactation periods increases the reproductive performance and alters fecal microbiota of the sows. Anim. Nutr. 2021, 7, 679–687. [Google Scholar] [CrossRef]
- Fu, Q.; Tan, Z.; Shi, L.; Xun, W. Resveratrol Attenuates Diquat-Induced Oxidative Stress by Regulating Gut Microbiota and Metabolome Characteristics in Piglets. Front. Microbiol. 2021, 12, 695155. [Google Scholar] [CrossRef]
- Wlodarska, M.; Willing, B.P.; Bravo, D.M.; Finlay, B.B. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci. Rep. 2015, 5, 9253. [Google Scholar] [CrossRef] [PubMed]
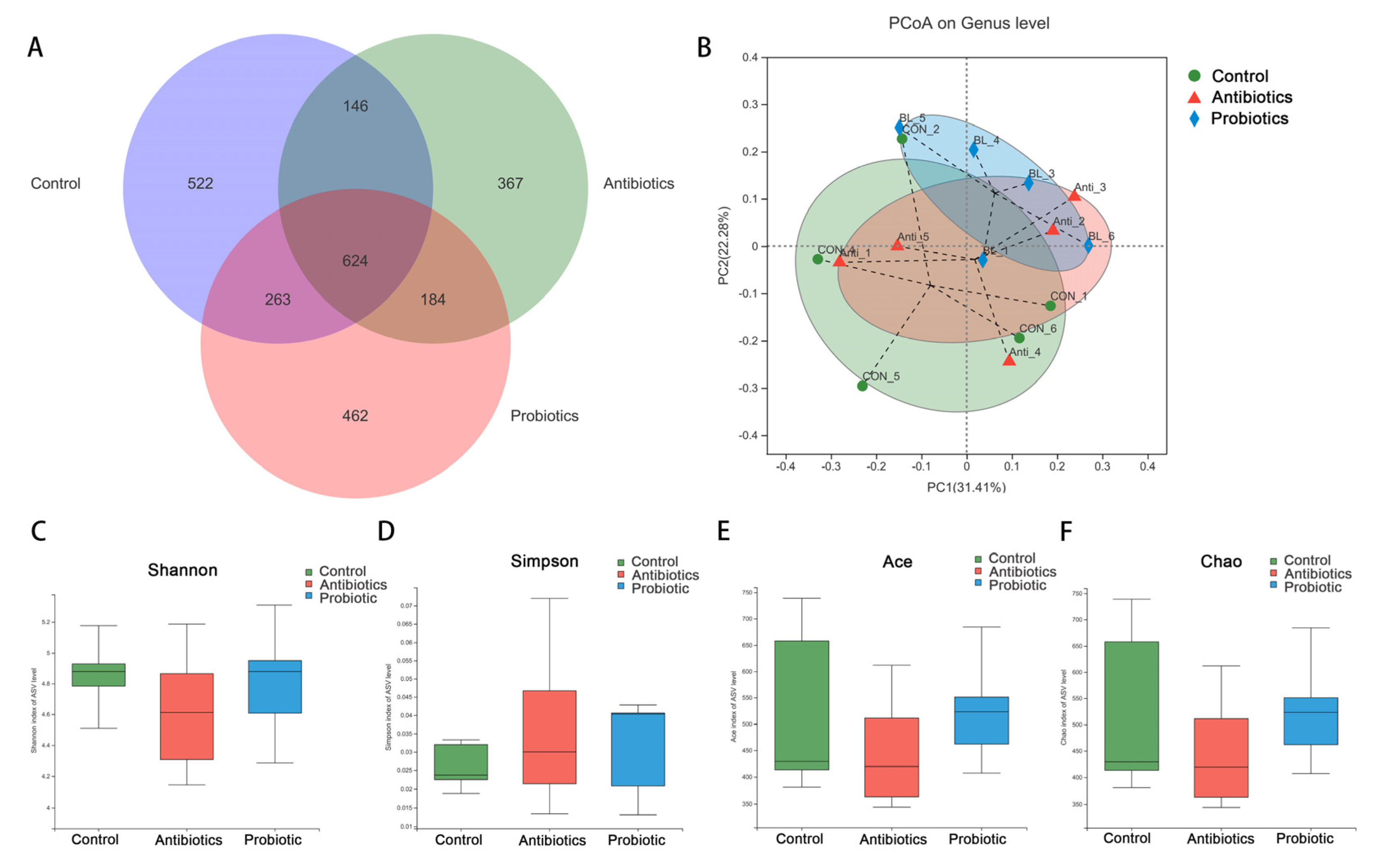
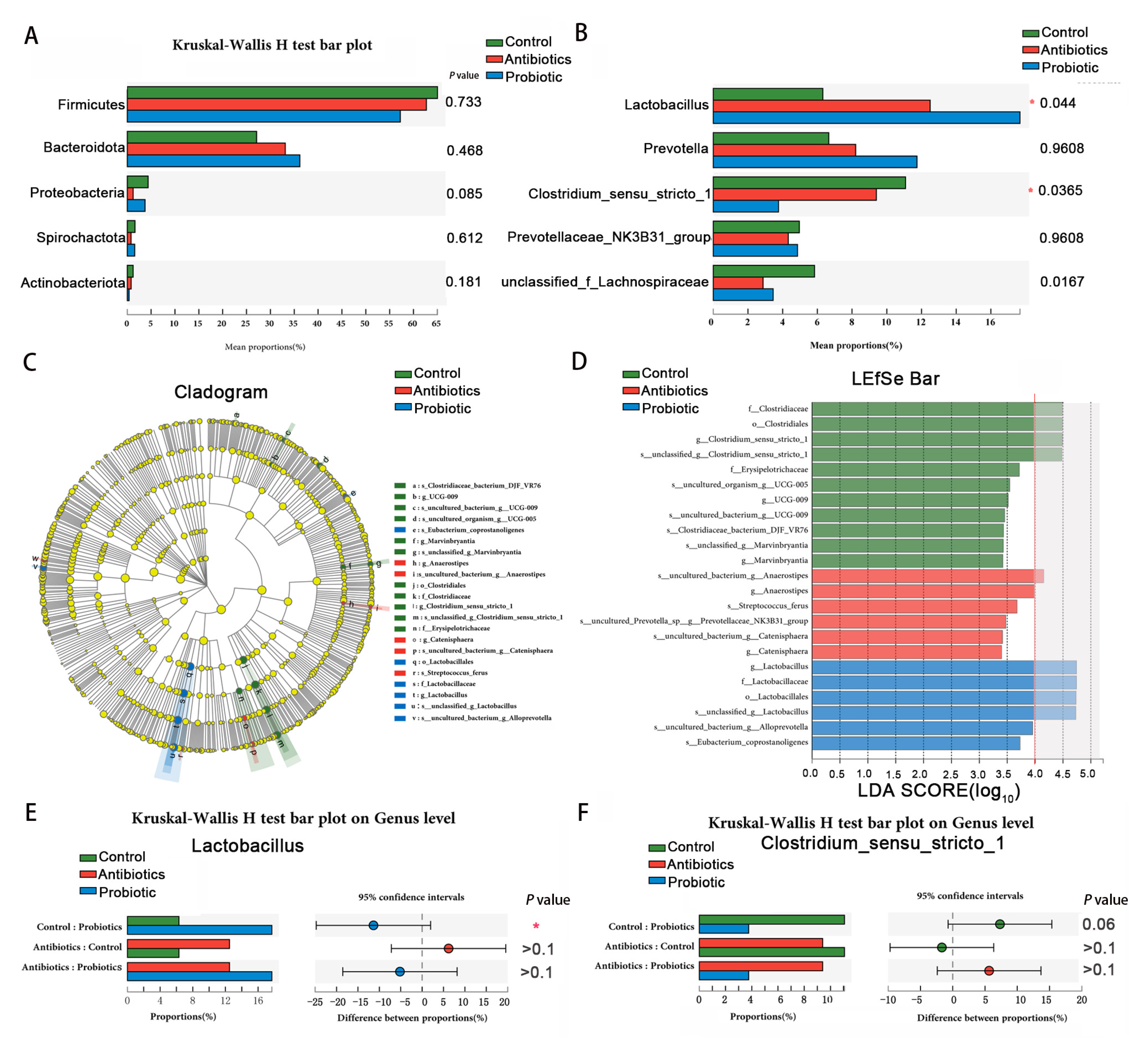
| Items | Control | Antibiotics | Probiotic | SEM | p Value |
|---|---|---|---|---|---|
| Weight, kg | |||||
| Day 0 | 6.50 | 6.50 | 6.50 | 0.21 | 0.97 |
| Day 14 | 8.1 b | 7.94 b | 8.52 a | 0.24 | <0.001 |
| ADG, g | 115 b | 103 b | 144 a | 5.2 | <0.001 |
| ADFI, g | 195 ab | 182 b | 231 a | 7.7 | 0.02 |
| FCR | 1.71 | 1.77 | 1.60 | 0.03 | 0.21 |
| Diarrhea incidence, % | |||||
| Day 0–14 | 14.85 a | 7.34 b | 10.71 b | - | 0.001 |
| Items | Control | Antibiotics | Probiotic | SEM | p Value |
|---|---|---|---|---|---|
| Lactate | 150 b | 153 b | 468 a | 162 | 0.024 |
| Formic acid | 15.8 y | 21.2 xy | 28.6 x | 6.7 | 0.064 |
| Acetic acid | 5552 | 5256 | 4972 | 312 | 0.215 |
| Propionic acid | 1315 y | 1273 xy | 1541 x | 185 | 0.062 |
| Isobutyric acid | 105 xy | 91 y | 118 x | 12 | 0.068 |
| Butyrate | 1076 | 1008 | 914 | 214 | 0.921 |
| Isovaleric acid | 93 | 66 | 78 | 16 | 0.393 |
| Valeric acid | 199 | 188 | 220 | 26 | 0.200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Chen, W.; Meng, K.; Cai, L.; Li, G.; Li, X.; Jiang, X. Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology 2023, 12, 238. https://doi.org/10.3390/biology12020238
Sun W, Chen W, Meng K, Cai L, Li G, Li X, Jiang X. Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology. 2023; 12(2):238. https://doi.org/10.3390/biology12020238
Chicago/Turabian StyleSun, Wenjuan, Wenning Chen, Kun Meng, Long Cai, Guiguan Li, Xilong Li, and Xianren Jiang. 2023. "Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets" Biology 12, no. 2: 238. https://doi.org/10.3390/biology12020238
APA StyleSun, W., Chen, W., Meng, K., Cai, L., Li, G., Li, X., & Jiang, X. (2023). Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology, 12(2), 238. https://doi.org/10.3390/biology12020238






